Abstract
Background
Attention-deficit hyperactivity disorder (ADHD) is an important psychiatric condition in terms of its prevalence and impact on quality of life. It has one of the highest heritabilities found in psychiatric disorders. A number of association studies exploring several candidate genes in different populations around the world have been carried out. The objective of the present study was to carry out a meta-analysis for 8 common variants located in 5 top candidate genes for ADHD (BDNF, HTR1B, SLC6A2, SLC6A4 and SNAP25); these genes are known to be involved in synaptic transmission and plasticity.
Methods
We performed a search for published genetic association studies that analyzed the candidate polymorphisms in different populations, and we applied state-of-the-art meta-analytical procedures to obtain pooled odds ratios (ORs) and to evaluate potential basis of heterogeneity. We included 75 genetic association studies in these meta-analyses.
Results
A major part of the previously postulated associations were nonconsistent in the pooled odds ratios. We observed a weak significant association with a single nucleotide polymorphism (SNP) located in the 3′ UTR region of the SNAP25 gene (rs3746544, T allele, OR 1.15, 95% confidence interval 1.01–1.31, p = 0.028, I2 = 0%). In addition to the low coverage of genetic variability given by these variants, phenotypic heterogeneity between samples (ADHD subtypes, comorbidities) and genetic background may explain these differences.
Limitations
Limitations of our study include the retrospective nature of our meta-analysis with the incorporation of study-level data from published articles.
Conclusion
To our knowledge, the present study is the largest meta-analysis carried out for ADHD genetics; previously proposed cumulative associations with common polymorphisms in SLC6A4 and HTR1B genes were not supported. We identified a weak consistent association with a common SNP in the SNAP25 gene, a molecule that is known to be central for synaptic transmission and plasticity mechanisms.
Introduction
Attention-deficit hyperactivity disorder (ADHD) has been identified as an important psychiatric condition in terms of its prevalence (around 5% worldwide) and its impact on quality of life for patients and their families.1 It has one of the highest heritabilities found in psychiatric disorders (around 0.76), which has supported the development of many genetic association studies exploring a number of candidate genes in different populations around the world.2 These studies have compared allele and genotype frequencies between patients and controls or their transmission within nuclear families.3
A large number of these genetic association studies have focused on the analysis of variations in dopaminergic and serotoninergic genes.4 A recent meta-analysis of 3 dopaminergic genes found a significant association with polymorphisms in the DRD4 and DRD5 genes.5 It has been postulated that a dysfunction of neuroplasticity mechanisms can be involved in the pathophysiology of ADHD and other common neuropsychiatric disorders.6–8 In this respect, a recent general review about ADHD genetics suggested the possibility that variations in some candidate genes such as SNAP25, SLC6A4 and HTR1B could be important risk factors for ADHD in different populations;4 these genes are known to be involved in neurotransmission and neural plasticity. In addition, in recent years a number of publications have explored other polymorphisms in genes of relevance for synaptic function and plasticity such as BDNF and SLC6A2, as susceptibility factors for ADHD.2 Recent genome-wide association studies of ADHD and related phenotypes show additional evidence supporting the role of neuroplasticity genes in the etiology of this disorder.9,10
To explore the possibility that some of these common variants may have a role as susceptibility factors for ADHD, we applied meta-analytic strategies to data from available association studies and considered possible factors that could account for heterogeneity between studies.
General information about the biological features of the 5 candidate genes analyzed herein is shown in Table 1. The BDNF gene encodes one of the main neurotrophic factors in the brain, HTR1B encodes one of the serotonin receptors, SLC6A2 encodes the noradrenalin transporter, SLC6A4 encodes the serotonin transporter and SNAP25 encodes one of the main presynaptic proteins. All these genes are known to be involved in synaptic and neural plasticity, a central molecular mechanism responsible for many behavioural phenomena in normal and pathological conditions in humans and animals.12
Table 1.
General information about candidate genes for attention-deficit hyperactivity disorder
| Gene | Symbol | Location | Size | Protein | Isoforms* | Exons | SNPs | nsSNPs | Blocks† | tSNPs | CNVs | Expression‡ | No. studies§ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain-derived neurotrophic factor | BDNF | 11p13 | 66 857 | 247 aa | 9/24 | 2 | 235 | 5 | 2 | 18 | N |
|
189 |
| 5-HT receptor 1B | HTR1B | 6q13 | 1260 | 390 aa | 1/1 | 1 | 29 | 4 | 3 | 21 | Y |
|
49 |
| Solute carrier family 6 (neurotransmitter transporter, noradrenalin), member 2 | SLC6A2 | 16q12.2 | 48 629 | 617 aa | 5/7 | 14 | 447 | 17 | 9 | 47 | Y |
|
39 |
| Solute carrier family 6 (neurotransmitter transporter, serotonin), member 4 | SLC6A4 | 17q11.1-q12 | 41 379 | 630 aa | 1/4 | 15 | 424 | 14 | 2 | 16 | N |
|
589 |
| Synaptosomal-associated protein, 25kDa | SNAP25 | 20p12-p11.2 | 88 589 | 206 aa | 3/31 | 8 | 704 | 6 | 13 | 55 | N |
|
15 |
5-HT = serotonin; CNVs = copy number variants; nsSNPs = nonsynonymous single nucleotide polymorphisms; SNPs = single nucleotide polymorphisms; tSNPs = r2-based tagging single nucleotide polymorphisms.
Isoforms were identified in the UCSC genes track/AceView track.
Haplotype blocks.
Expression information in brain tissues was retrieved from the SymAtlas database.11
Number of published papers exploring variations in the respective genes.
Methods
We searched for genetic association studies analyzing polymorphisms in the SLC6A4 (rs4795541 and intronic VNTR), SNAP25 (rs3746544 and rs1051312), BDNF (rs6265 and rs56164415), HTR1B (rs6296) and SLC6A2 (rs998424) genes in the PubMed database. We combined disease search terms “ADHD or attention-deficit hyperactivity disorder” with the respective search terms for the genes of interest: “BDNF or brain-derived neurotrophic factor,” “HTR1B or serotonin receptor 1B,” “SLC6A2 or norepinephrine transporter,” “SLC6A4 or serotonin transporter” and “SNAP25 or synaptosomal-associated protein, 25 kDa.” In addition, we searched reference lists of relevant review and original papers and checked the supplementary files of high-throughput association studies of ADHD to identify additional papers not covered by the electronic search of abstracts.
We included articles published in English in peer-reviewed journals that described results from case–control or transmission disequilibrium test (TDT) studies analyzing the association of the selected candidate polymorphisms with ADHD in children or adults in different ethnic populations (the TDT was the main analytical approach used in the family-based studies). However, we did not include studies of quantitative measures of ADHD, response to medications or analyses of other markers (different from the selected candidate polymorphisms) in the candidate genes.
We extracted information about general features of the studies (e.g., sample sizes, phenotyping scales, genotyping methodologies, subtypes analyzed) from each article. In all cases of missing data, we contacted the respective authors to ask for allele frequencies that were not available in the main text of the papers or in their supplementary files.
For the meta-analysis procedures, we used the Catmap program (http://cran.r-project.org/web/packages/catmap/index.html); it is a freely available package specifically created for the meta-analysis of genetic association studies. It runs in the R statistical platform and allows for the joint analysis of case–control and family-based association studies and other advanced analysis approaches (e.g., implementation of fixed-effects or random-effects models, sensitivity analysis, cumulative meta-analysis).13 We calculated the Q statistic for heterogeneity and, following recommendations in the area,14 we used random-effects models for the calculations of the pooled odds ratios (ORs).
We downloaded genotype data for the different candidate genes from the HapMap database (release23a; using additional 20 kb as both flanking 5′ and 3′ regions) for the Caucasian (CEU, from Northern Europe living in Utah, US), Asian (JPT+CHB, from Japanese living in Tokyo and Chinese living in Beijing) and African (YRI, Yoruba living in Nigeria) populations,15 and we calculated the haplotype block and linkage disequilibrium structures with the Haploview and GOLD programs.16,17 To enhance the visualization of haplotype block structure we modified the output from Haploview, incorporating physical distances into the linkage disequilibrium map. We retrieved physical positions of analyzed markers and their locations with respect to single nucleotide polymorphisms (SNPs) typed in the HapMap project from the UCSC genome browser18 and retrieved information about candidate genes from the UCSC genome browser, the NCBI dbSNP database, GeneCards, SymAtlas and HuGE navigator.11,18–21 In addition, we retrieved data about protein–protein interactions from the UniHI server.22
We assessed the evidence from the current meta-analysis by applying the Venice interim criteria,23 as implemented by Allen and colleagues,24 which are based on 3 main features: amount of evidence, consistency of replication and protection from bias (further details about the numerical analyses supporting these assessments are provided in Appendix 1, available at www.cma.ca/jpn).
Results
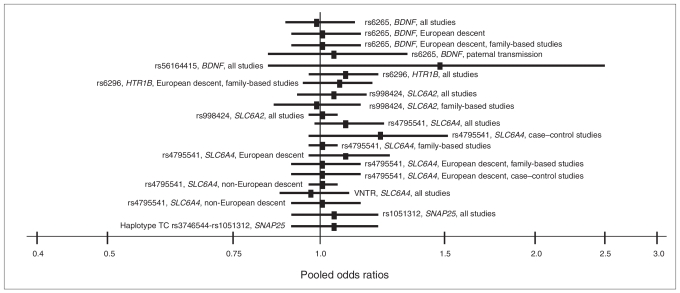
Information about the genetic markers included in this meta-analysis is shown in Table 2. We applied meta-analytical procedures to data from 75 studies that analyzed these 8 common variants in 5 candidate genes involved in neural transmission and plasticity. We included a number of meta-analyses examining 17 studies on rs4795541 (SLC6A4; n = 16), 10 studies on the VNTR in SLC6A4 gene (n = 9), 14 studies on rs6265 (BDNF; n = 12), 3 studies on rs56164415 (BDNF; n = 2), 7 studies on rs3746544 (SNAP25; n = 6), 6 studies on rs1051312 (SNAP25; n = 5), 12 studies on rs6296 (HTR1B; n = 11) and 6 studies for the rs998424 polymorphism (SLC6A2; n = 6). Data about general features of the studies and the respective allele frequencies are provided in Appendix 2, available at www.cma.ca/jpn. Many of the studies used the TDT design, were mainly carried out in populations of European descent and were based on the DSM-IV diagnosis of ADHD. The composition of clinical samples was heterogeneous among studies, mainly in terms of ADHD subtypes and percents (e.g., the proportion of included patients with ADHD combined type varied between 29% and 100%) and types of comorbidities (e.g., the proportion of included patients with oppositional defiant disorder varied between 0% and 58%). Many of the studies reported a positive association in stratified analyses (e.g., in specific ADHD subtypes or parental transmissions) that were not evident in the total sample analyses (Fig. 1). Application of formal meta-analytical procedures using random-effects models showed that a major part of the pooled ORs were not significant for the total samples. In some cases, there was enough data (from 3 or more studies) to conduct a meta-analysis of stratified samples and the results were also mainly negative (Fig. 1). Respective pooled ORs for each association are presented in Appendix 3, available at www.cma.ca/jpn.
Table 2.
General information about candidate polymorphisms for attention-deficit hyperactivity disorder
| Polymorphism | Alias | Gene | Position | Region | Alleles | MAF | Conservation* | Functional† | Coverage, %‡ | Year§ |
|---|---|---|---|---|---|---|---|---|---|---|
| rs6265 | Val66Met | BDNF | chr11:27636492 | Exon | A/G | 0.18 | Yes | Yes | 84.71 | 2002 |
| rs56164415 | C270T | BDNF | chr11:27,678,311 | Intron | C/T | 0.06 | Yes | No | 84.71 | 2001 |
| rs6296 | G861C | HTR1B | chr6:78228979 | Exon | C/G | 0.34 | Yes | No | 36.76 | 1995 |
| rs998424 | MnlI | SLC6A2 | chr16:54289447 | Intron | C/T | 0.33 | No | No | 0.00 | 1996 |
| rs4795541 | HTTLPR | SLC6A4 | chr17:25588443–25588485 | 5′ region | s/l | 0.4 | No | Yes | 0.00 | 1996 |
| HTT VNTR | SLC6A4 | chr17:25,572,552–25,572,650 | Intron | 9/10/12 | 0.44 | No | Yes | 53.23 | 1996 | |
| rs3746544 | MnlI | SNAP25 | chr20:10235084 | 3′UTR | A/C | 0.41 | No | No | 1.12 | 2000 |
| rs1051312 | DdeI | SNAP25 | chr20:10235088 | 3′UTR | C/T | 0.41 | Yes | No | 1.12 | 2000 |
MAF = minor allele frequency.
Genomic position conserved in mouse.
Evidence about allele-specific functional effects.
Fraction of haplotype block variation in the genomic region covered by polymorphism.
Year in which the polymorphism was discovered.
Fig. 1.
Pooled odds ratios for different genotype/phenotype correlations that were nonsignificant in our meta-analysis.
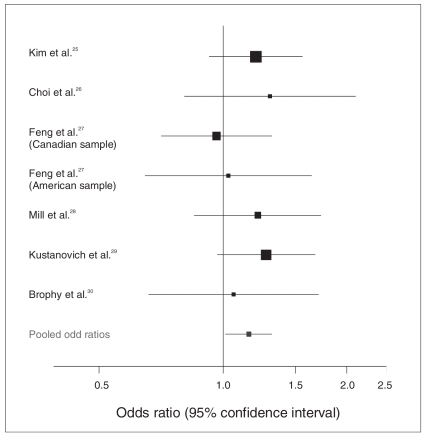
There was a significant pooled OR for the T allele of the rs3746544 SNP in the SNAP25 gene after including all available studies: OR 1.15, 95% confidence interval (CI) 1.01–1.31, p = 0.028 (460 transmissions v. 402 nontransmissions in 6 TDT studies; τ2 ≤ 0, Q statistic p = 0.86, I2 = 0%; Fig. 2).25–30 In addition, tau2 (an estimate of the between-study variance used for the calculation of random-effects models) was equal to or less than 0 for this pooled OR; in these cases, the fixed-effects and random-effects models are mathematically equivalent. In terms of the Venice interim criteria,23 the evidence for this association with a marker in SNAP25 can be defined as weak (AAC grades; Appendix 1).
Fig. 2.
Odds ratios for the A allele of the rs3746544 polymorphism in the SNAP25 gene of individuals with attention-deficit hyperactivity disorder.
We did not find evidence for the paternal overtransmission of the G allele of the rs6296 SNP in the HTR1B gene (OR 1.37, 95% CI 0.99–1.90, p = 0.06, Q statistic p = 0.045, I2 = 62.6%, Appendix 4, available at www.cma.ca/jpn [Fig. S9]), which contrasts with a positive pooled OR that was previously reported for a smaller set of studies.31
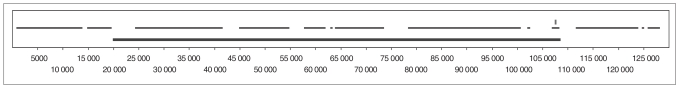
Analysis of HapMap data for these candidate genes showed that several of the analyzed markers cover a minor fraction of the genetic variability in those genomic regions. Data about haplotype block structures and linkage disequilibrium patterns in different populations for the candidate genes are presented in Figure 3 and Appendix 4, available at www.cma.ca/jpn (Fig. S3–S8).
Fig. 3.
Haplotype block structure of the SNAP25 gene region (around 130 kb) in the Caucasian population (Northern European descent, living in Utah) from HapMap release 23a. The solid line indicates the position of the SNAP25 gene, the vertical line shows the position of the 2 studied single nucleotide polymorphisms in the 3′ UTR region and the broken line indicates the location of haplotype blocks.
Discussion
An examination of publication trends in PubMed and HuGE navigator (Appendix 4, Fig. S1) showed that there were 198 publications about 61 candidate genes for ADHD. The first was published in 1995. These publications mainly involved populations of European descent; there is a clear need for the analysis of ADHD candidate genes in other regions, including Asia, Latin America and Africa. In addition to these 198 publications, there were 4 published meta-analyses for 4 of the genes: DAT1, DRD4, DRD5 and COMT2,32–34 (see Appendix 1 for a comparison between genetic association studies of ADHD and schizophrenia).
It has been postulated that a dysfunction of neuroplasticity mechanisms can be involved in the pathophysiology of ADHD and other common neuropsychiatric disorders.6–8 We have applied meta-analytical procedures14 to available data examining 8 common polymorphisms in 5 candidate genes for ADHD. The functional interactions for these genes involved in neural plasticity are shown in Appendix 4, Figure S2 (SNAP25 is part of the SNARE complex and appears as central in terms of interactions with other neural proteins). Several of the papers reported associations only in the stratified analysis and not in the total samples of ADHD, which makes comparisons among the studies difficult. It has been shown that analysis of several subtypes may be an important contributing factor to false-negatives arising from multiple testing.35 We found a significant cumulative association with the T allele of the rs3746544 SNP in the SNAP25 gene (population attributable risk 6%). In terms of the Venice interim criteria, the cumulative evidence for this association can be considered as being weak. This marker is not known to have allele-dependent functional effects; it is possible that it is in linkage disequilibrium with genetic variations of functional relevance (located in protein-coding or regulatory regions).
Faraone and colleagues4 in their general review about ADHD genetics stated that they found significant ORs of 1.19 (1.03–1.38) for rs3746544 (SNAP25) in family-based studies, 1.31 (1.09–1.59) for HTTLPR in case–control studies and 1.44 (1.14–1.83) for rs6296 (HTR1B) in family-based studies; however, there were no data or details about the specific studies included or the meta-analytic procedures that were used.
Phenotypic heterogeneity is an evident issue in the analyzed studies. There were important differences in terms of clinical features such as the relative compositions of included subtypes and comorbidities.2 A major part of the genetic markers analyzed in the present study were identified in the pre-HapMap era (and in some cases before the completion of the Human Genome Project)36 and do not capture the complete variability in those genomic regions; however, some of them are genetic variations with described allele-specific functional effects.2 In terms of implications for ongoing genome-wide association studies for ADHD,9,10 it is important to highlight that some candidate genes such as HTR1B are underrepresented in commonly used genotyping chips (0 and 1 SNPs present in the Illumina 650K and the Affymetrix 6.0 genotyping platforms, respectively, compared with 21 tagging SNPs in that genomic region).15,18
Sample sizes of many of the studies included in our meta-analysis are in the low range compared with genetic studies in other psychiatric diseases (meta-analyses for schizophrenia are based on an average size of more than 3500 participants). This can be explained in part by the use of family-based approaches (e.g., the TDT), which have the inherent disadvantage of the effective samples being substantially smaller than the initial samples.3
To our knowledge, the present study is the largest meta-analysis of ADHD genetics. A recent meta-analysis of 3 dopaminergic genes found a significant association with common polymorphisms in the DRD4 (OR 1.34, 95% CI 1.23–1.45, p < 0.001 for the 7-repeat, 33 studies, population attributable risk 5%) and DRD5 genes (OR 1.34, 95% CI 1.21–1.49, p < 0.001 for the 148 bp allele, 9 studies, population attributable risk 12%).5 Our current results suggest that a common variation in SNAP25, in addition to polymorphisms in the DRD4 and DRD5 genes, may be significantly associated with ADHD (out of 9 genes formally tested in meta-analyses).5,32–34
Limitations
Our study has 3 main limitations, which are also inherent in many meta-analyses14 of association studies in psychiatric genetics. The first was the retrospective nature of our meta-analysis, incorporating data from published studies. The second was the inclusion of study-level data. The third was the study of polymorphisms with limited coverage of the respective candidate genes. The development of prospective meta-analyses will facilitate the control of possible publication biases and the inclusion of individual-level data.14 Ongoing studies using information of genome-wide linkage disequilibrium patterns from HapMap data15 will lead to studies of multiple polymorphisms with a better coverage of the candidate genes.
It is expected that future systematic analysis of animal models of ADHD will identify additional variations in genes involved in synaptic plasticity (synaptogenomics)7,37 as important genetic risk factors for ADHD (see Appendix 1 for an overview of recent results from genome-wide association studies of ADHD related to neural plasticity). In this context, HapMap-based analysis of common variants, including further genome-wide association studies, to complement the exploration of rare variants in different populations around the world will be fundamental.36
Acknowledgements
This work was supported by grants from the Fundación Banco de la Republica and DIB-UNAL. We thank the researchers who answered the requests about allele frequencies not available in the original articles.
Footnotes
Competing interests: None declared.
Contributors: Drs. Forero, Vasquez and H. Arboleda designed the study. Drs. Forero and Vasquez acquired the data, which Drs. Forero, G. Arboleda and H. Arboleda analyzed. Drs. Forero, G. Arboleda and H. Arboleda wrote the article, which Dr. Vasquez reviewed. All authors approved the final version for publication.
References
- 1.Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–8. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 2.Albayrak O, Friedel S, Schimmelmann BG, et al. Genetic aspects in attention-deficit/hyperactivity disorder. J Neural Transm. 2008;115:305–15. doi: 10.1007/s00702-007-0839-9. [DOI] [PubMed] [Google Scholar]
- 3.Cordell HJ, Clayton DG. Genetic association studies. Lancet. 2005;366:1121–31. doi: 10.1016/S0140-6736(05)67424-7. [DOI] [PubMed] [Google Scholar]
- 4.Faraone SV, Perlis RH, Doyle AE, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–23. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Li D, Sham PC, Owen MJ, et al. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD) Hum Mol Genet. 2006;15:2276–84. doi: 10.1093/hmg/ddl152. [DOI] [PubMed] [Google Scholar]
- 6.Jensen V, Rinholm JE, Johansen TJ, et al. N-methyl-d-aspartate receptor subunit dysfunction at hippocampal glutamatergic synapses in an animal model of attention-deficit/hyperactivity disorder. Neuroscience. 2009;158:353–64. doi: 10.1016/j.neuroscience.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Forero DA, Casadesus G, Perry G, et al. Synaptic dysfunction and oxidative stress in Alzheimer’s disease: emerging mechanisms. J Cell Mol Med. 2006;10:796–805. doi: 10.1111/j.1582-4934.2006.tb00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramocki MB, Zoghbi HY. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455:912–8. doi: 10.1038/nature07457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesch KP, Timmesfeld N, Renner TJ, et al. Molecular genetics of adult ADHD: converging evidence from genome-wide association and extended pedigree linkage studies. J Neural Transm. 2008;115:1573–85. doi: 10.1007/s00702-008-0119-3. [DOI] [PubMed] [Google Scholar]
- 10.Neale BM, Lasky-Su J, Anney R, et al. Genome-wide association scan of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1337–44. doi: 10.1002/ajmg.b.30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su AI, Wiltshire T, Batalov S, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101:6062–7. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant SG, Marshall MC, Page KL, et al. Synapse proteomics of multiprotein complexes: en route from genes to nervous system diseases. Hum Mol Genet. 2005;14(Spec No. 2):R225–34. doi: 10.1093/hmg/ddi330. [DOI] [PubMed] [Google Scholar]
- 13.Nicodemus KK. Catmap: case-control and TDT meta-analysis package. BMC Bioinformatics. 2008;9:130. doi: 10.1186/1471-2105-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kavvoura FK, Ioannidis JP. Methods for meta-analysis in genetic association studies: a review of their potential and pitfalls. Hum Genet. 2008;123:1–14. doi: 10.1007/s00439-007-0445-9. [DOI] [PubMed] [Google Scholar]
- 15.International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abecasis GR, Cookson WO. GOLD–graphical overview of linkage disequilibrium. Bioinformatics. 2000;16:182–3. doi: 10.1093/bioinformatics/16.2.182. [DOI] [PubMed] [Google Scholar]
- 17.Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 18.Karolchik D, Kuhn RM, Baertsch R, et al. The UCSC Genome Browser Database: 2008 update. Nucleic Acids Res. 2008;36:D773–9. doi: 10.1093/nar/gkm966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safran M, Chalifa-Caspi V, Shmueli O, et al. Human Gene-Centric Databases at the Weizmann Institute of Science: GeneCards, UDB, CroW 21 and HORDE. Nucleic Acids Res. 2003;31:142–6. doi: 10.1093/nar/gkg050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheeler DL, Barrett T, Benson DA, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2008;36:D13–21. doi: 10.1093/nar/gkm1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu W, Gwinn M, Clyne M, et al. A navigator for human genome epidemiology. Nat Genet. 2008;40:124–5. doi: 10.1038/ng0208-124. [DOI] [PubMed] [Google Scholar]
- 22.Chaurasia G, Iqbal Y, Hanig C, et al. UniHI: an entry gate to the human protein interactome. Nucleic Acids Res. 2007;35:D590–4. doi: 10.1093/nar/gkl817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ioannidis JP, Boffetta P, Little J, et al. Assessment of cumulative evidence on genetic associations: interim guidelines. Int J Epidemiol. 2008;37:120–32. doi: 10.1093/ije/dym159. [DOI] [PubMed] [Google Scholar]
- 24.Allen NC, Bagade S, McQueen MB, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–34. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 25.Kim JW, Biederman J, Arbeitman L, et al. Investigation of variation in SNAP-25 and ADHD and relationship to co-morbid major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:781–90. doi: 10.1002/ajmg.b.30522. [DOI] [PubMed] [Google Scholar]
- 26.Choi TK, Lee HS, Kim JW, et al. Support for the MnlI polymorphism of SNAP25; a Korean ADHD case-control study. Mol Psychiatry. 2007;12:224–6. doi: 10.1038/sj.mp.4001922. [DOI] [PubMed] [Google Scholar]
- 27.Feng Y, Crosbie J, Wigg K, et al. The SNAP25 gene as a susceptibility gene contributing to attention-deficit hyperactivity disorder. Mol Psychiatry. 2005;10:998–1005. doi: 10.1038/sj.mp.4001722. [DOI] [PubMed] [Google Scholar]
- 28.Mill J, Richards S, Knight J, et al. Haplotype analysis of SNAP-25 suggests a role in the aetiology of ADHD. Mol Psychiatry. 2004;9:801–10. doi: 10.1038/sj.mp.4001482. [DOI] [PubMed] [Google Scholar]
- 29.Kustanovich V, Merriman B, McGough J, et al. Biased paternal transmission of SNAP-25 risk alleles in attention-deficit hyperactivity disorder. Mol Psychiatry. 2003;8:309–15. doi: 10.1038/sj.mp.4001247. [DOI] [PubMed] [Google Scholar]
- 30.Brophy K, Hawi Z, Kirley A, et al. Synaptosomal-associated protein 25 (SNAP-25) and attention deficit hyperactivity disorder (ADHD): evidence of linkage and association in the Irish population. Mol Psychiatry. 2002;7:913–7. doi: 10.1038/sj.mp.4001092. [DOI] [PubMed] [Google Scholar]
- 31.Smoller JW, Biederman J, Arbeitman L, et al. Association between the 5HT1B receptor gene (HTR1B) and the inattentive subtype of ADHD. Biol Psychiatry. 2006;59:460–7. doi: 10.1016/j.biopsych.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 32.Cheuk DK, Wong V. Meta-analysis of association between a catechol-O-methyltransferase gene polymorphism and attention deficit hyperactivity disorder. Behav Genet. 2006;36:651–9. doi: 10.1007/s10519-006-9076-5. [DOI] [PubMed] [Google Scholar]
- 33.Yang B, Chan RC, Jing J, et al. A meta-analysis of association studies between the 10-repeat allele of a VNTR polymorphism in the 3′-UTR of dopamine transporter gene and attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:541–50. doi: 10.1002/ajmg.b.30453. [DOI] [PubMed] [Google Scholar]
- 34.Faraone SV, Doyle AE, Mick E, et al. Meta-analysis of the association between the 7-repeat allele of the dopamine D(4) receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1052–7. doi: 10.1176/appi.ajp.158.7.1052. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan PF. Spurious genetic associations. Biol Psychiatry. 2007;61:1121–6. doi: 10.1016/j.biopsych.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Bodmer W, Bonilla C. Common and rare variants in multifactorial susceptibility to common diseases. Nat Genet. 2008;40:695–701. doi: 10.1038/ng.f.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forero DA, Benitez B, Arboleda G, et al. Analysis of functional polymorphisms in three synaptic plasticity-related genes (BDNF, COMT and UCHL1) in Alzheimer’s disease in Colombia. Neurosci Res. 2006;55:334–41. doi: 10.1016/j.neures.2006.04.006. [DOI] [PubMed] [Google Scholar]