Abstract
Background
Chronic ethanol intake is a significant risk factor for the development of cirrhosis and hepatocellular carcinoma (HCC). The effects of ethanol on extracellular signal-regulated kinase (ERK) activation, transforming growth factor alpha (TGF-α) and HCC growth were examined in this study.
Methods
HepG2, SKHep, Hep3B human HCC cells or normal human hepatocytes were treated with ethanol (0-100mM), exogenous TGF-α, TGF-α neutralization antibody or the MEK inhibitor U0126. TGF-α levels were quantified by ELISA. Growth was determined by trypan blue-excluded cell counts. Cell cycle phase distribution was determined by flow cytometry. Protein expression was determined by Western blot.
Results
Ethanol treatment (10-40mM) increased ERK activation in HepG2 and SKHep HCC cells but not in Hep3B or human hepatocyte cells. Growth increased in HepG2 (174 ± 29 %, P<0.05) and SKHep (149 ± 12 %, P<0.05) cells in response to ethanol treatment. Correspondingly, ethanol increased S phase distribution in these cells. U0126 suppressed ethanol-induced growth increases. Ethanol treatment for 24 hours also raised TGF-α levels in HepG2 cells (118-198 %) and SKHep cells (112-177 %). Exogenous administration of recombinant TGF-α mimicked the ethanol-induced growth in HepG2 and SKHep cells; TGF-α neutralization antibody effectively abrogated this effect. The TGF-a neutralization antibody also prevented ERK activation by ethanol in HepG2 cells.
Conclusion
These data demonstrate that clinically relevant doses of ethanol stimulate ERK-dependent proliferation of HCC cells. Ethanol up-regulates TGF-α levels in HCC cells and enhances growth through cell cycles changes, which appear to be mediated through TGF-α-MEK-ERK signaling. Ethanol-MEK signaling in normal hepatocytes is absent, suggesting that ethanol promotion of HCC growth may in part depend upon the acquisition of cancer-specific signaling by hepatocytes.
Keywords: Hepatocellular carcinoma, MEK, ERK, Ethanol, TGF-α, HCC
INTRODUCTION
The incidence of hepatocellular carcinoma (HCC) is increasing at an alarming rate in the United States and on a global scale (1-4). Unlike other common malignancies, HCC usually occurs within the bounds of known risk factors of which underlying cirrhosis arising from viral (hepatitis B [HBV] and C [HCV]) infection, exposure to aflatoxin, or chronic ethanol intake is the most common precursor for HCC development (3-5). Considerable research effort over the past 20 years has led to significant advances in our understanding of the physiological and pathophysiological effects of moderate and chronic ethanol intake on liver function (3, 5). However, the effects of ethanol on mechanisms that regulate HCC proliferation and progression following initial hepatocyte transformation remain poorly defined. In the clinical setting, this may have significant consequences with regard to patients that are already at higher risk for HCC development who continue to consume ethanol in either moderation or excess (3, 5, 6). That is, while viral hepatitis-related, macronodular cirrhosis poses a greater HCC risk than ethanol-induced, micronodular cirrhosis, a clear risk with alcohol exists that increases the risk of both accelerated cirrhosis development and subsequent HCC development (3, 7, 8).
Several studies have identified a role for numerous cytokines, growth factors and signaling pathways during tumor initiation and progression (9, 10). Despite the diverse nature of many of the signaling pathways believed to be important during hepatic tumorigenesis, increasing evidence suggests the proliferative HCC phenotype may be caused and sustained, at least in part, by increased expression and activity of extracellular signal-regulated kinase-mitogen-activated protein kinase (ERK-MAPK) (11-15). Others studies have corroborated that alcohol activates MAPK and nuclear factor kappa-B (NF-κB) signaling pathways as well as stimulates the release of cytokines and growth factors including TNF-α, TGF-α, TGF-β1, and IL-6, Il-1β (16-19). Furthermore, an increasing body of evidence suggests the effects of ethanol on normal and transformed hepatocytes may be dependent on altered expression and/or activity of ERK-MAPK-dependent signaling pathways (20-22).
The ERK-MAPK’s are serine/threonine kinases that represent a point of convergence for diverse signaling pathways originating at the cell membrane, the small G-protein p21ras being identified as a common intermediate for diverse receptor signaling pathways (11-13, 23). Activation of p21ras initiates a kinase phosphorylation cascade through a series of structurally similar protein isoforms culminating in ERK activation. Phosphorylation of ERK proteins resulting in cellular and/or nuclear responses depends upon the nature of the stimulus and the specific ERK isoform activated.
The aims of the current study were to determine the effects of ethanol treatment on the expression and activity of ERK-MAPK signaling cascades in HCC and establish whether these changes affect cell growth. We report that the promotion of cell growth by ethanol is likely related to P-ERK modulation of the cell cycle. Furthermore, ethanol treatment increases TGF-α levels in association with ERK activation in HCC cells but without any change in AKT expression or activation.
MATERIAL AND METHODS
Materials
Human hepatocellular carcinoma cell lines (Hep3B, HepG2, and SKHep) were purchased from American Type Culture Collection (ATCC; Manassas, VA). Freshly isolated human hepatocytes from patients undergoing resection for benign/precancerous lesions of the liver without a concomitant diagnosis of hepatitis or alcohol-associated liver disease were purchased from Cellzdirect (Pittsboro, NC). Alpha minimum essential medium (MEMα), fetal calf serum (FCS), and ITS-X were purchased from Gibco-Invitrogen (Carlsbad, CA). Antibiotics (penicillin and streptomycin) were purchased from BioWhittaker (Walkersville, MD). Dexamethasone and 200 proof ethanol were purchased from Sigma-Aldrich (St Louis, MO).
Cell Culture conditions and treatments
Human HCC cell lines were cultured in MEMα containing 10% (v/v) FCS, supplemented with penicillin (100U/ml) and streptomycin (100mg/ml). Freshly isolated human hepatocytes (Cellzdirect, Pittsboro, NC) were cultured in William’s E Medium supplemented with insulin (6.25 μg/mL), transferrin (6.25 μg/mL), selenium (6.25 ng/mL), dexamethasone (0.1 mM), 100 U/mL penicillin and 100 mg/mL streptomycin (Cellzdirect, Pittsboro, NC). For all cell types, cells were plated into T25 flasks with 0.22μm-vented, screw caps for ethanol experiments or 6-well plates. 24 hours after initial plating, the cell culture medium was removed and replaced with the appropriate high serum (10% (v/v) FCS) medium containing ethanol (0-100mM). Ethanol concentrations were prepared via serial dilutions for each experiment. Cells were treated with the following reagents in varying combinations: ethanol, U0126 (Calbiochem, La Jolla, CA), LY294002 (Calbiochem, La Jolla, CA), TGF-α (Calbiochem, La Jolla, CA), and TGF-α antibody (Calbiochem, La Jolla, CA).
Cell Counts
At the end of the specified treatment times, cell viability was determined by staining with trypan blue and counting using a hemacytometer. The mean number of cells for each treatment was then compared to control (untreated) or vehicle (DMSO) treated cells and expressed as a percentage relative to control (set equal to 100%).
Western blot analysis
Following ethanol treatment (0-100mM, 24 hours) cells were lysed using RIPA buffer (PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1mM phenylmethylsulfonyl fluoride, 10μg/ml aprotinin, 1mM Na3VO4) and cell debris removed by centrifugation (10,000 x g, 10 minutes, 4°C). Supernatants were collected and lysates resolved by SDS-PAGE (10μg total protein). Following transfer and blocking (5% (w/v) non-fat dry milk [NFDM] in 0.1% (v/v) Tween-20-TBS [TTBS]), membranes were incubated with primary antibody (1:1000 dilution) overnight at 4°C. Membranes were then washed (x3, TTBS) and incubated with a horseradish peroxidase-conjugated IgG secondary antibody (1:5000) for 60 minutes at room temperature. Detection was performed using enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ). The primary antibodies employed included a specific phospho-p42/44 MAP Kinase (Thr202/Tyr204) antibody (Cell Signaling, Beverly, MA), total ERK1/2 (K-23) antibody (Santa Cruz Biotech, Santa Cruz, CA), phospho-Akt (Ser473) monoclonal antibody (Cell Signaling), total AKT antibody (Cell Signaling), and actin (C-11) (Santa Cruz Biotech, Santa Cruz, CA). Densitometric analysis was performed using Totallab software (Nonlinear Dynamics, Durham, NC).
TGF-α ELISA
Conditioned media samples were taken at indicated timepoints after treatment with ethanol. TGF-α levels were measured in control and conditioned cell culture media via ELISA according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN).
Cell Cycle Analysis
Cell cycle phase distribution was determined by flow cytometry. Cells were plated in T-25 flasks at a density of 10 × 105 cells per ml. The following day, cells were washed and then incubated in serum-free media for 24 hours. After synchronization, cells were washed and media containing 10% FBS with or without alcohol was added. After 24 hours, the cells were trypsinized, washed, pelleted, and then treated with RNase A (2 μg/μl) and stained with propidium iodide (50ng/μl) on ice for at least 30 minutes. Samples were analyzed by flow cytometry. Cell cycle phase distribution was determined using Modfit software (Verity Software House, Inc., Tupshin, ME) to analyze DNA content histograms.
Statistics
Statistical analyses were performed using T-test and one-way analysis of variance (ANOVA) with Tukey post-hoc.
RESULTS
Ethanol treatment increases MEK activity in human HCC but not in non-transformed cells in the absence of significant ethanol metabolism
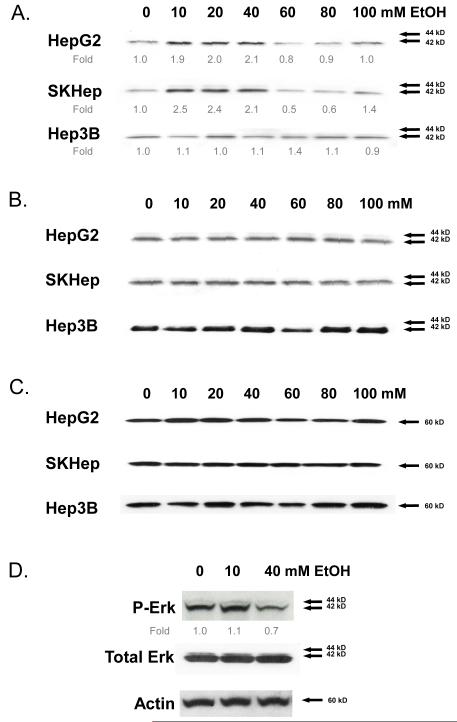
HepG2, SKHep, and Hep3B human hepatocellular carcinoma lines were treated with different concentrations of ethanol (0-100 mM) for 24 hours. Western blot analysis of cell lysates using antibodies specific for active, phosphorylated ERK (P-ERK) demonstrated increased P-ERK levels in HepG2 and SKHep HCC cells but not in Hep3B cells (Figure 1A). Cumulative densitometric analysis (normalized to β-actin) demonstrated that in the HepG2 and SKHep cells, ethanol treatment maximally stimulated ERK activation between 10-40 mM ethanol; no significant increases in ERK activation were detected at concentrations higher than 40 mM (Figure 1A). Similar results were observed at earlier 30 and 180 minute timepoints at the 10 mM and 40 mM concentrations of ethanol (data not shown). Interestingly, the 10-40 mM ethanol concentration range corresponds to physiologic blood alcohol levels of between 0 and 0.18 mg/dl. Ethanol did not significantly alter total ERK or actin expression at any of the concentrations employed (0-100 mM) in all three HCC cell lines (Figures 1B & 1C). Given that many of deleterious effects of ethanol are associated with hepatic metabolism (3), we measured the ethanol concentration in cell culture medium before and after treating the three HCC cell lines for 24 hours and detected no significant changes in ethanol concentration (data not shown).
Fig. 1.
ERK activation in human hepatocellular carcinoma (HCC) cell lines (Hep3B, SKhep, and HepG2) following treatment with ethanol (0-100 mM) for 24 hours. Cell lysates were analyzed by Western immunoblotting to detect (A) phosphorylated ERK1,2 - fold expression relative to β-actin averaged from three independent experiments is shown; (B) total ERK1,2; and (C) β-actin. (D) Human hepatocytes treated with ethanol (0-40 mM) for 24 hours. Phosphorylated ERK1,2 with fold expression relative to β-actin, total ERK, and β-actin are shown. Representative Western immunoblots are presented; similar results were obtained in at least two independent experiments.
Having demonstrated that ethanol treatment alters ERK activation in HCC cells, we next sought to determine whether similar effects were evidenced in normal human hepatocytes. Human hepatocytes were treated with ethanol (0-40 mM) for 24 hours and showed no change in P-ERK levels at 10 mM ethanol and a slight decrease at 40 mM ethanol relative to β-actin (Figure 1D). The effect of ethanol on MEK activity (P-ERK) seen in human HCC, therefore, is not apparent in normal hepatocytes. Similar results were observed in normal (NMH) and/or precancerous (TAMH) murine hepatocytes (data not shown).
Ethanol treatment increases growth of human HCC cells
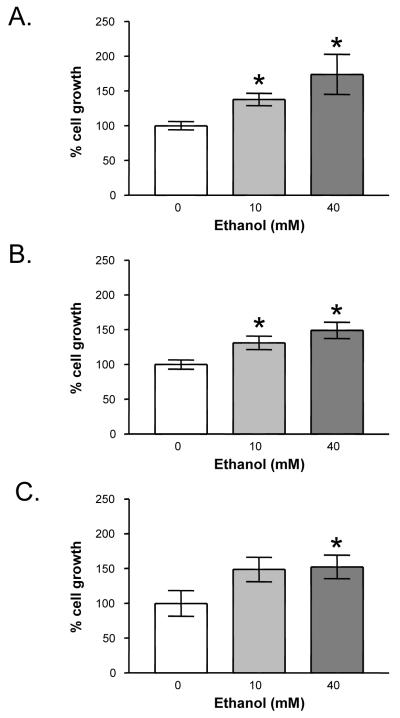
To determine the effects of ethanol on HCC progression, we next measured cell growth by performing cell counts in the three human HCC cell lines in the absence (control) or presence of ethanol (10 and 40 mM). Treatment of HepG2 and SKHep cells with 10 and 40 mM ethanol led to significant increases in cell growth versus control (131±8% and 174±29% [HepG2] and 131±10% and 149±12% [SKHep], Figures 2A & B). Ethanol treatment also increased Hep3B cell growth, but significant differences were only detected at 40 mM ethanol (143 ± 14%, Figure 2C).
Fig. 2.
Effect of ethanol on HCC cell growth: (A) HepG2, (B) SKHep, and (C) Hep3B. Actively dividing cells were treated for 24 hours with ethanol (0 to 40 mM). Trypan blue excluded cell counts were performed to determine growth relative to untreated, control cells. Each point on the graph represents the mean of triplicate samples +/- SEM. Similar results were obtained in at least three independent experiments. * = P < 0.05 compared to vehicle control.
The effect of ethanol on cell cycle phase distribution in HCC
We hypothesized that ethanol-induced growth may be mediated by increased cell cycling through promotion of the G1-S phase transition. HepG2 cells were treated with ethanol for 24 hours and then cell cycle phase distribution was determined by flow cytometric analysis. A representative cell cycle phase distribution of HepG2 cells treated with 10 or 40 mM ethanol is shown in Table 1. Interestingly, 10 mM ethanol precipitated decreases in G0/G1 (-4%) and G2M (-3%) and concomitant increases in S (+7%) phase distribution. At 40 mM ethanol, further decreases in G0/G1 (-5%) and G2M (-10%) and increase in S (+15%) were noted indicating a concentration-dependent effect of ethanol on cell cycle phase distribution. This data suggests that ethanol’s effect on growth may in part be mediated through significant reductions in the percentage of cells in G0/G1 and G2M with corresponding increases in S phase.
Table 1.
Cell cycle phase distribution of HepG2 Cells after treatment with ethanol (0 - 40 mM) in the presence or absence of MEK inhibitor, U0126 (10 μM) for 24 hours. Values from a representative experiment are shown
| Treatment | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|
| Control | 38 | 40 | 22 |
| U012610μM | 39 | 42 | 19 |
| EtOH10mM | 34 | 47 | 19 |
| EtOH40mM | 33 | 55 | 12 |
| EtOH40mM + U012610μM | 36 | 45 | 19 |
Ethanol-dependent increases in HCC growth are ERK-MAPK-dependent
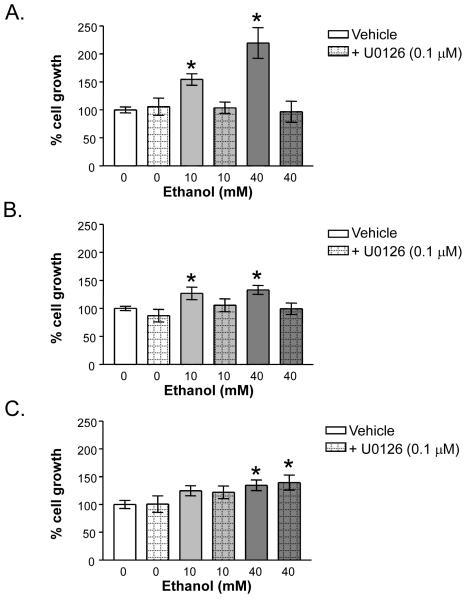
The three human HCC cell lines were next treated with the MEK inhibitor U0126 (0.1-10μM) in the absence or presence of ethanol. Western blot analysis of P-ERK expression confirmed that U0126 inhibited ethanol-induced ERK activation to a level not significantly different from control cells in all three human HCC cell lines (data not shown). To determine whether inhibition of ethanol-induced MEK-ERK activity in HCC cell lines translates to altered cell growth, we performed parallel cell count studies in which the three HCC cell lines were treated with ethanol in the presence or absence of U0126 (0.1μM). These data demonstrated that inhibition of MEK activity abrogated the effects of ethanol treatment on growth in all three cell lines (Figure 3). Furthermore, the ethanol-induced accumulation of cells in S phase was successfully blocked with U0126 (Table 1).
Fig. 3.
Effect of U0126 on ethanol-induced growth in HCC cells: (A) HepG2, (B) SKHep, and (C) Hep3B. Cells were treated for 24 hours with ethanol (0 to 40 mM) alone or in combination with U0126 (0.1 μM). Trypan blue excluded cell counts were used to determine growth relative to control untreated cells. Each point on the graph represents the mean of triplicate samples +/- SEM. Similar results were obtained in at least three independent experiments. * = P < 0.05 compared to vehicle control.
TGF-α levels are increased by ethanol in human HCC
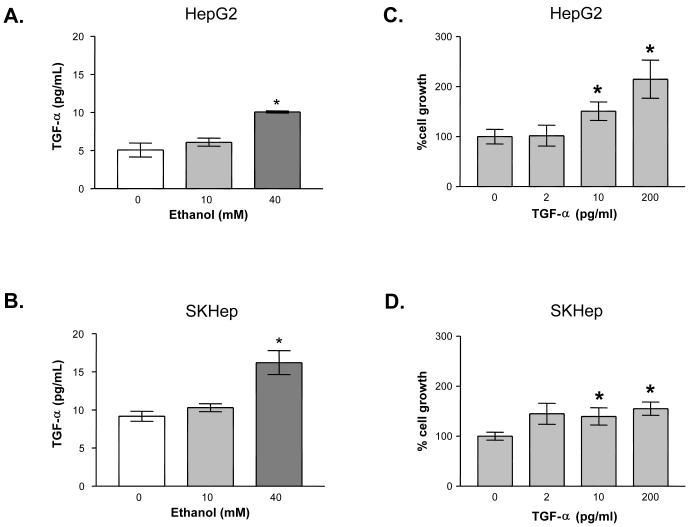
TGF-α ligand interaction with the EGFR receptor has been reported to signal through both the MEK/ERK and Pi3K/AKT pathways (24, 25). The effects of alcohol on TGF-α levels in HCC were therefore examined (Figures 4A & B). Conditioned cell culture media from HepG2 and SKHep cells was assayed for the presence of TGF-α following 24 hour exposure to ethanol (10 - 40 mM). In the HepG2 cell line, a baseline TGF-α level of approximately 5 pg/ml was detected. Addition of 10 mM ethanol increased the level 118% compared to vehicle control; 40 mM ethanol increased TGF-α levels 198% compared to vehicle control, equivalent to approximately 10 pg/ml. In the SKHep cell line, the baseline level of TGF-α detected was 1.8 fold higher than in the HepG2 cells (9 pg/ml). The TGF-α level increased to 112% and 177% of control with 10 mM and 40 mM ethanol, respectively. Increases in TGF-α by 40 mM ethanol were statistically significant in both cell lines.
Fig 4.
Effect of ethanol on transforming growth factor-alpha (TGF-α) levels in cell culture media: (A) HepG2 and (B) SKHep. Cells were treated for 24 hours with ethanol (0 to 40 mM). A TGF-α ELISA was performed to measure TGF-α (pg/ml) in culture media. Each point on the graph represents the mean of triplicate samples +/- SEM. Similar results were obtained in at least three independent experiments. * = P < 0.01 compared to vehicle control. Effect of exogenous TGF-α on cell proliferation: (C) HepG2 and (D) SKHep. Cells were treated for 24 hours with exogenous TGF-α (0 to 200 pg/ml). Trypan blue excluded cell counts were used to determine growth relative to control untreated cells. Each point on the graph represents the mean of triplicate samples +/- SEM. Similar results were obtained in at least three independent experiments. * = P < 0.05 compared to vehicle control.
The effect of exogenous TGF-α on human HCC growth
After finding that ethanol induced TGF-α levels in the media, we sought to mimic this by adding exogenous TGF-α to untreated HCC cells to determine if (the expected) increases in growth exhibited were comparable in scale to ethanol-induced growth. Human HCC cells were treated with exogenous TGF-α (0-200 pg/ml) for 24 hours (Figures 4C & D). Significant growth increases were observed at 10 and 200 pg/ml treatments in the HepG2 cell line. Exogenous TGF-α produced increases in growth of 102 ± 21 percent with 2 pg/ml, 151 ± 18 percent with 10 pg/ml, and 215 ± 38 percent with 200 pg/ml as compared to control. The SKHep cell line showed growth increases of 145 ± 21 percent at 2 pg/ml, 140 ± 17 percent at 10 pg/ml, and 155 ± 13 percent at 200 pg/ml of exogenous TGF-α treatments as compared to control. Thus, growth increases with exogenous TGF-α mimicked the scale of the ethanol-induced growth in the SKHep cells. In HepG2 cells, the effects of ethanol on growth were greater than that accounted for solely by the increase in TGF-α based upon these experiments.
The effect of TGF-α neutralization antibodies on ethanol-induced growth in human HCC
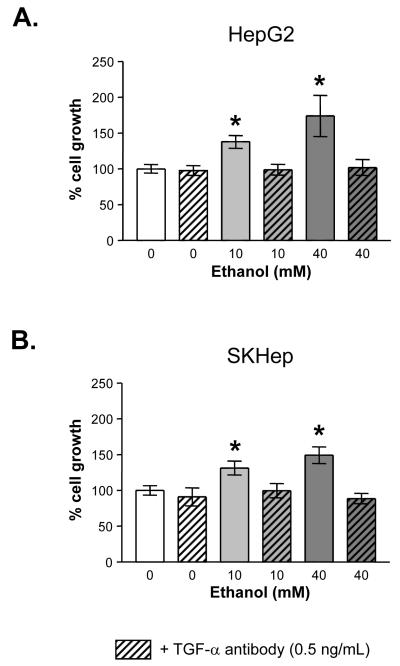
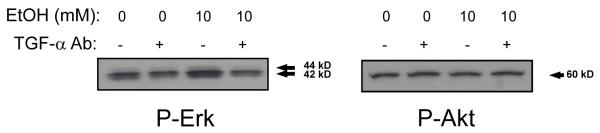
At this point, we wished to determine whether ethanol-TGF-α signaling (whether from existing cellular TGF-α or “de novo” ethanol-induced TGF-α) would account for the ethanol-induced increases in growth. With this in mind, TGF-α neutralization antibodies were employed to block all TGF-α signaling. HepG2 and SKHep human HCC cell lines were treated with 10 or 40 mM ethanol and simultaneously given either vehicle control or 0.5 μg/ml of TGF-α neutralization antibody. Growth was determined after 24 hours of treatment by trypan blue excluded cell counts (Figures 5A & B). Growth increased in HepG2 (174 ± 29 %, P<0.05) and SKHep (149 ± 12 %, P<0.05) cells in response to ethanol (10 - 40 mM) treatment. Addition of TGF-α neutralization antibody completely reversed the ethanol-induced growth in both cell lines. Specifically, in the HepG2 cells the ethanol-mediated growth increase at 10 mM ethanol (138 ± 9 %) was reduced to basal levels (99 ± 8 %); growth with 40 mM of ethanol (174 ± 29 %) was reduced to basal levels (102 ± 11 %, p<0.05). In the SKHep cells, the ethanol-mediated growth increase seen with 10 mM ethanol (131 ± 10 %) was reduced to basal level (100 ± 10%); at 40 mM of ethanol, growth (149 ± 12 %) was brought back to or below basal level (88 ± 7 %, p<0.05). Furthermore, treatment with TGF-α neutralization antibody or the MEK inhibitor U0126 blocked ethanol-induced increases in P-ERK (Figure 6 and data not shown). Thus, ethanol-induced cell growth appears to be mediated through ethanol-TGF-α-MEK-ERK signaling.
Fig. 5.
Effect of TGF-α antibody on ethanol-induced growth: (A) HepG2 and (B) SKHep. Actively dividing cells were treated for 24 hours with ethanol (0 to 40 mM) alone or in combination with TGF-α antibody (0.5 ng/ml). Trypan blue excluded cell counts were used to determine proliferation relative to control untreated cells. Data represent the mean +/- SD from a representative experiment. * = P < 0.05 compared to vehicle control.
Fig. 6.
Phosphorylated ERK and AKT levels in HepG2 cells following treatment with ethanol (10 mM) and TGF-α antibody for 30 minutes. Cell lysates were prepared for analysis by Western immunoblotting to detect phosphorylated ERK and AKT as indicated. A representative Western immunoblot is shown; similar results were obtained in at least two independent experiments.
The effect of ethanol on Pi3Kinase activity in human HCC
In the previous section, we investigated the effect of ethanol-mediated increases in TGF-α on downstream MEK-ERK. We also examined Pi3Kinase activity (P-AKT) and total AKT. Western immunoblot studies were performed on both HepG2 and SKHep cell lines following exposure to ethanol (0 - 40 mM) and TGF-α neutralization antibody for 30 minutes. P-AKT levels were unchanged with ethanol or the addition of the TGF-α neutralization antibody (Figure 6). Total AKT levels were likewise unchanged (data not shown). Thus, the ethanol-TGF-α-growth signaling in HepG2 and SKHep HCC cells appears to be MEK-ERK dependent and Pi3Kinase/AKT independent.
DISCUSSION
The incidence of HCC is on the rise in both the United States and Europe (2). Recent trends suggest alcohol is the main etiological factor in a significant number of these cases (22). The hepatotoxic effects of alcohol are well defined (10, 21), and it is accepted that alcohol-induced cirrhosis contributes to the development of HCC. Less clear is whether direct effects of ethanol on cellular growth and survival signaling pathways contribute to hepatocarcinogenesis. The purpose of these studies was to determine the effects of ethanol on MEK-ERK growth signaling in HCC as well as normal hepatoctyes.
It has been previously shown that HCC increases activation of p42/p44 MAPK (MEK-ERK) pathway intermediates (15, 26-28). In the present study, we showed that MEK, a key enzyme in this pathway, was activated after 24 hours of ethanol treatment (10 - 40 mM) in human HCC lines. Noticeably, normal human hepatocyte cells showed no changes in MEK activity in the presence of ethanol. This suggests that normal hepatocyte mitogenic signaling may not be enhanced by ethanol, while neoplastic cells are in some way primed for this potentiation of their signaling. Thus, we hypothesize that ethanol promotion of HCC may depend upon hepatocytes acquiring this cancer specific signaling.
Ethanol-induced activation of the MEK-ERK pathway also correlated with accelerated HCC growth. Interestingly, although Hep3B cells did not show increased MEK activity in the presence of ethanol, cell growth was still induced by ethanol. Consistent with this, MEK inhibition failed to block ethanol-induced growth in Hep3B cells. Thus ethanol-mediated growth appears to be MEK-independent in the Hep3B cell line. In contrast, ethanol-mediated growth in the HepG2 and SKHep cell lines was associated with increased MEK activity that was completely blocked with MEK inhibition, suggesting dependence on MEK.
In a review by Aroor and Shukla, the authors highlight the important role that MAPK pathways play in the signaling effects of ethanol (29). These signaling effects vary among cell types and can often be correlated with length of ethanol exposure. In liver, in vitro studies demonstrate that ethanol enhances ERK activity in fetal hepatoctyes (30). Based upon our results, one may speculate that HCC cells have reverted to this fetal hepatocyte signaling. In a rodent model of HCC, ethanol treatment has been associated with higher expression/activity of inhibitory guanine nucleotide regulatory proteins (Gi-proteins) and enhanced Gi-protein agonist-stimulated MAPK activity (31). This is an example of how ethanol may indirectly affect signaling through changes in protein expression. Interestingly, ethanol has also been shown to increase levels of TGF-α (18) which is a positive regulator of the p42/p44 MAPK and Pi3K/AKT pathways (32).
Similarly, in our studies, alcohol increased TGF-α levels in human HCC cells up to four fold. In a previous study, induction of TGF-α expression by ethanol in HepG2 cells stimulated collagen production by stellate cells (18). This study showed baseline TGF-α levels of approximately 200 pg/ml compared with our baseline levels of 5 pg/ml in HepG2 cells. This difference is likely explained by their use of dialysis and concentration of cell media prior to assaying for TGF-α. We believe that our findings are not inconsistent with this prior study.
In normal hepatocytes, little TGF-α expression is present (33). TGF-α expression, however, is increased in precancerous conditions such as hepatitis and regenerative nodules found in cirrhotic livers (34, 35). Hepatitis viral proteins have been implicated in the upregulation of TGF-α in HCC (33, 36, 37). Importantly, TGF-α levels are elevated in the liver of rats administered ethanol chronically, and this is thought to be due to a decreased ability to internalize and degrade the cytokine (38). Finally, TGF-α transgenic mice, which overexpress human TGF-α, have a 70% incidence of hepatocellular carcinoma at 15 months of life suggesting that overexpression of human TGF-α may be sufficient to cause HCC (39).
TGF-α is a well known ligand of the epidermal growth factor receptor (40). Downstream pathways that are affected by TGF-α activation include the p42/p44 MAPK pathway as well as the Pi3K-AKT pathway (24, 25). The studies presented here demonstrate that ethanol-induced increases in TGF-α in HCC are associated with increased MEK-ERK signaling, increased growth and cell accumulation in S phase. Exogenous TGF-α reproduces these alcohol-induced endpoints of growth while TGF-α neutralization abrogates them. Furthermore, ethanol-induced MEK-ERK signaling, growth and cell cycle changes are blocked with MEK inhibition. Thus, ethanol enhances HCC growth through cell cycle regulatory changes mediated through TGF-α-MEK-ERK signaling. In addition, the effects of TGF-α are independent of the Pi3K/AKT pathway in these two human HCC lines.
CONCLUSION
The findings of this study suggest that the mechanism of alcohol-induced HCC may involve increases in TGF-α and TGF-α MEK-ERK signaling. Increased TGF-α levels have been noted in a variety of cancers (41-47). Thus, the findings of our study may have broader relevance to other cancers, particularly cancers which have alcohol as a known etiologic factor (22, 48).
In conclusion, physiologically relevant doses of ethanol (10 - 40 mM) which correspond to blood alcohol levels (BAL) of 0.05 to 0.18, were sufficient to induce increases in ERK activation and TGF-α levels in HCC. Ethanol-TGF-α-MEK-ERK signaling resulted in increased growth in HCC through cell cycle regulatory changes. Thus, TGF-α may be an important molecule for therapeutic targeting in alcohol-induced HCC and/or patients at high risk of alcohol-induced HCC. Future studies will focus on in vivo models to better understand how ethanol-TGF-α-MEK-ERK signaling relates to hepatocarcinogenesis.
Acknowledgments
Financial support
This work was supported by a grant from the NIH-NIAAA (P50 AA07611-16-20) and Clarian Values Fund for Research (VFR-121).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.McKillop IH, Schrum LW. Alcohol and liver cancer. Alcohol. 2005;35:195–203. doi: 10.1016/j.alcohol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Okuda K. Natural history of hepatocellular carcinoma including fibrolamellar and hepato-cholangiocarcinoma variants. J Gastroenterol Hepatol. 2002;17:401–405. doi: 10.1046/j.1440-1746.2002.02734.x. [DOI] [PubMed] [Google Scholar]
- 5.Longnecker MP. Alcohol consumption and risk of cancer in humans: an overview. Alcohol. 1995;12:87–96. doi: 10.1016/0741-8329(94)00088-3. [DOI] [PubMed] [Google Scholar]
- 6.Kew MC. Epidemiology of hepatocellular carcinoma. Toxicology. 2002;181-182:35–38. doi: 10.1016/s0300-483x(02)00251-2. [DOI] [PubMed] [Google Scholar]
- 7.Smart RG, Mann RE, Suurvali H. Changes in liver cirrhosis death rates in different countries in relation to per capita alcohol consumption and Alcoholics Anonymous membership. J Stud Alcohol. 1998;59:245–249. doi: 10.15288/jsa.1998.59.245. [DOI] [PubMed] [Google Scholar]
- 8.Stickel F, Schuppan D, Hahn EG, Seitz HK. Cocarcinogenic effects of alcohol in hepatocarcinogenesis. Gut. 2002;51:132–139. doi: 10.1136/gut.51.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombo M, Sangiovanni A. Etiology, natural history and treatment of hepatocellular carcinoma. Antiviral Res. 2003;60:145–150. doi: 10.1016/j.antiviral.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Hoek JB, Pastorino JG. Cellular signaling mechanisms in alcohol-induced liver damage. Semin Liver Dis. 2004;24:257–272. doi: 10.1055/s-2004-832939. [DOI] [PubMed] [Google Scholar]
- 11.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 12.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 13.Matozaki T, Nakanishi H, Takai Y. Small G-protein networks: their crosstalk and signal cascades. Cell Signal. 2000;12:515–524. doi: 10.1016/s0898-6568(00)00102-9. [DOI] [PubMed] [Google Scholar]
- 14.McKillop IH, Schmidt CM, Cahill PA, Sitzmann JV. Altered expression of mitogen-activated protein kinases in a rat model of experimental hepatocellular carcinoma. Hepatology. 1997;26:1484–1491. doi: 10.1002/hep.510260615. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt CM, McKillop IH, Cahill PA, Sitzmann JV. Increased MAPK expression and activity in primary human hepatocellular carcinoma. Biochem Biophys Res Commun. 1997;236:54–58. doi: 10.1006/bbrc.1997.6840. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez-Ruiz MC, Quiroz SC, Souza V, Bucio L, Hernandez E, Olivares IP, Llorente L, Vargas-Vorackova F, Kershenobich D. Cytokines, growth factors, and oxidative stress in HepG2 cells treated with ethanol, acetaldehyde, and LPS. Toxicology. 1999;134:197–207. doi: 10.1016/s0300-483x(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 17.Jeong HJ, Hong SH, Park RK, An NH, Kim HM. Ethanol induces the production of cytokines via the Ca2+, MAP kinase, HIF-1alpha, and NF-kappaB pathway. Life Sci. 2005;77:2179–2192. doi: 10.1016/j.lfs.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Kato J, Sato Y, Inui N, Nakano Y, Takimoto R, Takada K, Kobune M, Kuroiwa G, Miyake S, Kohgo Y, Niitsu Y. Ethanol induces transforming growth factor-alpha expression in hepatocytes, leading to stimulation of collagen synthesis by hepatic stellate cells. Alcohol Clin Exp Res. 2003;27:58S–63S. doi: 10.1097/01.ALC.0000078614.44983.97. [DOI] [PubMed] [Google Scholar]
- 19.McClain CJ, Shedlofsky S, Barve S, Hill DB. Cytokines and alcoholic liver disease. Alcohol Health Res World. 1997;21:317–320. [PMC free article] [PubMed] [Google Scholar]
- 20.Clemens DL, Halgard CM, Miles RR, Sorrell MF, Tuma DJ. Establishment of a recombinant hepatic cell line stably expressing alcohol dehydrogenase. Arch Biochem Biophys. 1995;321:311–318. doi: 10.1006/abbi.1995.1400. [DOI] [PubMed] [Google Scholar]
- 21.Higuchi H, Gores GJ. Mechanisms of liver injury: an overview. Curr Mol Med. 2003;3:483–490. doi: 10.2174/1566524033479528. [DOI] [PubMed] [Google Scholar]
- 22.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87–96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Seger R, Krebs EG. The MAPK signaling cascade. Faseb J. 1995;9:726–735. [PubMed] [Google Scholar]
- 24.Kawamura K, Fukuda J, Shimizu Y, Kodama H, Tanaka T. Survivin contributes to the anti-apoptotic activities of transforming growth factor alpha in mouse blastocysts through phosphatidylinositol 3′-kinase pathway. Biol Reprod. 2005;73:1094–1101. doi: 10.1095/biolreprod.105.042754. [DOI] [PubMed] [Google Scholar]
- 25.Sewell JM, Smyth JF, Langdon SP. Role of TGF alpha stimulation of the ERK, PI3 kinase and PLC gamma pathways in ovarian cancer growth and migration. Exp Cell Res. 2005;304:305–316. doi: 10.1016/j.yexcr.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Feo F, Pascale RM, Simile MM, De Miglio MR, Muroni MR, Calvisi D. Genetic alterations in liver carcinogenesis: implications for new preventive and therapeutic strategies. Crit Rev Oncog. 2000;11:19–62. [PubMed] [Google Scholar]
- 27.Ito Y, Sasaki Y, Horimoto M, Wada S, Tanaka Y, Kasahara A, Ueki T, Hirano T, Yamamoto H, Fujimoto J, Okamoto E, Hayashi N, Hori M. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998;27:951–958. doi: 10.1002/hep.510270409. [DOI] [PubMed] [Google Scholar]
- 28.Wiesenauer CA, Yip-Schneider MT, Wang Y, Schmidt CM. Multiple anticancer effects of blocking MEK-ERK signaling in hepatocellular carcinoma. J Am Coll Surg. 2004;198:410–421. doi: 10.1016/j.jamcollsurg.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339–2364. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Reddy MA, Shukla SD. Potentiation of mitogen-activated protein kinase by ethanol in embryonic liver cells. Biochem Pharmacol. 1996;51:661–668. doi: 10.1016/s0006-2952(95)02239-2. [DOI] [PubMed] [Google Scholar]
- 31.McKillop IH, Vyas N, Schmidt CM, Cahill PA, Sitzmann JV. Enhanced Gi-protein-mediated mitogenesis following chronic ethanol exposure in a rat model of experimental hepatocellular carcinoma. Hepatology. 1999;29:412–420. doi: 10.1002/hep.510290218. [DOI] [PubMed] [Google Scholar]
- 32.Thoresen GH, Guren TK, Sandnes D, Peak M, Agius L, Christoffersen T. Response to transforming growth factor alpha (TGFalpha) and epidermal growth factor (EGF) in hepatocytes: lower EGF receptor affinity of TGFalpha is associated with more sustained activation of p42/p44 mitogen-activated protein kinase and greater efficacy in stimulation of DNA synthesis. J Cell Physiol. 1998;175:10–18. doi: 10.1002/(SICI)1097-4652(199804)175:1<10::AID-JCP2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Wang WL, Li Q, Qiao Q. Expression of transforming growth factor-alpha and hepatitis B surface antigen in human hepatocellular carcinoma tissues and its significance. World J Gastroenterol. 2004;10:830–833. doi: 10.3748/wjg.v10.i6.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hufnagl K, Parzefall W, Marian B, Kafer M, Bukowska K, Schulte-Hermann R, Grasl-Kraupp B. Role of transforming growth factor alpha and prostaglandins in preferential growth of preneoplastic rat hepatocytes. Carcinogenesis. 2001;22:1247–1256. doi: 10.1093/carcin/22.8.1247. [DOI] [PubMed] [Google Scholar]
- 35.Kira S, Nakanishi T, Suemori S, Kitamoto M, Watanabe Y, Kajiyama G. Expression of transforming growth factor alpha and epidermal growth factor receptor in human hepatocellular carcinoma. Liver. 1997;17:177–182. doi: 10.1111/j.1600-0676.1997.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 36.Chung YH, Kim JA, Song BC, Lee GC, Koh MS, Lee YS, Lee SG, Suh DJ. Expression of transforming growth factor-alpha mRNA in livers of patients with chronic viral hepatitis and hepatocellular carcinoma. Cancer. 2000;89:977–982. doi: 10.1002/1097-0142(20000901)89:5<977::aid-cncr6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi J, Aoki H, Kajino K, Moriyama M, Arakawa Y, Hino O. Hepatitis C virus core protein activates the MAPK/ERK cascade synergistically with tumor promoter TPA, but not with epidermal growth factor or transforming growth factor alpha. Hepatology. 2000;32:958–961. doi: 10.1053/jhep.2000.19343. [DOI] [PubMed] [Google Scholar]
- 38.Tuma DJ, Todero SL, Barak-Bernhagen M, Sorrell MF. Effects of chronic ethanol administration on the endocytosis of cytokines by rat hepatocytes. Alcohol Clin Exp Res. 1996;20:579–583. doi: 10.1111/j.1530-0277.1996.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 39.Wu JC, Merlino G, Fausto N. Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proc Natl Acad Sci U S A. 1994;91:674–678. doi: 10.1073/pnas.91.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Washington B, Mtshali C, Williams S, Smith H, Li JD, Shaw B, Gwathmey J. Ethanol-induced mitogen activated protein kinase activity mediated through protein kinase C. Cell Mol Biol (Noisy-le-grand) 2003;49:1351–1356. [PubMed] [Google Scholar]
- 41.Doraiswamy V, Parrott JA, Skinner MK. Expression and action of transforming growth factor alpha in normal ovarian surface epithelium and ovarian cancer. Biol Reprod. 2000;63:789–796. doi: 10.1095/biolreprod63.3.789. [DOI] [PubMed] [Google Scholar]
- 42.More E, Fellner T, Doppelmayr H, Hauser-Kronberger C, Dandachi N, Obrist P, Sandhofer F, Paulweber B. Activation of the MAP kinase pathway induces chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) expression in human breast cancer cell lines. J Endocrinol. 2003;176:83–94. doi: 10.1677/joe.0.1760083. [DOI] [PubMed] [Google Scholar]
- 43.Piyathilake CJ, Frost AR, Manne U, Weiss H, Bell WC, Heimburger DC, Grizzle WE. Differential expression of growth factors in squamous cell carcinoma and precancerous lesions of the lung. Clin Cancer Res. 2002;8:734–744. [PubMed] [Google Scholar]
- 44.Sawhney RS, Sharma B, Humphrey LE, Brattain MG. Integrin alpha2 and extracellular signal-regulated kinase are functionally linked in highly malignant autocrine transforming growth factor-alpha-driven colon cancer cells. J Biol Chem. 2003;278:19861–19869. doi: 10.1074/jbc.M213162200. [DOI] [PubMed] [Google Scholar]
- 45.Vail ME, Pierce RH, Fausto N. Bcl-2 delays and alters hepatic carcinogenesis induced by transforming growth factor alpha. Cancer Res. 2001;61:594–601. [PubMed] [Google Scholar]
- 46.Wu M, Putti TC, Bhuiya TA. Comparative study in the expression of p53, EGFR, TGF-alpha, and cyclin D1 in verrucous carcinoma, verrucous hyperplasia, and squamous cell carcinoma of head and neck region. Appl Immunohistochem Mol Morphol. 2002;10:351–356. doi: 10.1097/00129039-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Zhou R, Skalli O. Identification of cadherin-11 down-regulation as a common response of astrocytoma cells to transforming growth factor-alpha. Differentiation. 2000;66:165–172. doi: 10.1046/j.1432-0436.2000.660402.x. [DOI] [PubMed] [Google Scholar]
- 48.Boyle P, Macfarlane GJ, Blot WJ, Chiesa F, Lefebvre JL, Azul AM, de Vries N, Scully C. European School of Oncology Advisory report to the European Commission for the Europe Against Cancer Programme: oral carcinogenesis in Europe. Eur J Cancer B Oral Oncol. 1995;31B:75–85. doi: 10.1016/0964-1955(95)00007-5. [DOI] [PubMed] [Google Scholar]