Abstract
Introduction
Cytolethal distending toxin (Cdt) is potentially one of several virulence factors of Aggregatibacter actinomycetemcomitans, the prime etiological agent of localized aggressive periodontitis (LAP). Little is known regarding the Cdt-specific antibody response in humans. The current study is a quantitative and qualitative evaluation of the toxin-specific antibody response in a cohort of LAP patients and age-, race- and sex-matched controls.
Methods
Ninety-five subjects provided a total of 692 serum samples. Sera were analysed by enzyme-linked immunosorbent assays to determine the titers of antibody against the intact Cdt holotoxin as well as the individual subunit proteins (CdtA, CdtB, and CdtC). Neutralization of growth inhibition mediated by Cdt was evaluated in a modified colony-forming assay using Chinese hamster ovary cells.
Results
Fourteen of the 95 subjects exhibited significant serum Cdt-binding activity. There were no differences in the percentages of seropositive individuals or in the mean antibody titers between the control and LAP groups. Binding activity was detected against each of the three Cdt subunit proteins in all of the positive samples. Neutralization of Cdt-mediated growth inhibition was detected in samples from all of the seropositive subjects (range 20–75%).
Conclusions
Cdt, a recently identified A. actinomycetemcomitans virulence factor, is capable of inducing a neutralizing antibody response indicating that the toxin is produced during natural infection of humans. The failure of a vast majority (20 of 23) of the LAP subjects to mount a significant anti-Cdt response may in part explain their relative susceptibility to the disease.
Keywords: Aggregatibacter (Actinobacillus) actinomycetemcomitans, antibody, cytolethal distending toxin, localized aggressive periodontitis
Aggregatibacter actinomycetemcomitans is a gram-negative coccobacillus that is believed to be a primary etiological agent of localized aggressive periodontitis (LAP) (16). LAP is a relatively rare form of periodontal disease that occurs at a higher incidence in African Americans relative to other racial groups within the United States (17). A. actinomycetemcomitans produces a number of virulence factors including adhesins, endotoxin [lipopolysaccharide (LPS)], leukotoxin (Lkt), and cytolethal distending toxin (Cdt) (7, 28). Certain of these factors facilitate colonization of the periodontal microenvironment, others are proinflammatory and still others act in an immunomodulatory fashion. Collectively, many of these molecules have been implicated as playing a role in the pathogenesis of LAP. A large body of data suggests that inherited anomalies in host immunity to bacterial infection may regulate susceptibility to LAP. Despite this knowledge, the role of specific A. actinomycetemcomitans virulence factors and the acquired immune responses they elicit in the pathogenesis of LAP remain unclear.
Virtually all LAP patients have been shown to mount a humoral immune response against at least one of a variety of A. actinomycetemcomitans antigens including LPS, outer membrane proteins, pili/fimbriae, exopolysaccharides (serotype antigens), and Lkt (14). In general, LAP patients were typically found to have higher titers of antibody relative to appropriate control populations. This has been observed with respect to antibody binding to intact bacterial cells as well as purified virulence factors including LPS and Lkt (4, 21, 25). The purely quantitative nature of these data and the cross-sectional manner in which they were collected make it very difficult to determine whether the antibodies found in LAP patients are protective, destructive, or irrelevant in terms of disease pathogenesis. Therefore, to delineate the role of antibodies directed against bacterial virulence factors in disease regression and/or progression it is necessary to evaluate not only the magnitude of the humoral response but also the functional attributes of the antibody molecules. Although there are studies addressing this issue relative to other A. actinomycetemcomitans virulence factors the literature contains minimal information regarding the humoral immune response against Cdt.
The Cdt is a recently characterized genotoxin that blocks cell cycle progression in specific classes of eukaryotic cells and cell lines. The toxin is a heterotrimer structurally similar to A–B-type toxins. The Cdt holotoxin is made up of three subunit proteins designated CdtA, CdtB, and CdtC. Several other pathogenic bacterial species produce a Cdt including Escherichia coli (10), Helicobacter hepaticus (2), Haemophilus ducreyi (5, 22), Campylobacter jejuni (8) and Shigella spp. (11). The Cdt has, to date, only been found in A. actinomycetemcomitans among bacterial species indigenous to the human oral cavity (29). Several types of oral epithelial cells and T lymphocytes are particularly susceptible to the A. actinomycetemcomitans Cdt. In contrast, human periodontal ligament cells and gingival fibroblasts seem considerably less vulnerable to the toxin (3, 12). Therefore, expression of this relatively unique secreted bacterial virulence factor may in part play a role in the close association between A. actinomycetemcomitans and LAP. This association is supported by the recent work of Tan et al. (27) showing that a majority of diseased sites examined (77%) in LAP patients contained A. actinomycetemcomitans strains that were of the cdt+ genotype.
Very little is known regarding the significance of Cdt-specific antibodies with respect to the pathogenesis of LAP. Thus, in the current study we evaluated the Cdt-specific humoral immune response in a cohort of LAP patients. The primary goal of this study was to test the hypothesis that subjects with a history of LAP or active LAP mount a quantitatively greater antibody response to Cdt than age-, race- and sex-matched controls. Secondarily, we attempted to characterize qualitatively the fine antigen specificity and functional activity of antibodies produced by these two groups of subjects.
Materials and methods
Study population
Serum samples were obtained from 95 of the 118 family members and control subjects who participated in an earlier study described in DiRienzo et al. (6). Sufficient amounts of serum for analysis were not available from the remaining 23 individuals. Twenty-one of the families had at least one member (child or adult) with clinical signs of LAP or a history of the disease and at least one sibling 13 years old or younger with no clinical evidence of LAP. The siblings with no periodontal disease were defined as the LAP-susceptible individuals and the persons with a history of disease or active disease were defined as the probands. Individuals from families with no history of LAP were also included as healthy controls and were matched by age, sex and race to the disease-susceptible subjects. There were a total of 48 male participants (baseline ages 6–41 years) and 47 female (baseline ages 7–42 years) participants. At the initiation of the study 76 of the subjects were periodontally healthy (62 of whom were under 18 years of age), 11 were diagnosed with LAP and eight with chronic periodontitis. Clinical examinations were conducted and blood samples were drawn periodically throughout the 7-year evaluation period. The number of blood draws varied between subjects depending upon the time of their entry into the study and their compliance with appointment dates (ranging from 1 to 28 samples per subject). Patients received treatment as deemed necessary. The clinical examination included evaluation of the plaque index, gingival index, probing depths, clinical attachment levels, and furcation involvement. Full-mouth radiographs were taken at the baseline visit and supplemented with bitewing and/or peri-apical radiographs as needed at subsequent visits. Based on the results of sequential examinations a change in status from periodontal health to LAP was referred to as a conversion. Such an event was defined as an increase of 2 mm or more relative to the baseline level in the distance measured from the cementoenamel junction to the bottom of the periodontal pocket or an increase of at least 2 mm in probing depth associated with the permanent incisors and or first molars. This change was confirmed over a 3-month interval and had to coincide with a loss of clinical attachment greater than or equal to 1 mm in relation to the baseline examination. Based on these criteria, 12 subjects developed LAP over the course of the 7-year study. Sera were isolated from blood drawn during this time and stored at −20°C. A total of 692 serum samples from the 95 subjects were analysed in this study. The serum samples were identified by coded numbers from 1 to 692 and the determination of anti-Cdt antibody titers was performed in a blinded manner.
Isolation of recombinant Cdt extracts and subunit protein antigens
Escherichia coli BL21(DE3) F− ompT hsdSB (rB− mB−) gal dcm (DE3) (pET15bCdt) was cultured in 10 ml of Luria Bertani medium containing 50 μg/ml ampicillin and grown overnight with vigorous shaking at 37°C as described previously (19). Briefly, bacterial suspensions were sonicated for 1 min at 4°C (Braun, Norwalk, CT), then centrifuged at 12,000 g for 10 min (Spinco model SS-34 rotor) to remove unbroken cells and sterilized by passage through a 0.22-μm pore-size filter (Whatman Inc, Clifton NJ). The Micro BCA protein assay kit (Pierce, IL) was used to determine total protein concentration.
Recombinant clones E. coli BL-21(DE3) (pET15bCdtA), E. coli BL-21(DE3) (pET15bCdtB), and E. coli BL-21(DE3) (pET15bCdtC) were used to prepare the three amino-terminal histidine-tagged wild-type His6-CdtA, His6-CdtB and His6-CdtC proteins, respectively (18). The proteins were obtained from isopropyl β-D-1-thiogalactopyranoside-induced cultures by affinity chromatography on nickel-iminodiacetic acid columns (EMD Chemicals [Novagen], Gibbstown, NJ) as described previously (18). Yields averaged 25–30 μg protein/ml of culture. The final protein preparations were dialysed to remove urea while promoting protein refolding, passed through 45-μm filters and quantified with the Micro BCA protein assay kit (Pierce, Rockville, IL). Purity was assessed by analysis on 10–20% sodium dodecyl sulfate–polyacrylamide gels (BioRad, Hercules, CA) stained with Coomassie Brilliant Blue. Aliquots of the quantified protein samples were stored at −70°C in a buffer containing 10 mM Tris–HCl (pH 7), 100 mM NaCl, 5 mM MgCl2, and 5 mM imidazole. The proteins were also analysed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis as previously described (15). Briefly, the samples were run at a room temperature in a 10–20% Tris–HCl Ready Gel (Bio-Rad) at 100 V. The gel was stained with Coomassie Blue for 1 h and then analysed.
Preparation of reconstituted toxin
Cdt was reconstituted in vitro by mixing equimolar concentrations of the individual subunit proteins in a coating buffer containing 0.1 M NaHCO3 (pH 8.6) and incubated at 4°C for at least 1.5 h.
Enzyme-linked immunosorbent assays (ELISA)
To determine serum antibody titers either the reconstituted recombinant Cdt (0.5 μg/well) or individual subunit proteins (166 ng/well) in 0.1 M NaHCO3 (pH 8.6) were used to coat 96-well microtiter plates overnight at 4°C. The plates were then washed three times with 0.1% phosphate-buffered saline (PBS) Tween-20 in an automatic washer (Ultrawash Plus, Dyna-tech Laboratories, Chantilly, VA). Blocking buffer (1% bovine serum albumin, 0.02% NaN3) was added and the plates were incubated for 1 h. The plates were washed three times. Sera were serially diluted from 1/100 to 1/3200 in 0.1% PBS-Tween 20 and the dilutions were added to triplicate wells. The plates were incubated for 1 h and washed three times. Bound human immunoglobulin G (IgG) was detected with a monoclonal antibody (mAb) anti-human IgG alkaline phosphatase conjugate (Amersham Biosciences, Piscataway, NJ) at 1/5000 and 1/25,000 dilutions in 0.1% PBS-Tween 20 for 1 h. The working dilutions of the anti-human IgG mAb were determined in preliminary experiments by obtaining titration curves. All incubations were performed in a humid atmosphere at 37°C. The plates were washed and developed using SIGMA FAST™ p-nitrophenylphosphate tablet sets (pNPP alkaline phosphatase substrate) in the dark for 30 min. A 1 M NaOH stop solution (30 μl/well) was added and the absorbance was read at 405 nm in an HTS 7000 BioAssay Plate Reader (Perkin Elmer, Norwalk, CT). Serum titers were expressed as the reciprocal of the dilution that yielded a half-maximal value of absorbance at 405 nm (A405). Enzyme immunological units (EIU) were calculated from the absorbance values using the formula:
(17, 23). Those samples with EIU scores two standard deviations above the mean for the entire set of sera were considered positive for reactivity against Cdt.
To determine antibody responses against each of the individual subunit proteins dose–response curves were obtained. Bound subunit proteins were detected with a 1:3000 dilution of anti-His-Tag mAb (Novagen) in PBS-1% bovine serum albumin followed by a 1:3000 dilution of anti-mouse IgG horseradish peroxidase conjugate (Amersham Biosciences, Piscataway, NJ) for 1 h at room temperature. The plates were developed with 100 μl horseradish peroxidase substrate ABTS-100 (Rockland, Inc., Gilbertsville, PA) and read as described above. Based on the dose–response data a subunit protein concentration of 166 ng/well was chosen for titration of the human sera.
Serum neutralization assay
The ability of serum antibodies to neutralize the Cdt was determined using a modification of a previously developed cell growth inhibition assay (19). Sonic extracts prepared from E. coli BL21(DE3)(pET15bCdt) were used as the source of Cdt. Longitudinally collected samples from each subject were pooled at a 1:1 ratio (based on volume) to obtain enough serum for analysis. The total volume of the pooled serum samples was adjusted with Ham’s F12 medium to yield a 1:5 dilution and sterilized by passage through a 0.45-μm filter. Two aliquots of each of the sterile serum preparations were mixed with appropriate volumes of Cdt-containing sonic extracts and adjusted to a volume of 1 ml yielding a final serum dilution of 1:10 and total protein concentrations of 15 and 35 ng protein/μl. These were then incubated at 4°C on a shaker for 1.5 h. Chinese hamster ovary (CHO) K-1 cells were grown in T-75 tissue culture flasks in Ham’s F12 medium, containing 5% fetal calf serum (FCS), at 37°C in the presence of 5% CO2. After 3–4 days of growth the cells were harvested, counted and adjusted to 1 × 104 cells per ml of medium. The CHO cells were seeded in six-well tissue culture plates (300 cells in 3 ml medium per well in triplicate) and were allowed to attach to the plates. One milliliter of each of the Cdt-serum preparations was added to different wells of CHO cells and incubated for 30 min at 37°C. The wells were washed with PBS and 2 ml fresh Ham’s F12 medium containing 5% FCS was added to each well. The plates were then incubated for 6 days at 37°C to allow colonies to form. The medium was then removed and the cells were fixed with 10% formalin, stained with crystal violet for 10 min, dried and counted. The number of colonies per well was expressed as colony-forming units (CFUs). A dose–response curve for wild-type reconstituted holotoxin was also prepared using 0–2.5 μg of total protein/ml. Each sample was analysed three times.
Statistical analyses
Data obtained from ELISA were analysed with the software package SAS version 9.1 and descriptive findings were produced using SAS Proc Univariate. One-way repeated measures analysis of variance with post-hoc paired comparisons was applied to determine whether there were any statistically significant differences in the antibody response against the different clinical groups as well as against each of the subunits. Regression analysis was applied for the neutralization assay.
Results
Humoral antibody response of the study subjects to the Cdt
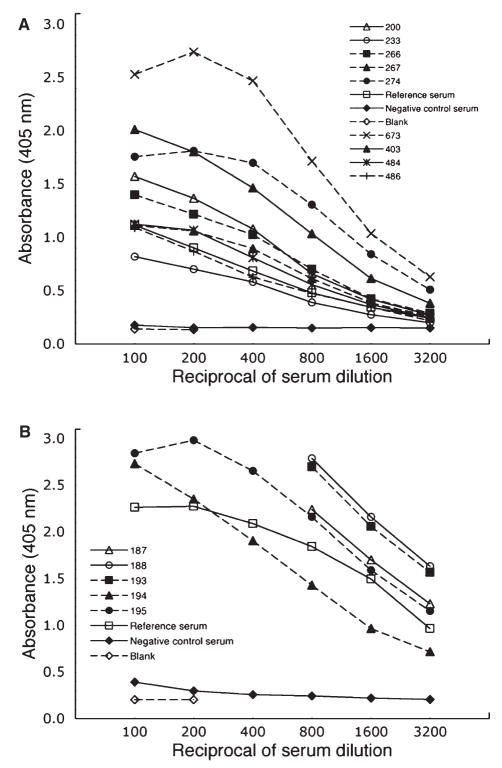
An ELISA was used to analyse 692 longitudinally collected serum samples originally obtained from 95 previously described subjects for Cdt-binding antibody (6). All of the available samples provided by each subject were evaluated (ranging in number from 1 to 28). A subset of 113 samples was randomly chosen for analysis to standardize the assay as well as to identify internal positive (reference serum) and negative (no Cdt-specific antibody) control sera. A representative sampling of the results of this initial screen are shown in Fig. 1A, B. Serum samples 692 and 30 were selected to serve as positive and negative controls, respectively, throughout subsequent analyses. The titers (reciprocal of the dilution that yielded a half-maximal A405 value) of the individual samples were distributed over a wide range of values. For example, sample 267 had a relatively low titer (approximately 800) in contrast to samples 187, 188 and 193 which had titers greater than 3200. There was significant variability in the absorbance values of the same reference and negative control sera among the ELISA plates.
Fig. 1.
Quantification of serum antibody binding to the cytolethal distending toxin (Cdt) holotoxin. Sera were serially diluted from 100 to 3200 and added to enzyme-linked immunosorbent assay plates coated with reconstituted Cdt. Values of optical density at 405 nm (OD405) are plotted against the reciprocal of the corresponding dilution factor. (A) and (B) show representative titration curves of samples chosen from the 692 that were analysed (designated by number).
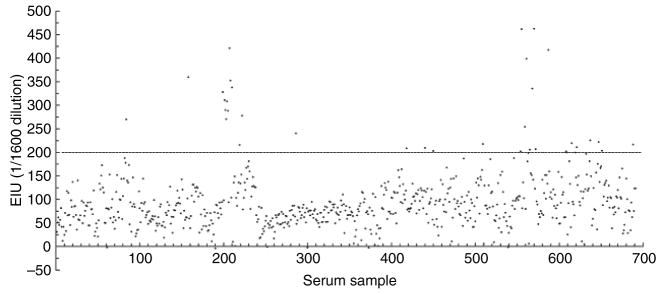
To normalize the responses of all of the serum samples to the Cdt and to control for the variability inherent in the examination of large numbers of ELISA plates EIU scores were calculated and used to express the results. Serum dilutions from 1:800 to 1:3200 were evaluated. A statistical analysis was performed by evaluating the EIU scores with the Proc Univariate report with a cut-off value of 95%. The reference and negative control sera were excluded from the statistical analysis because it was necessary to use them to calculate the EIU scores. Fig. 2 shows the distribution of the EIU scores at the dilution 1:1600; this dilution was chosen because it was at this dilution that the EIU scores approached a normal distribution. A majority of the samples exhibited EIU scores between 50 and 150 while a limited number had relatively higher values. Only those samples with EIU scores in the 95th percentile and higher were considered seropositive. The horizontal line in Fig. 2 depicts the cut-off value of EIU 200 (95th percentile) and separates the seropositive from the seronegative samples. Using this approach it was determined that 35 out of 692 samples contained relatively high levels of Cdt-binding antibody (EIU scores ranging from 203 to 462). These sera originated from 14 of the 95 individuals from whom blood was collected. These will be referred to as seropositive subjects 1 through 14. Therefore, only 14.7% of the 95 subjects exhibited significant serum Cdt-binding activity as defined by the criteria established for the ELISA.
Fig. 2.
Distribution of enzyme immunological units (EIU) scores representing cytolethal distending toxin (Cdt) -binding activity in sera at a 1:1600 dilution. To account for day-to-day variations in the enzyme-linked immunosorbent assays all data were converted EIU scores. The scores measured at a 1:1600 were chosen for evaluation because they most closely approached a normal distribution. The horizontal line depicts the cut-off value of EIU 200 (95th percentile) and separates the positive from the negative samples.
Distribution of the seropositive and seronegative sera among the diagnostic categories is shown in Fig. 3. Although the majority of positive samples (10/14) were obtained from clinically healthy individuals, the percentages of seropositive subjects within each group were very similar. Interestingly, only a small percentage of the LAP subjects (12.5%) were seropositive with respect to Cdt-binding antibody. The serum sample from each subject exhibiting the highest EIU score was further analysed by ELISA extending the dilutions up to 1:204,800 (data not shown). Whereas most of the positive sera lost binding activity at dilutions between 1:6400 to 1:12,800, there were two samples (324 and 688) that exhibited binding activity up to a dilution slightly greater than 1:51,200. These sera were from a healthy subject (324) and a conversion patient (688).
Fig. 3.
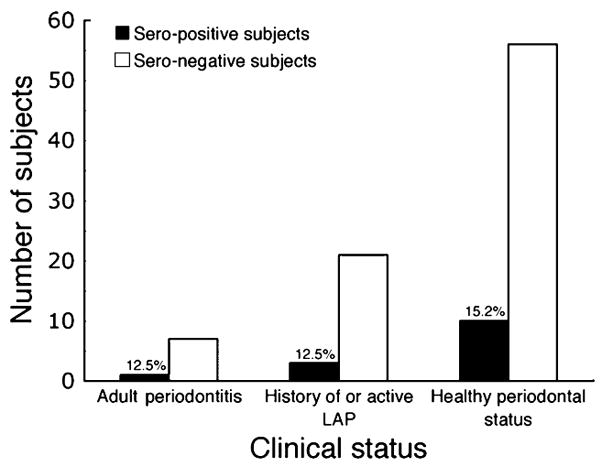
Distribution of seropositive individuals among the three diagnostic categories. Of the 14 seropositive subjects, 10 remained clinically healthy, three were diagnosed with localized aggressive periodontitis (LAP) and one with chronic periodontitis. The differences in the percentages of seropositive subjects detected in each category were not statistically significant (P > 0.05).
With the significant disparity in the number of samples collected per seropositive subject (ranging from two to 16) no consistent pattern of anti-Cdt antibody production was detected over time. Only two of the 14 seropositive subjects provided three or more positive samples: seropositive subject 7 (nine out of 14) and seropositive subject 11 (10 out of 11) (data not shown). Interestingly, seropositive subject 7 developed LAP during the study and eight of the nine positive samples were obtained after the single conversion event. In contrast, seropositive subject 11 was a control subject who remained clinically healthy throughout the study.
Binding of serum IgG antibody to individual Cdt subunits
The initial phase of our analysis allowed us to identify serum samples that exhibited significant Cdt-binding activity. These experiments were conducted with intact holotoxin so it is unclear whether the antibodies were specific for conformation-dependent epitopes formed via the interaction of the three subunits or, alternatively, for epitopes unique to the individual subunits. Therefore, the 14 serum samples with the highest EIU scores (dilution of 1:1600) against the reconstituted toxin were further analysed by ELISA for IgG antibodies against each one of the subunits. This approach not only allowed us to map the specificity of the antisera but also to determine whether the antibody responses were skewed towards any one of the subunits. Equal volumes of serum from all the positive samples were pooled and used as the reference serum in these analyses. The negative control serum remained the same as in the previous ELISA experiments. EIUs were determined for each of the individual high-titer antisera (diluted 1:1600) to the individual Cdt subunits (Fig. 4). Statistical analysis showed that the mean EIU scores for CdtA, CdtB, and CdtC were not significantly different (α > 0.05).
Fig. 4.
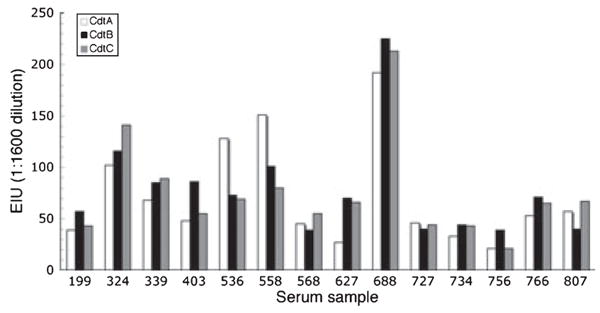
Antibody binding to the individual cytolethal distending toxin (Cdt) subunit proteins (CdtA, CdtB, and CdtC). Samples from the 14 seropositive individuals were further evaluated by enzyme-linked immunosorbent assay on plates coated with the individual Cdt subunit proteins. Mean enzyme immunological unit (EIU) scores were calculated for the samples provided by each subject at a 1:1600 dilution. Although the patterns of reactivity against the three proteins differed between individuals there was not a statistically significant difference in the overall binding to the proteins (P > 0.05).
Serum neutralization of Cdt
The results derived from the ELISAs are purely quantitative in nature because they do not provide information regarding the functional characteristics of the Cdt-binding antibody. To address this issue experiments were conducted to determine whether the serum antibodies could neutralize the toxin. Neutralizing activity was defined as the ability of the sera to block Cdt-mediated growth inhibition of CHO cells as measured in a colony-forming assay. Growth inhibition was determined by calculating the number of CFUs in Cdt-treated cultures relative to untreated control cultures. To have sufficient volumes of antisera to conduct this series of experiments it was necessary to pool the sera collected at different time-points for each of the 14 seropositive subjects. The CHO cells were exposed to recombinant Cdt, Cdt preincubated with negative control antisera (1:10 final dilution) or Cdt preincubated with pooled positive antisera (1:10 final dilution). Untreated CHO cells served as the negative control. Additionally, extract prepared from wild-type E. coli BL21(DE3) had no effect on the growth of CHO cells (data not shown). Preliminary assays showed that 15 ng protein/μl extract consistently resulted in a 50% reduction in CFU relative to controls and was therefore chosen as the appropriate concentration for the neutralization assays (data not shown). The cells were exposed to the various preparations for 30 min. A representative assay is shown in Fig. 5A. In this example, there was a slight reduction in the number of CFUs in cultures exposed to Cdt extract preincubated with one of the 14 pooled positive antisera (Panels a versus d). Cells exposed to Cdt extract preincubated with the negative control serum exhibited a significant reduction in the number of CFUs (Panels a versus c). To eliminate tissue culture plate-to-plate variation, the percentage of neutralization of the cytotoxic effect, relative to that of the positive control, was calculated for each of the 14 pooled serum samples. All of the samples exhibited some neutralizing activity ranging from an approximately 20% to a 75% reduction of the cytotoxic effect observed in the absence of serum (Fig. 5B). There was no correlation between the extent of serum neutralizing activity and the clinical status of each of the 14 seropositive subjects. When the percentage inhibition of cell death was plotted against the mean EIU score for each subject an inverse relationship was observed; higher percentages of neutralizing activity correlated with lower mean EIU scores (P = 0.02, data not shown).
Fig. 5.
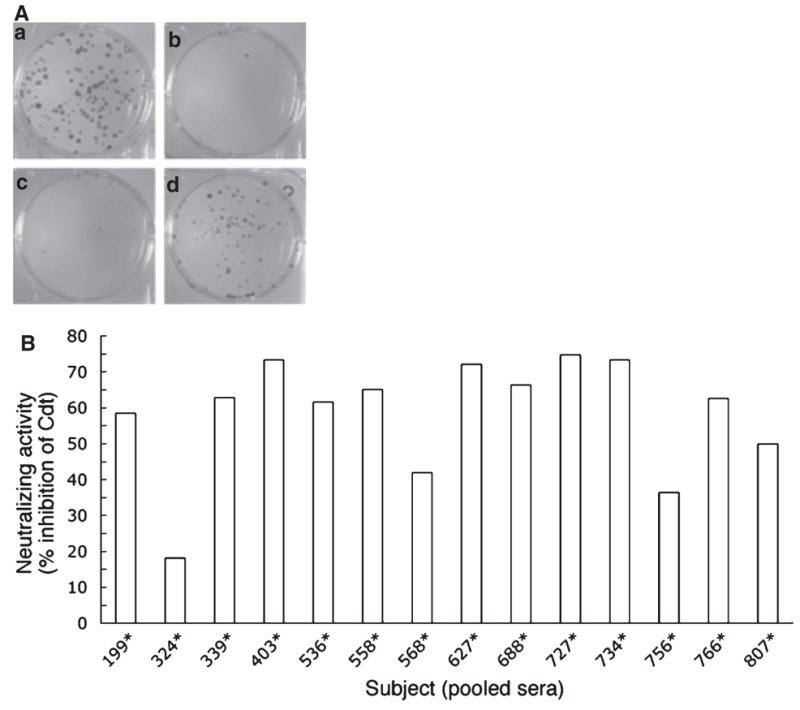
Neutralization of cytolethal distending toxin (Cdt) -mediated growth inhibition of Chinese hamster ovary (CHO) cells. A modified colony-forming assay was used to evaluate Cdt neutralizing activity in pooled samples of serum from each of the 14 seropositive subjects. (A) A typical assay in which wells contained: (a) untreated CHO cells, (b) Cdt-treated cells, (c) cells treated with Cdt that had been preincubated with negative control serum, and (d) cells treated with Cdt that had been preincubated with serum from a seropositive subject. (B) The percentage neutralization of Cdt-mediated growth inhibition of CHO cells for each of the 14 seropositive subjects.
Discussion
It is the general consensus that patients with LAP have elevated levels of serum antibodies against various antigens of A. actinomycetemcomitans (14). The goal of this study was to determine whether this observation also pertains to a recently identified A. actinomycetemcomitans virulence factor, Cdt. The results showed no statistically significant difference in the mean Cdt-specific antibody levels between subjects with a history of LAP or with active LAP and healthy individuals. Therefore, the hypothesis that individuals with a history of LAP or with active LAP have higher levels of antibody to Cdt was rejected. There are no other reports in the literature addressing the A. actinomycetemcomitans Cdt-specific antibody response in LAP patients. However, our finding is in agreement with that reported by Johansson et al. (9), who reported that the levels of Cdt-specific serum antibodies detected in the patients with chronic periodontitis were essentially equivalent to those of age-matched periodontally-healthy control subjects.
Data derived from ELISA provides only a quantitative measurement of antibody levels in serum. This type of information, though certainly of use, does not provide an indication of the functional activity of the antibody. There are numerous human infectious diseases in which microbes elicit quantitatively robust systemic humoral immune responses that fail to provide protection, as is the case with infection by the human immunodeficiency virus (13). Hence, more detailed analyses of the seropositive samples were conducted. They all exhibited activity against each of the subunit proteins (CdtA, CdtB, and CdtC). There were no statistically significant differences in the absolute amounts of antibody against the three subunits (α > 0.05). The only report in the literature regarding the subunit specificity of the human antibody response to Cdt is that of Mbwana et al. (20) who evaluated responses against the Cdt from H. ducreyi in patients with chancroid. The results of that study showed that the only subunit recognized by all of the sera was CdtA. Conversely, our results indicated a far more diverse response composed of antibody reactive with each of the three subunits. This suggests that none of the subunits exhibit so-called immunodominance. With regards to neutralizing activity, all 14 samples were found to neutralize the A. actinomycetemcomitans Cdt in a colony-forming assay, though some more efficiently than others. This observation is similar to that reported by Abuoun et al. (1) who detected Cdt-neutralizing antibody in humans naturally infected with toxin-producing strains of C. jejuni.
In an attempt to further elucidate the implications of the results described in this paper the data were considered in relation to microbiological data previously reported by our laboratory (6). That study characterized A. actinomycetemcomitans isolated from the same individuals who provided sera for the current study. The bacterium was isolated from five of the 10 seropositive periodontally healthy subjects and all of the isolates belonged to restriction fragment length polymorphism (RFLP) groups found to produce minimal if any Cdt. In contrast, A. actinomycetemcomitans belonging to RFLP groups associated with high levels of Cdt production were isolated from six of nine seronegative conversion subjects and eight of 12 seronegative probands diagnosed with LAP. Taken together these observations suggest that individuals who mount a significant anti-Cdt neutralizing antibody response directed against all three subunits of the holotoxin are immune to highly cytotoxic strains of A. actinomycetemcomitans. With respect to the three seropositive subjects who converted to disease a majority of the positive samples (nine out of 11) were obtained at time-points following their clinical conversions. None of these individuals exhibited further breakdown during the study. This may in part explain why a majority of the seropositive subjects did not develop LAP and of those that did, so-called ‘burnout’ of the disease occurred following induction of the anti-Cdt response. Additionally, the presence of neutralizing antibody in the serum of individuals known to be colonized by Cdt-producing strains of A. actinomycetemcomitans indicates that the toxin is produced during natural infection of humans.
Why some individuals develop a protective anti-Cdt response and others do not is a matter of conjecture. In all likelihood both bacterial and host factors are involved in this process. Since Cdt is known to have a cytotoxic effect on lymphocytes and plasmacytoid cells it is possible that the toxin itself down-modulates the production of antibody by both direct and indirect effects on B cells (23, 24, 26). Clearly, genetic regulation of the anti-Cdt antibody response must be considered. The specificity of an antibody molecule is determined by the amino acid sequences of the variable regions of the light and heavy chain proteins. It is therefore plausible that some individuals inherit the genetic elements which encode ‘protective’ specificities while others produce antibody against biologically irrelevant portions of the Cdt (or the component subunit proteins). This can only be investigated via a molecular genetic evaluation of the genes encoding the variable regions of ‘protective’ antibodies, which is well beyond the scope of the current study.
Despite the fact that a larger sample size would allow a more powerful analysis of the A. actinomycetemcomitans Cdt-specific antibody response in LAP, there are points to be taken from this study. First, the fact that an individual is diagnosed with the disease and known to be infected with a toxin-producing strain of the organism does not infer the presence of Cdt-specific serum antibody. Second, and possibly more important, high titers of anti-Cdt antibody do not imply potent functional activity. It could be argued that high titers of antibody against the Cdt result from constant stimulation of the response as the result of an inability to neutralize the toxin and/or eliminate the bacterium. Finally, when considered along with the previously reported microbiological data, as described above, the results of the current study suggest that low titer sera with potent Cdt-neutralizing activity may represent one aspect of a protective response against A. actinomycetemcomitans.
In summary, these results demonstrate that the Cdt is capable of inducing a neutralizing antibody response in humans naturally infected with A. actinomycetemcomitans. The data from this study provide a foundation for extending studies of the humoral antibody response to the A. actinomycetemcomitans Cdt. Such endeavors will help clarify the role of Cdt and toxin-specific antibody in the pathogenesis of LAP.
Acknowledgments
This work was supported by USPHS grants DE012593 and DE017679 from the National Institute of Dental and Craniofacial Research. The authors would like to thank Dr Mark Cohen (Statistician, Naval Institute of Dental and Biomedical Research, Great Lakes, IL) for performing the statistical analysis of the data.
References
- 1.AbuOun M, Manning G, Cawthraw SA, et al. Cytolethal distending toxin (CDT) -negative Campylobacter jejuni strains and anti-CDT neutralizing antibodies are induced during human infection but not during colonization in chickens. Infect Immun. 2005;73:3053–3062. doi: 10.1128/IAI.73.5.3053-3062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avenaud P, Castroviejo M, Claret S, Rosenbaum J, Megraud F, Menard A. Expression and activity of the cytolethal distending toxin of Helicobacter hepaticus. Biochem Biophys Res Comm. 2004;318:739–745. doi: 10.1016/j.bbrc.2004.04.089. [DOI] [PubMed] [Google Scholar]
- 3.Belibasakis G, Johansson A, Wang Y, et al. Inhibited proliferation of human periodontal ligament cells and gingival fibroblasts by Actinobacillus actinomycetemcomitans: involvement of the cytolethal distending toxin. Eur J Oral Sci. 2002;110:366–373. doi: 10.1034/j.1600-0722.2002.21350.x. [DOI] [PubMed] [Google Scholar]
- 4.Califano JV, Pace BE, Gunsolley JC, Schenkein HA, Lally ET, Tew JG. Antibody reactive with Actinobacillus actinomycetemcomitans leukotoxin in early-onset periodontitis patients. Oral Microbiol Immunol. 1997;12:20–26. doi: 10.1111/j.1399-302x.1997.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 5.Cope LD, Lumbey S, Latimer JL, et al. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiRienzo JM, Slots J, Sixou M, et al. Specific genetic variants of Actinobacillus actinomycetemcomitans correlate with disease and health in a regional population of families with localized juvenile periodontitis. Infect Immun. 1994;62:3058–3065. doi: 10.1128/iai.62.8.3058-3065.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fives-Taylor PM, Meyer DH, Mintz KP, Brisette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontol 2000. 1999;20:136–167. doi: 10.1111/j.1600-0757.1999.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 8.Hassane DC, Lee RB, Picket CL. Campylobacter jejuni cytolethal distending toxin promotes DNA repair responses in normal human cells. Infect Immun. 2003;71:541–545. doi: 10.1128/IAI.71.1.541-545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson A, Buhlin K, Koski R, Gustafsson A. The immunoreactivity of systemic antibodies to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in adult periodontitis. J Eur Oral Sci. 2005;113:197–202. doi: 10.1111/j.1600-0722.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson WM, Lior H. Response of Chinese hamster ovary cells to a cytolethal distending toxin (CDT) of Escherichia coli and possible misinterpretation as heat-labile (LT) enterotoxin. FEMS Microb Lett. 1987;43:19–23. [Google Scholar]
- 11.Johnson WM, Lior H. Production of Shiga toxin and a cytolethal distending toxin (CLDT) by serogroups of Shigella spp. FEMS Microb Lett. 1987;48:235–238. [Google Scholar]
- 12.Kanno F, Volgina A, Korostoff J, DiRienzo JM. Differential response of human oral cells to the cytolethal distending toxin of Actinobacillus actinomycetemcomitans. J Periodontol. 2005;76:1189–1201. doi: 10.1902/jop.2005.76.7.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlsson Hedestam GB, Fouchier RAM, Phogat S, Burton DR, Sodroski J, Wyatt RT. The challenge of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat Rev Microbiol. 2008;6:143–155. doi: 10.1038/nrmicro1819. [DOI] [PubMed] [Google Scholar]
- 14.Kinane DF, Mooney J, Ebersole JL. Humoral immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontal disease. Periodontol 2000. 1999;20:289–340. doi: 10.1111/j.1600-0757.1999.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1972;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Lang N, Bartold PM, Cullinan M, et al. Consensus report: aggressive periodontitis. Ann Periodontol. 1999;4:53. [Google Scholar]
- 17.Löe H, Brown LJ. Early onset periodontitis in the United States of America. J Periodontol. 1991;62:608–616. doi: 10.1902/jop.1991.62.10.608. [DOI] [PubMed] [Google Scholar]
- 18.Mao X, DiRienzo JM. Functional studies of the recombinant subunits of a cytolethal distending holotoxin. Cell Microbiol. 2002;4:245–255. doi: 10.1046/j.1462-5822.2002.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer MPA, Bueno LC, Hansen EJ, DiRienzo JM. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect Immun. 1999;67:1227–1237. doi: 10.1128/iai.67.3.1227-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mbwana J, Ahmed HJ, Ahlman K, et al. Specificity of antibodies directed against the cytolethal distending toxin of Haemophilus ducreyi in patients with chancroid. Microb Pathog. 2003;35:133–137. doi: 10.1016/s0882-4010(03)00111-6. [DOI] [PubMed] [Google Scholar]
- 21.Okuda JG, Kato T, Naito Y, Takazoe I. Precipitating antibody against lipopolysaccharide of Haemophilus actinomycetemcomitans in human serum. J Clin Microbiol. 1986;24:846–848. doi: 10.1128/jcm.24.5.846-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purven M, Falsen E, Lagergård T. Cytotoxin production in 100 strains of Haemophilus ducreyi from different geographic locations. FEMS Microb Lett. 1995;129:221–224. doi: 10.1111/j.1574-6968.1995.tb07583.x. [DOI] [PubMed] [Google Scholar]
- 23.Sato T, Koseki T, Yamato K, et al. p53-Independent expression of p21CIP1/WAF1 in plasmacytic cells during G2 cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal distending toxin. Infect Immun. 2002;70:528–534. doi: 10.1128/IAI.70.2.528-534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shenker BJ, McKay T, Datar S, Miller M, Chowhan R, Demuth D. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2-arrest in human T cells. J Immunol. 1999;162:244–251. [PubMed] [Google Scholar]
- 25.Sims TJ, Moncla BJ, Darveau RP, Page RC. Antigens of Actinobacillus actinomycetemcomitans recognized by patients with juvenile periodontitis and periodontally-normal subjects. Infect Immun. 1991;59:913–924. doi: 10.1128/iai.59.3.913-924.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svensson LA, Tarkowski A, Thelestam M, Lagergård T. The impact of Haemophilus ducreyi cytolethal distending toxin on cells involved in the immune response. Microbial Pathog. 2001;30:157–166. doi: 10.1006/mpat.2000.0422. [DOI] [PubMed] [Google Scholar]
- 27.Tan KS, Song KP, Ong G. Cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Occurrence and association with periodontal disease. J Periodontal Res. 2002;37:268–272. doi: 10.1034/j.1600-0765.2002.01618.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilson M, Henderson B. Virulence factors of Actinobacillus actinomycetemcomitans relevant to the pathogenesis of inflammatory periodontal diseases. FEMS Microbiol Rev. 1995;17:365–379. doi: 10.1111/j.1574-6976.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 29.Yamano R, Ohara M, Nishikubo S, et al. Prevalence of a cytolethal distending toxin production in periodontopathogenic bacteria. J Clin Microb. 2003;41:1391–1398. doi: 10.1128/JCM.41.4.1391-1398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]