Abstract
Background
Partial or total overnight sleep deprivation produces immediate mood improvement in about 50% of patients with depression, but not in healthy controls. Our objectives were to compare the neurochemical changes that accompanied partial overnight sleep deprivation in healthy and depressed participants, and to compare baseline neurochemical profiles and overnight neurochemical changes between those depressed participants who did and did not respond to sleep loss with mood improvement.
Methods
We studied 2 brain regions (left dorsal prefrontal area and pons) in 12 women with unipolar depression and in 15 healthy women using proton magnetic resonance spectroscopy acquired at 1.5 T. The scans took place at baseline and 24 hours later after a night with sleep restricted to a maximum of 2.5 hours (22:30–01:00). We assessed 3 neurochemical signals (referenced to internal water): N-acetylaspartate (NAA), choline compounds (Cho) and creatine-plus-phosphocreatine (tCr).
Results
In both groups combined, sleep restriction caused a 20.1% decrease in pontine tCr (F1–16 = 5.07, p = 0.039, Cohen’s d = 0.54) and an 11.3% increase in prefrontal Cho (F1–21 = 5.24, p = 0.033, Cohen’s d = 0.46). Follow-up tests revealed that prefrontal Cho increases were significant only among depressed participants (17.9% increase, t9 = −3.35, p = 0.008, Cohen’s d = 1.06). Five depressed patients showed at least 30% improvement in mood, whereas 6 showed no change or worsening in mood after sleep restriction. Baseline pontine Cho levels distinguished subsequent responders from nonresponders to sleep restriction among depressed participants (z = 2.61, p = 0.008).
Limitations
A limitation of this study is the relatively small sample size.
Conclusion
Sleep restriction altered levels of pontine tCr and prefrontal Cho in both groups combined, suggesting effects on phospholipid and creatine metabolism. Baseline levels of pontine Cho were linked to subsequent mood responses to sleep loss, suggesting a role for pontine phospholipid metabolism in mood effects of sleep restriction.
Introduction
Investigating the brain changes that accompany depression and recovery from depression is complicated by the fact that treatments typically take days or weeks to have substantial effects on mood.1 During the interval between depressed state and mood improvement, lengthy chains of regulatory changes may occur (e.g., in neurotransmitter levels, gene expression cascades, synaptic and other structural changes). Overnight sleep deprivation therapy can reliably produce an antidepressant response in some patients within a 24-hour period.2–4 Its rapid effects create an opportunity to use sleep deprivation as a model for studying rapid neurochemical processes in the brain that correlate with, and may underlie, mood regulation in depression. Unlike many other treatment modalities, sleep deprivation can also be applied ethically to healthy controls so that neurochemical changes specific to mood regulation in depression can be distinguished from nonspecific changes accompanying sleep loss in healthy participants. In addition, since half of patients typically respond to sleep deprivation,2–4 neurochemical differences between responders and nonresponders can be assessed.
Previous studies have reported effects of sleep deprivation on brain chemistry. Two proton magnetic resonance spectroscopy (1H-MRS) studies of healthy volunteers conducted at 1.5 T reported decreases in occipital levels of N-acetylaspartate (NAA) and choline-containing compounds (Cho) after sleep deprivation5 and increases in unresolved γ-aminobutyric acid, glutamate and glutamine in the pons.6 Two phosphorus MRS (31P-MRS) studies (which provide information on membrane phospholipids and on metabolism of high-energy phosphates) of healthy volunteers after sleep deprivation, conducted at 1.5 T (using a surface coil), reported no changes in measures of brain chemistry in the frontal lobes7 and in a large medial prefrontal region.8 A single 1H-MRS study of inpatients with major depression reported increases in levels of creatine-plus-phosphocreatine (tCr) and Cho in the left dorsal prefrontal region after sleep deprivation.9
In the present study, we used 1H-MRS to identify the neurochemical correlates of sleep restriction in 2 selected volumes of interest: the left dorsal prefrontal region and the pons (Fig. 1). Functional brain imaging studies have consistently reported a reduction in metabolic rate in dorsal prefrontal and frontal regions in depression,10–14 which is normalized after successful treatment.15,16 The pons was selected because it is involved in regulation of sleep and arousal17 and because neurotransmitter systems in this region are involved in mood regulation.18
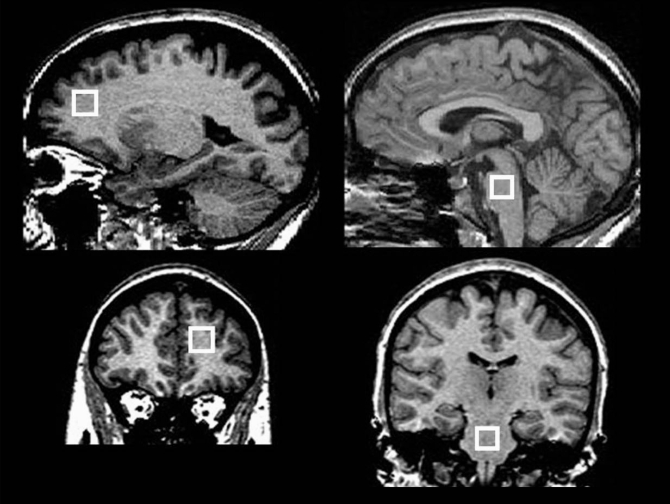
Fig. 1.
Positions of the 2 spectroscopic volumes of interest (VOI): the left dorsal prefrontal region and the pons. We first acquired data from a 16 × 16 × 16–mm VOI in the left dorsal prefrontal region (left panels) and then from a 13 × 13 × 13–mm VOI in the pons (right panels). The VOIs are illustrated in the sagittal plane (above) and the coronal plane (below).
Based on previous studies,2–4 we anticipated that about half of depressed participants would show substantial mood improvement after sleep loss. We predicted that clinical and neurochemical responses to sleep loss would differ between depressed participants and healthy controls and that responders and nonresponders to sleep restriction would differ in their neurochemical characteristics.
Methods
Participants
We recruited women with a current diagnosis of moderate unipolar depression and healthy controls. We diagnosed depression and ruled out potential comorbidities according to the criteria of the DSM-IV, text revised,19 using the Mini-International Neuropsychiatric Interview.20 Using the Hamilton Depression Rating Scale (HDRS),21 we assessed the severity of depressive symptoms. We obtained self-reports of depressive symptoms using the Profile of Mood States (POMS)22 and the Hamilton Depression Inventory (HDI).23
A score of 18 or higher on the 17-item HDRS was required for inclusion in the depressed group. We applied the following exclusion criteria to all participants: first-degree relatives with bipolar disorder, presence of neurologic disorders, current suicidal plans, psychotic symptoms, use of prescribed medication other than a stable antidepressant treatment and current use of illegal drugs. An abstinence period of 1 year was required of any participant reporting previous alcohol or substance dependence; however, all participants denied such dependence. We sought depressed women not currently receiving antidepressant drug therapy preferentially, but permitted current stable pharmacotherapy. Previous studies have reported substantial mood improvement in depressed patients after sleep deprivation, regardless of medication status.4 A psychiatrist (S.D.) interviewed all selected participants to confirm that they met inclusion and exclusion criteria.
The Capital District Health Authority Research Ethics Board approved our study protocol, and we obtained informed consent in writing from each participant after the nature of the procedures had been fully explained.
Procedures
We asked participants to maintain their usual sleep habits during the week before the study. All participants reported that they had a normal night’s sleep on the night preceding the experiment. At 11:00 on the baseline day, they completed the POMS and HDI and underwent a brain scan at either 12:00 or 13:00 (scan A). We then instructed participants to go about their usual activities but to avoid napping. That evening, participants completed the POMS at 22:00 and were allowed to rest or sleep in bed from 22:30 until 01:00 the next morning; sleep was not monitored. Such partial sleep deprivation has been shown to be almost as effective as a night of total sleep deprivation.24 During the postsleep restriction day, we obtained self-reports on the POMS at 01:00, 04:00, 07:30 and 11:30; the HDI was administered at 7:30 and 11:30. A second brain scan took place that day at 12:00 or 13:00 (scan B). To help participants stay awake during brain scans, we did not give them a blanket in the scanner, and we prompted them to respond to short questions during the scan.
Image acquisition protocols
We performed magnetic resonance acquisitions with a 1.5 T GE scanner and a standard quadrature head coil. We acquired coronal T1-weighted 3-dimensional fast spoiled gradient recall images with the following parameters: flip angle 40°, echo time (TE) 5 ms, repetition time (TR) 25 ms, field of view 24 × 18 cm, matrix 256 × 160 pixels, number of excitations 1, no interslice gap, 124 images, slice thickness 1.5 mm. We acquired single-volume 1H-MRS spectra with a point-resolved spectroscopy sequence: TE 30 ms, TR 2000 ms, 320 acquisitions, 2500 Hz spectral bandwidth, 2048 data points, duration 11.5 minutes; a water unsuppressed spectrum was acquired with the same parameters but only 16 acquisitions. Automated shimming took place before MRS acquisitions, and we applied outer volume suppression bands about 3 mm from the outside borders of each volume of interest (VOI). We initially positioned a 16 × 16 × 16 mm VOI in the left dorsal prefrontal region encompassing both white and grey matter: the posterior border was immediately anter ior to the slice containing the corpus callosum (sagittal view), the inferior border was 2 slices below the most superior slice containing the corpus callosum (sagittal view) and the VOI was centred left-to-right within the hemisphere (coronal view). Subsequently, we positioned a 13 × 13 × 13 mm VOI in the mesopontine region centrally in each direction (Fig. 1). The Montreal Neurological Institute (MNI) coordinates for the central point of a representative VOI were −18 mm (left), +44 mm (anterior) and +21 mm (superior) for the prefrontal region; and +1 mm (right), −29 mm (posterior) and −41 mm (inferior) for the pons.
Data processing
The focus of the analysis was on the 3 main spectral peaks: NAA 2.0 ppm, Cho 3.0 ppm and tCr 3.2 ppm. Spectra were line-shape corrected to restore the Lorentzian line shape using combined QUALITY deconvolution and eddy current correction25 to remove artifacts caused by gradient coil vibration and residual eddy current distortions. We removed the residual water peak using an operator-independent singular value decomposition fitting algorithm.26 We then fitted spectroscopy data in the time domain using fitMAN analysis software.27
We fitted data using 3 peaks, 1 for each resonance of interest. To minimize the influence of signals coming from short T2 and J-coupled spectral peaks, we omitted the first 16.4 ms at the beginning of the time domain signal.28 We also omitted data beyond 200 ms from the fit because the signal had decayed to noise by 200 ms. We scaled metabolite levels using the unsuppressed water as an internal standard and adjusted for the partial volumes of cerebral spinal fluid (CSF) in the prefrontal VOI. Tissue-type segmentation was not possible in the pons; it was performed in the prefrontal region using the 3dAnhist script provided by the program Analysis of Functional NeuroImages.29,30 We did not adjust for potential between-group differences in T1 and T2 relaxation times, therefore neurochemical levels are reported in institutional units (IU) rather than as molar concentrations. Only spectra with a line width narrower than 7 Hz and a signal-to-noise ratio for NAA above 10 are included in the analyses.
Statistical analyses
We performed statistical analyses with SPSS software, version 11.5 (SPSS Inc.). The significance threshold was p < 0.05, 2-tailed. If the assumption of normality was violated (tested with the Shapiro–Wilk test), we used the appropriate nonparametric test. Analyses of variance (ANOVAs) assessed the effects of group and of scan time on levels of each neurochemical in each VOI and on stability of tissue volumes in the prefrontal VOI. We considered each neurochemical independently; we did not apply a Bonferroni correction owing to the pilot nature of our study. We conducted follow-up tests for each group, contrasting scans A and B, and we calculated Cohen’s d to estimate effect sizes.
Results
Participants
Fifteen healthy women and 12 women with a current diagnosis of moderate unipolar depression participated in our study. The demographic characteristics of both groups are summarized in Table 1, and the clinical characteristics of the depressed patients are provided in Table 2. One depressed participant withdrew during the night of sleep restriction; her baseline data are included in our analyses where appropriate.
Table 1.
Demographic characteristics of women in the depressed and control groups
| Group; no.* |
||
|---|---|---|
| Characteristic | Control (n = 15) | Depressed (n = 12)† |
| Age, mean (range), yr | 24 (19–30) | 25 (21–30) |
| Education level | ||
| Undergraduate | 7 | 8 |
| Graduate student | 8 | 4 |
| Handedness | ||
| Right | 15 | 11 |
| Left | 0 | 1 |
Unless otherwise indicated.
One depressed participant quit the study during the sleep deprivation night.
Table 2.
Clinical characteristics of 11 women in the depressed group who completed the sleep deprivation night, by participant subgroup
| Age, yr | Current medication (duration) | Duration of current episode | Start of first episode | HDRS score* at screening | POMS score† at screening |
|---|---|---|---|---|---|
| Responder | |||||
| 22 | Citalopram (2 yr) | 2 yr | 13 yr ago | 21 | 79 |
| 26 | None (1 yr drug-free) | 4 yr | 4 yr ago | 21 | 150 |
| 30 | Citalopram (2 mo) | 6 mo | 15 yr ago | 20 | 98 |
| 30 | None (7 mo w/o sertraline) | 13 yr | 13 yr ago | 22 | 76 |
| 28 | Venlafaxine and bupropion (3 yr) | 3 yr | 18 yr ago | 26 | 58 |
| Nonresponder | |||||
| 27 | Venlafaxine (1 mo) | 3 yr | 12 yr ago | 20 | 52 |
| 21 | None (drug-naïve) | 11 yr | 11 yr ago | 22 | 50 |
| 22 | Paroxetine (1 yr) | 1 yr | 1 yr ago | 24 | 108 |
| 23 | Citalopram (6 mo) | 9 yr | 9 yr ago | 25 | 115 |
| 25 | None (drug-naïve) | 12 yr | 12 yr ago | 18 | 56 |
| 21 | None (drug-naïve) | 2 yr | 5 yr ago | 20 | 128 |
Cutoff for inclusion in the study was 18 on the HDRS. Group mean (SD) scores at screening were 22.0 (2.3) for subsequent responders and 21.5 (2.7) for nonresponders.
Group (mean) scores on the POMS at screening were 92.2 (35.3) for subsequent responders and 84.8 (35.9) for nonresponders.
Mood scores
Analyses of depression scores are provided in Appendix 1, available at www.cma.ca/jpn. We classified depressed patients as responders to sleep restriction if they showed (1) improvement in HDI total score after sleep restriction and (2) at least a 30% improvement in HDI mood score. Based on these criteria, 5 of 11 participants were responders and 6 were non-responders. Mann–Whitney U tests showed that responders did not differ from nonresponders in baseline mood scores (z = 1.792, p = 0.10) nor baseline HDI total scores (z = 0.940, p = 0.42).
Imaging data
The mean line width (standard deviation [SD]) of the water peaks from all spectra was 5.46 (0.59) Hz. The fractional volumes of tissue types in prefrontal VOIs did not vary between groups at either scan time. However, within the depressed group, small differences in positioning of prefrontal VOIs resulted in an increase from 66.7% to 76.2% in white matter partial volume and a decrease from 32.4% to 23.3% in grey matter partial volume from scan A to B; CSF partial volumes were stable at 0.9% (scan A) and 0.4% (scan B) of the VOI (Table 3).
Table 3.
Analyses of variance for neurochemicals (in institutional units) and for fractional content of tissues
| Scan time* |
Group† |
Interaction‡ |
|||||
|---|---|---|---|---|---|---|---|
| Variable | Degrees of freedom | F | p value | F | p value | F | p value |
| Prefrontal | |||||||
| NAA | (1–21) | 0.196 | 0.66 | 0.121 | 0.73 | 0.795 | 0.38 |
| Cho | (1–21) | 5.242 | 0.033** | 0.116 | 0.74 | 0.942 | 0.34 |
| tCr | (1–21) | 0.063 | 0.81 | 0.849 | 0.37 | 0.746 | 0.40 |
| Grey matter | (1–21) | 9.048 | 0.007†† | 0.992 | 0.33 | 7.352 | 0.013** |
| White matter | (1–21) | 9.642 | 0.005†† | 0.872 | 0.36 | 7.657 | 0.012** |
| Cerebrospinal fluid | (1–21) | 3.180 | 0.09 | 0.122 | 0.73 | 1.987 | 0.17 |
| Pons§ | |||||||
| NAA | (1–16) | 1.719 | 0.21 | 0.698 | 0.42 | 2.216 | 0.16 |
| Cho | (1–16) | 1.555 | 0.23 | 3.966 | 0.06 | 0.058 | 0.81 |
| Cho¶ | (2–15) | 1.603 | 0.22 | 4.072 | 0.039** | 2.299 | 0.14 |
| tCr | (1–16) | 5.070 | 0.039** | 3.804 | 0.07 | 0.090 | 0.77 |
Cho = choline compounds; NAA = N-acetylaspartate; tCr = creatine-plus-phosphocreatine.
Within-subject factor with 2 levels: A (baseline) and B (post–sleep restriction).
Between-subject factor with 2 levels: control and depressed.
Scan time by group.
Accurate segmentation of tissue types was not possible in the pons.
Variable for which the between-subject factor has 3 levels: control, responder and nonresponder.
Significant at p < 0.05.
Significant at p < 0.01.
Prefrontal region
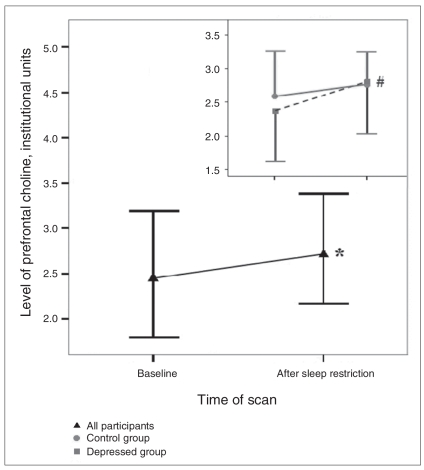
We found a significant main effect of scan time for prefrontal Cho, indicating an 11.3% increase (Cohen’s d = 0.46) in Cho levels after sleep restriction in both groups combined (Fig. 2, Table 3, Table 4). Follow-up paired t tests revealed a 17.9% increase in the depressed group (t9 = −3.352, p = 0.008, Cohen’s d = 1.06) after sleep restriction, but no change in controls (t9 = −0.835, p = 0.42). We observed no clustering of values with respect to medication status. We evaluated whether the increase in prefrontal Cho could be an artifact of the observed increase in white matter partial volume during the second scan in the depressed group (see the Discussion section and Appendix 1).
Fig. 2.
Concentration levels of choline compounds in institutional units (IU) acquired in the left prefrontal region for both groups combined and for the control (n = 13) and depressed (n = 10) groups (inset) during the baseline scan and 24 h later after overnight sleep restriction. Levels increased significantly after sleep restriction for both groups combined (*p = 0.033, Cohen’s d = 0.46). There was no significant change in the control group alone, but a significant increase among depressed participants (#p = 0.008, Cohen’s d = 1.06). Error bars representing 1 standard deviation are shown in 1 direction only for clarity. The number of values varies because only participants with scans meeting quality criteria on both days are included.
Table 4.
Neurochemical values (in institutional units) for the pons and the left prefrontal region
| Magnetic resonance spectroscopy scan;* mean (SD)
|
||||
|---|---|---|---|---|
| Brain region; participant group | Neurochemical | Scan A | Scan B | n† |
| Pons | ||||
| All participants | Cho | 3.79 (1.01) | 3.32 (0.94) | 18 |
| tCr | 8.14 (2.02) | 6.50§ (1.91) | 18 | |
| NAA | 15.07 (1.93) | 16.03 (2.20) | 18 | |
| Controls | Cho | 4.12‡ (1.02) | 3.55 (0.80) | 8 |
| tCr | 8.86 (1.02) | 6.97 (1.57) | 8 | |
| NAA | 14.16 (1.61) | 16.52 (1.92) | 8 | |
| Depressed (all) | Cho | 3.53 (0.98) | 3.14 (1.04) | 10 |
| tCr | 7.56 (2.46) | 6.12 (2.15) | 10 | |
| NAA | 15.80 (1.93) | 15.65 (2.43) | 10 | |
| Responders | Cho | 4.31‡ (0.74) | 2.94 (0.94) | 5 |
| tCr | 7.80 (3.25) | 5.31 (2.22) | 5 | |
| NAA | 16.32 (1.64) | 15.51 (1.73) | 5 | |
| Nonresponders | Cho | 2.74‡ (0.21) | 3.34 (1.21) | 5 |
| tCr | 7.32 (1.72) | 6.93 (1.96) | 5 | |
| NAA | 15.28 (2.23) | 15.79 (3.20) | 5 | |
| Left prefrontal region | ||||
| All participants | Cho | 2.49 (0.70) | 2.78§ (0.61) | 23 |
| tCr | 8.21 (1.86) | 8.14 (1.51) | 23 | |
| NAA | 10.07 (0.87) | 9.88 (1.31) | 23 | |
| Controls | Cho | 2.58 (0.68) | 2.76 (0.49) | 13 |
| tCr | 8.22 (1.77) | 8.53 (1.14) | 13 | |
| NAA | 10.26 (0.97) | 9.79 (1.28) | 13 | |
| Depressed (all) | Cho | 2.38 (0.75) | 2.80¶ (0.76) | 10 |
| tCr | 8.20 (2.08) | 7.63 (1.83) | 10 | |
| NAA | 9.83 (0.68) | 9.99 (1.42) | 10 | |
| Responders | Cho | 2.21 (1.00) | 2.80 (1.01) | 5 |
| tCr | 7.21 (1.40) | 7.33 (2.60) | 5 | |
| NAA | 9.44 (0.12) | 10.14 (1.30) | 5 | |
| Nonresponders | Cho | 2.54 (0.45) | 2.80 (0.54) | 5 |
| tCr | 9.19 (2.30) | 7.93 (0.74) | 5 | |
| NAA | 10.23 (0.79) | 9.85 (1.66) | 5 | |
Cho = choline compounds; NAA = N-acetylaspartate; tCr = creatine-plus-phosphocreatine.
Scan A took place on the baseline day at 12:00; scan B took place the following day at 12:00 after restriction of sleep to a maximum of 2.5 h.
The number of values varies because only those with scans meeting quality criteria on both days are included.
Analysis of variance showed that the between-group factor was significant (p < 0.05). Follow-up t tests showed that nonresponders at scan A differed from both controls and responders (p < 0.01).
Analysis of variance showed that the repeated measures factor was significant (p < 0.01).
Follow-up t test was significant (p < 0.01).
Pontine region
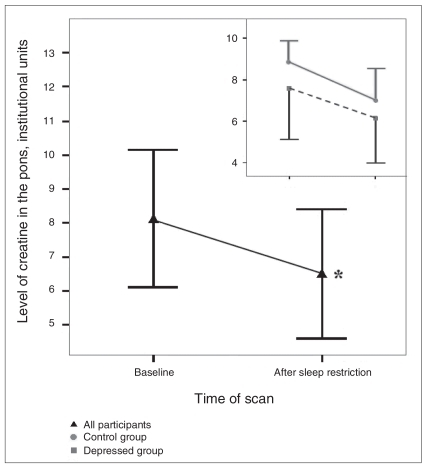
We observed a significant main effect of scan time for pontine tCr (Fig. 3, Table 3, Table 4), revealing a 20.1% decrease (Cohen’s d = 0.54) from scan A to scan B. Follow-up Wilcoxon tests showed no significant change in controls (z = 1.023, p = 0.32) or depressed participants alone (t9 = 1.236, p = 0.25). This main effect was attributable to both groups combined.
Fig. 3.
Concentration levels of creatine-plus-phosphocreatine (tCr) in the pons for both groups combined (n = 18) and separately for the control (n = 8) and depressed (n = 10) groups (inset) during the baseline scan and 24 hours later after overnight sleep restriction. In both groups combined, tCr levels declined by 20.1% after sleep restriction relative to baseline values (*p = 0.039, Cohen’s d = 0.54). We observed similar patterns in the 2 groups (inset), but neither group alone was statistically significant. Error bars representing 1 standard deviation are shown in 1 direction only for clarity. The number of values varies because only participants with scans meeting quality criteria on both days are included.
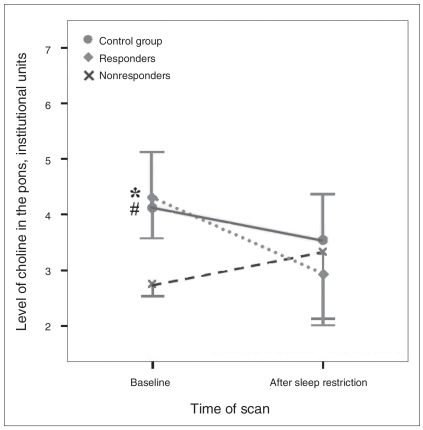
Exploratory mixed ANOVAs conducted to compare neurochemical levels between scan times and between controls and depressed responders/nonresponders to sleep restriction revealed a significant main effect of group for baseline pontine Cho levels (Fig. 4, Table 3, Table 4). Follow-up Mann–Whitney U tests showed that subsequent nonresponders had reduced baseline Cho levels relative to responders (z = 2.611, p = 0.008) and controls (z = 2.951, p = 0.001), whereas subsequent responders did not differ from controls (z = 0.843, p = 0.44).
Fig. 4.
Concentration levels of choline compounds (Cho) acquired in the pons during the baseline scan and 24 hours later after overnight sleep restriction. Data are shown for the control group (n = 10) and separately for depressed participants showing at least 30% mood improvement after sleep loss (responders, n = 5) and those failing to show such improvement (nonresponders, n = 5). The omnibus test showed a significant effect of group (p = 0.039). Follow-up tests showed that Cho levels were significantly lower at baseline for nonresponders compared with both responders (*p = 0.008) and controls (#p = 0.001). Error bars representing 1 standard deviation are shown in 1 direction only for clarity. The number of values varies because only participants with scans meeting quality criteria on both days are included.
Discussion
Pons
The pons contains nuclei and monoamines that are implicated in mood disorders and targeted in their pharmacotherapy.18,31 It is also critically involved in sleep–wake regulation.17 Our results indicate that pontine levels of tCr were reduced after sleep restriction in all participants combined. Both depressed and control participants showed similar trends, but there may have been insufficient power to demonstrate significant changes for each group separately (Fig. 3). Since the creatine kinase/phosphocreatine network is involved in connecting sites of production and consumption of adenosine triphosphate (ATP), the primary source of metabolic energy in the brain,32 these findings suggest changes in ATP-related metabolic activity. Consistent with this interpretation, a positron emission tomography study of sleep deprivation effects in healthy people reported lower metabolic rates in the mesopontine and pontine regions after sleep deprivation.33
Levels of pontine Cho during the baseline scan did not differ between the depressed and control groups. However, pontine Cho values in depressed women at baseline predicted subsequent mood responses to sleep restriction: all depressed women who subsequently met the criterion for mood improvement had higher baseline Cho levels than those who did not (Fig. 4). This result should be regarded with caution because of the small size of these subgroups.
Membrane-bound phosphatidylcholine modulates concentrations of phosphorylcholine and glycerophosphorylcholine, the 2 major constituents of the Cho signal.34 Thus, changes in the Cho peak may reflect changes in metabolic activity of phosphatidylcholine. Since phospholipid metabolic activity consumes about 20% of total ATP in the brain,35 these data may also reflect differences in pontine metabolic activity.
Prefrontal region
We found that Cho levels increased in the prefrontal region after sleep restriction for both groups combined; this increase was statistically significant for depressed participants alone but not for controls. The selectivity of this effect should be considered tentative given the small number of participants; however, previous studies also reported that sleep deprivation did not affect brain chemistry in the frontal and prefrontal regions of healthy volunteers,7,8 whereas it marginally increased concentrations of Cho and tCr in the left dorsal prefrontal region of depressed inpatients.9 Our results confirmed only the increases in Cho, perhaps because our sample consisted of outpatients with moderate levels of depression rather than inpatients. In view of the relation between the subcomponents of the Cho peak and brain metabolism noted previously,34,35 these results suggest an increase in metabolic activity in this region after sleep loss in depressed patients. This interpretation is consistent with previous functional imaging studies that reported that remission of depressive symptoms after a course of antidepressant medication was associated with increased metabolic rates in this region.15,16
Despite the use of precise guidelines and landmarks in positioning VOIs, there is a risk that small differences in positioning VOIs in repeated-measures studies can alter the fractional content of white matter, grey matter and CSF. Neurochemical concentrations are known to differ among white matter, grey matter and CSF.36,37 Fractional CSF has the greatest effect on measured neurochemical concentrations, therefore we adjusted measured concentrations for fractional CSF in each VOI.
The prefrontal VOIs, however, had a 9.5% higher proportion of white matter in depressed participants during scan B compared with scan A (Table 3), so we considered whether this change could have contributed to the observed increase in Cho. Previous studies reported that absolute concentrations of Cho are somewhat lower in white matter relative to grey matter in the cerebrum.36,37 Whether Cho concentrations in each tissue type are stable or change after loss of sleep is unknown. Regardless, the increase in white matter proportion alone could not account for the 17.9% increase in Cho at scan B. The increase in prefrontal Cho thus appears to be attributable to the effects of sleep loss and not to the change in VOI tissue type content (Appendix 1).
Limitations
A number of limitations of this study should be addressed in future investigations of these issues, including studying larger numbers of participants. Some depressed participants were undergoing stable pharmacological treatments. Although they did not appear to differ from untreated participants in their neurochemical or mood characteristics, it would be preferable to include only medication-naïve participants. Monitoring sleep electrophysiologically at baseline and during the sleep-restriction period would allow a test of whether sleep differences among participants might have affected the results obtained.
Conclusion
In summary, the significant increase after sleep restriction in Cho levels in the dorsal, prefrontal region only in depressed women may reflect increased metabolic activity, consistent with numerous previous studies showing that metabolic activity in this region is correlated with mood state. In addition, the results demonstrate that the level of Cho in the pons is not correlated with mood state, but that its level at baseline strongly predicts responsiveness of depressed women to the mood-elevating effects of sleep restriction. These findings suggest that pontine Cho levels may be a marker for biological differences between responders and nonresponders to sleep loss. A better understanding of the physiologic processes contributing to acute mood improvement after sleep loss could provide insight into the mechanisms underlying depressive disorders and their treatment.
Acknowledgments
This research was supported by grants from the Nova Scotia Capital District Health Authority Research Fund and the Department of Psychiatry, Dalhousie University. It was submitted in partial fulfillment of the requirements for a PhD degree (D.B.) at Dalhousie University. We thank Carl Helmick, Mark Given, Matthew Rogers and Gregory McLean for their invaluable technical assistance; the late Vivek Kusumakar for his support and encouragement; Sonia Chehil and Marina Sokolenko for clinical support; Mohamed Abdolell and Wade Blanchard for their useful advice on statistical analyses; and three anonymous reviewers for numerous helpful suggestions.
Footnotes
Competing interests: None declared.
Contributors: Drs. Bernier, MacMaster and Rusak designed the study. Drs. Bernier, Devarajan, MacMaster and Schmidt acquired the data, which Drs. Bernier, Bartha, MacMaster, Schmidt and Rusak analyzed. Drs. Bernier, MacMaster and Rusak wrote the article, which all authors reviewed and approved for publication.
References
- 1.Stassen HH, Angst J, Hell D, et al. Is there a common resilience mechanism underlying antidepressant drug response? Evidence from 2848 patients. J Clin Psychiatry. 2007;68:1195–205. doi: 10.4088/jcp.v68n0805. [DOI] [PubMed] [Google Scholar]
- 2.Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147:14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: What do we know, where do we go? Biol Psychiatry. 1999;46:445–53. doi: 10.1016/s0006-3223(99)00125-0. [DOI] [PubMed] [Google Scholar]
- 4.Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6:361–77. [PubMed] [Google Scholar]
- 5.Urrila AS, Hakkarainen A, Heikkinen S, et al. Preliminary findings of proton magnetic resonance spectroscopy in occipital cortex during sleep deprivation. Psychiatry Res. 2006;147:41–6. doi: 10.1016/j.pscychresns.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Murck H, Struttmann T, Czisch M, et al. Increase in amino acids in the pons after sleep deprivation: a pilot study using proton magnetic resonance spectroscopy. Neuropsychobiology. 2002;45:120–3. doi: 10.1159/000054949. [DOI] [PubMed] [Google Scholar]
- 7.Murashita J, Yamada N, Kato T, et al. Effects of sleep deprivation: the phosphorus metabolism in the human brain measured by 31P-magnetic resonance spectroscopy. Psychiatry Clin Neurosci. 1999;53:199–201. doi: 10.1046/j.1440-1819.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- 8.Dorsey CM, Lukas SE, Moore CM, et al. Phosphorus31 magnetic resonance spectroscopy after total sleep deprivation in healthy adult men. Sleep. 2003;26:573–7. doi: 10.1093/sleep/26.5.573. [DOI] [PubMed] [Google Scholar]
- 9.Murck H, Schubert MI, Schmid D, et al. The glutamatergic system and its relation to the clinical effect of therapeutic-sleep deprivation in depression — an MR spectroscopy study. J Psychiatr Res. 2009;43:175–80. doi: 10.1016/j.jpsychires.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–31. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 11.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimized treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 12.Galynker II, Cai J, Ongseng F, et al. Hypofrontality and negative symptoms in major depressive disorder. J Nucl Med. 1998;39:608–12. [PubMed] [Google Scholar]
- 13.Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12:527–44. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 14.Aihara M, Ida I, Yuuki N, et al. HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Res. 2007;155:245–56. doi: 10.1016/j.pscychresns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy SH, Evans KR, Kruger S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 17.Boutrel B, Koob GF. What keeps us awake: the neuropharmacology of stimulants and wakefulness promoting medications. Sleep. 2004;27:1181–94. doi: 10.1093/sleep/27.6.1181. [DOI] [PubMed] [Google Scholar]
- 18.Ressler KJ, Nemeroff CB. Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol Psychiatry. 1999;46:1219–33. doi: 10.1016/s0006-3223(99)00127-4. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed, text Revised. Washington: The Association; 2000. [Google Scholar]
- 20.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 21.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–7. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 22.McNair DM, Lorr M, Droppleman LF, editors. Manual for the profile of mood states revised. San Diego (CA): Edits/Educational and Industrial Testing Service; 1992. [Google Scholar]
- 23.Reynolds WM, Kobak KA. HDI Hamilton Depression Inventory A self-report version of the Hamilton Depression Rating Scale (HDRS) Odessa (FL): Psychological Assessment Resources; 1995. [Google Scholar]
- 24.Giedke H, Klingberg S, Schwarzler F, et al. Direct comparison of total sleep deprivation and late partial sleep deprivation in the treatment of major depression. J Affect Disord. 2003;76:85–93. doi: 10.1016/s0165-0327(02)00071-x. [DOI] [PubMed] [Google Scholar]
- 25.Bartha R, Drost DJ, Menon RS, et al. Spectroscopic lineshape correction by QUECC: combined QUALITY deconvolution and eddy current correction. Magn Reson Med. 2000;44:641–5. doi: 10.1002/1522-2594(200010)44:4<641::aid-mrm19>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.van den Boogaart A, van Ormondt D, Pijnappel WW, et al. Removal of the water resonance from 1H magnetic resonance spectra. In: McWirter JG, editor. Mathematics in signal processing III. Oxford: Clarendon Press; 1994. pp. 175–95. [Google Scholar]
- 27.Bartha R, Drost DJ, Williamson PC. Factors affecting the quantification of short echo in-vivo 1H MR spectra: prior knowledge, peak elimination, and filtering. NMR Biomed. 1999;12:205–16. doi: 10.1002/(sici)1099-1492(199906)12:4<205::aid-nbm558>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Stanley JA, Panchalingam K, Keshavan MS, et al. A postprocessing method to accurately quantify N-acetyl-aspartate in short echo time in vivo 1H spectra. [(accessed 2009 Jul 9)];Proc ISMRM. 2002 10:2514. Available: http://brain.wayne.edu/jeff/naa_ismrm2002x.pdf. [Google Scholar]
- 29.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 30.Gispert JD, Reig S, Pascau J, et al. Method for bias field correction of brain T1-weighted magnetic resonance images minimizing segmentation error. Hum Brain Mapp. 2004;22:133–44. doi: 10.1002/hbm.20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery SA. Why do we need new and better antidepressants? Int Clin Psychopharmacol. 2006;21:S1–10. doi: 10.1097/01.yic.0000199455.39552.1c. [DOI] [PubMed] [Google Scholar]
- 32.Wallimann T, Wyss M, Brdiczka D, et al. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostatsis. Biochem J. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 34.Boulanger Y, Labelle M, Khiat A. Role of phospholipase A(2) on the variations of the choline signal intensity observed by 1H magnetic resonance spectroscopy in brain diseases. Brain Res Brain Res Rev. 2000;33:380–9. doi: 10.1016/s0165-0173(00)00037-0. [DOI] [PubMed] [Google Scholar]
- 35.Purdon AD, Rosenberger TA, Shetty HU, et al. Energy consumption by phospholipid metabolism in mammalian brain. Neurochem Res. 2002;27:1641–7. doi: 10.1023/a:1021635027211. [DOI] [PubMed] [Google Scholar]
- 36.Hetherington HP, Mason GF, Pan JW, et al. Evaluation of cerebral gray and white matter metabolite differences by spectroscopic imaging at 4.1T. Magn Reson Med. 1994;32:565–71. doi: 10.1002/mrm.1910320504. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Li S-J. Differentiation of metabolic concentrations between gray matter and white matter of human brain by in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 1998;39:28–33. doi: 10.1002/mrm.1910390107. [DOI] [PubMed] [Google Scholar]