Abstract
Background
Late-life depression is associated with decreased brain volumes, particularly in frontal and temporal areas. Evidence suggests that depressive symptoms at a subclinical level are also associated with brain atrophy in these regions, but most of these associations are based on cross-sectional data. Our objective was to investigate both cross-sectional and longitudinal relations between sub-threshold depressive symptoms and brain volumes in older adults and to examine whether these associations are modified by age.
Methods
In total, 110 dementia-free adults from the neuroimaging substudy of the Baltimore Longitudinal Study of Aging aged 56 years and older at baseline participated in this study. Participants received annual evaluations for up to 9 years, during which structural magnetic resonance imaging (MRI) scans were acquired and depressive symptoms were measured using the Center for Epidemiologic Studies Depression Scale.
Results
Mean depressive symptom scores over time were associated with grey matter volume reductions in the left temporal lobe. Depressive symptoms were associated with brain volume reductions with advancing age in the cingulate gyrus and orbitofrontal cortex. Moreover, individuals with higher mean depressive symptom scores showed a faster rate of volume decline in left frontal white matter. Depressive symptoms were not associated with hippocampus volumes.
Limitations
Limitations include the relative homogeneity of our primarily white and highly educated sample, the lack of information about age at onset of depressive symptoms and potential limitations of the automated brain volume registration.
Conclusion
Our results suggest that depressive symptoms, even at a subthreshold level, are associated with volume reductions in specific frontal and temporal brain regions, particularly with advancing age.
Introduction
Symptoms of depression in older adults that fall short of the criteria for major depressive disorder have received increasing attention in recent literature. These subthreshold depressive symptoms are associated with similar cognitive deficits and clinical correlates as major depression.1–5 The limited neuroimaging studies of depressive symptoms provide some evidence that neural correlates of depressive symptoms overlap with clinical depression. Consistent with the hypothesized role of frontolimbic circuits in the etiology of depression, the preponderance of cross-sectional studies of late-life clinical depression have revealed structural and functional changes in the frontal lobes,6–9 including subregions such as the anterior cingulate7,10–12 and orbitofrontal7,13–17 cortices, and in temporal lobe structures such as the hippocampus,13,18–24 although there are some exceptions.25 Pre-frontal atrophy has also been observed in individuals with subthreshold depression.8,9,26,27 In contrast, the lone study that examined subthreshold depression and hippocampus volumes found no evidence of an association between depressive symptoms and volume in that region.27
Longitudinal neuroimaging studies of late-life depression have primarily focused on changes in white and grey matter lesion volume over time and have yielded conflicting results.28,29 To our knowledge, the impact of subclinical depressive symptoms on volumetric decline over time in older adults has not been examined previously. Accordingly, the present study used structural magnetic resonance imaging (MRI) to examine the association between depressive symptoms and volumetric decline over as long as a 9-year interval in a sample of dementia-free older adults. We were also interested in whether the relation between depressive symptoms and changes in brain volume varies as a function of age. Based on previous cross-sectional studies, we predicted that depressive symptoms would be associated with smaller volumes and a faster rate of volumetric decline in frontal and temporal areas, specifically in the cingulate gyrus, orbitofrontal cortex and hippocampus. Moreover, we predicted that the effect of depressive symptoms on frontal and temporal volume reductions would increase with age.
Methods
Participants
Participants were from the neuroimaging substudy of the Baltimore Longitudinal Study of Aging,30 an ongoing longitudinal brain imaging study of older adults initiated in 1994 to identify brain changes that may be predictors of cognitive decline and Alzheimer disease. Participants in our analyses reflect a snapshot of visits for 154 volunteers, aged 56–85 at baseline, who returned for annual evaluation for up to 10 visits over as many as 9 years. Exclusionary criteria at initial evaluation included central nervous system disease, severe cardiovascular disease, severe pulmonary disease and metastatic cancer. We excluded data for participants with dementia or mild cognitive impairment at baseline or any follow-up evaluations, determined based on standard procedures,31 and data for participants with cerebrovascular disease. We included participants with 2 or more MRI and depressive symptoms assessments. The local institutional review boards and the National Institute on Aging Intramural Research Program approved our study, and all participants gave written informed consent at each visit.
Depressive symptomatology
The cognitive testers for the Baltimore Longitudinal Study of Aging administered the Center for Epidemiologic Studies Depression Scale (CES-D)32 at each visit as a measure of depressive symptoms. The CES-D is a widely-used 20-item self-report inventory that assesses the frequency and severity of depressive symptoms experienced in the previous week. In large longitudinal studies such as the Baltimore Longitudinal Study of Aging, the CES-D is useful in providing a measurement of depressive symptomatology that can be easily used to track changes in symptoms over time. Adequate validity of the CES-D in older community-dwelling adults has been demonstrated.33,34 For each participant, we averaged all CES-D scores during the study interval as a measure of chronic or persistent symptoms (average number of CES-D measurements = 6.37, standard deviation [SD] = 2.04). To avoid the inclusion of increases in scores that may be attributed to more transient life events, we excluded outlying scores (i.e., greater than 2 standard deviations above the mean). We used mean scores as continuous predictor variables in statistical analyses. We performed secondary analyses using the average CES-D depressed mood subscale when we observed significant overall CES-D effects. This subscale is 1 of 4 identified by factor analysis32,35,36 and provides a measure of negative mood independent of somatic complaints that are common in older adults. Thus, secondary analyses provided confirmation that findings were associated with depressed mood.
Image acquisition
MRI acquisition procedures for the Baltimore Longitudinal Study of Aging neuroimaging study are detailed elsewhere.30 A technician performed the scans on a General Electric Signa 1.5 T scanner using a high-resolution volumetric spoiled gradient recalled acquisition in a steady state (GRASS) series (axial acquisition, repetition time 35 ms, echo time 5 ms, flip angle 45°, field of view 24, matrix 256 × 256, number of excitations = 1, voxel dimensions of 0.94 × 0.94 × 1.5 mm slice thickness).
Image analysis
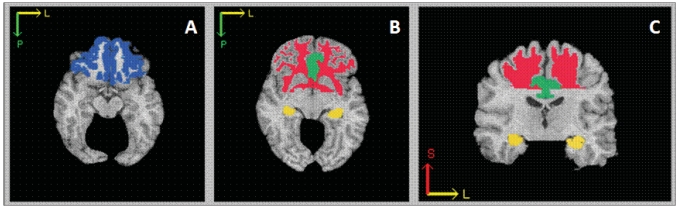
Image processing was based on a semiautomated approach with demonstrated validity and high reliability.37,38 An experienced technician first reformatted the images parallel to the intercommissural plane and then removed extracranial tissue, the cerebellum and brainstem structures inferior to the mammillary bodies using a semiautomated procedure. A computer algorithm segmented images into white matter, grey matter and cerebrospinal fluid using binary labels. We subsequently used an automated computer-based template warping method39 to obtain regional volumetric measurements of specific regions of interest (ROIs). This technique uses a digital atlas labelled for brain lobes and individual structures, including the cingulate gyrus, orbitofrontal cortex and hippocampus (Fig. 1). Atlas definitions are transferred to each MRI scan via an image warping algorithm performing pattern matching of anatomically corresponding brain regions. We obtained the volumes of grey matter, white matter and cerebrospinal fluid of each labelled brain region by summing the number of voxels falling within each region. Labelling of ROIs for the present study was highly stable; test–retest correlations based on year 1 and year 2 scans were all greater than or equal to 0.84.
Fig. 1.
Schematic of frontal and temporal regions of interest: (A) the orbitofrontal cortex is presented in blue, (B, C) white matter is in red, the cingulate gyrus is in green and the hippocampus is in yellow.
Statistical analyses
We used mixed-effects regression analyses (Appendix 1, available at www.cma.ca/jpn), performed using the PROC MIXED procedure in SAS 9.1 software (SAS Institute), to investigate the effects of age and average CES-D scores on longitudinal volume change for individual brain regions, including frontal and temporal ROIs described in detail below. Mixed-effects models account for correlations among repeated measurements on the same participant. Although the number of assessments varied across participants because the current data provide a snapshot of an ongoing longitudinal study, mixed-effects models also have the advantage of being unaffected by unequal numbers of assessments and intervals between visits among individuals, allowing use of all available data for each individual.
We hypothesized that increased depressive symptoms would be associated with smaller regional volumes in frontal and temporal areas of the brain. Thus, our primary analyses focused on grey and white matter volumes in the frontal and temporal lobes (left and right), orbitofrontal cortex volumes (collapsed across left and right) and grey matter volumes in the cingulate gyrus (collapsed across left and right) and in the left and right hippocampus. We collapsed estimates of orbitofrontal cortex and cingulate gyrus volumes across left and right hemispheres to increase the stability of measurement because preliminary examination of test–retest correlations for right and left regions separately indicated reduced stability for these structures. We performed exploratory analyses on total grey matter, total white matter and ventricular volumes, as well as grey and white matter volumes in the parietal and occipital lobes. All analyses included total intracranial volume as a covariate to control for global size differences. Baseline age (at first neuroimaging evaluation) and time interval were additional independent variables. Time interval represents years since baseline testing for each assessment and indexes longitudinal age change, whereas baseline age indexes cross-sectional age differences. We also entered 2-way interactions between CES-D and baseline age, interval and sex into the models. We modelled all independent variables and their interactions as fixed effects, whereas intercept, which represents the baseline value of the dependent variable, and interval were modelled as random effects. We employed a backward elimination procedure in which all lower order terms remained in the model, whereas nonsignificant interaction terms (p > 0.05) were eliminated from the model in stages until a final solution was reached.40 In addition to intracranial volume, we entered sex as a covariate; antidepressant use and the presence of diabetes, hypertension and heart disease were initially entered as covariates but were subsequently removed from the models because they were nonsignificant predictors in all analyses. We treated all variables as continuous variables, with the exception of sex, which we treated as a class variable.
Results are reported without correction for multiple comparisons to avoid type-II errors.41 Rather, effect sizes are presented as a measure of the magnitude of the effects.42 We calculated effect sizes as the regression estimate/standard error.
Results
Of the 154 participants from the neuroimaging substudy of the Baltimore Longitudinal Study of Aging who had annual evaluations for up to 10 visits, we excluded data for 19 participants with diagnoses of dementia or mild cognitive impairment at baseline or any follow-up evaluations. We also excluded data for participants with diagnoses of cerebrovascular disease for all sessions subsequent to diagnosis, which resulted in the complete exclusion of 1 participant. We excluded 12 participants with major depression, as indicated by history or medical record review. None of the remaining participants reported a history of major psychiatric disorder. We included only participants with 2 or more MRI and depressive symptoms assessments in our sample, which resulted in the exclusion of an additional 12 participants. Demographic characteristics for the final sample of 110 participants are presented in Table 1. Frequency data for the number of repeat MRI assessments in the sample are presented in Table 2. Although none of the participants reported current diagnoses of major depression, 12 participants reported taking antidepressant medication. Information regarding antidepressant use is provided in Table 3.
Table 1.
Characteristics of 110 older adults with depressive symptoms
| Group; mean (SD)* |
|||
|---|---|---|---|
| Characteristic | Men | Women | Total |
| No. of participants | 63 | 47 | 110 |
| Baseline age, yr | 69.72 (7.25) | 68.92 (8.33) | 69.38 (7.71) |
| Education, yr | 16.46 (2.94) | 16.21 (2.36) | 16.35 (2.70) |
| Handedness, right/left | 61/2 | 45/2 | 106/4 |
| Race, white/nonwhite | 57/6 | 39/8 | 96/14 |
| Follow-up interval, yr | 6.66 (2.34) | 7.18 (2.27) | 6.88 (2.31) |
| No. of repeat MRI assessments | 6.92 (2.35) | 7.38 (2.10) | 7.11 (2.25) |
| Average CES-D | 4.57 (4.24) | 4.19 (5.11) | 4.44 (4.54) |
| No. of CES-D measurements | 6.36 (2.13) | 6.38 (1.94) | 6.37 (2.04) |
| No. of medical conditions that developed during the study period | |||
| Diabetes | 9 | 0 | 9 |
| Hypertension | 36 | 12 | 48 |
| Heart disease† | 20 | 7 | 27 |
CES-D = Center for Epidemiologic Studies Depression Scale;32 MRI = magnetic resonance imaging; SD = standard deviation.
Unless otherwise indicated.
Heart disease included myocardial infarction, coronary artery disease and congestive heart failure.
Table 2.
Number of participants at each visit who had repeat magnetic resonance imaging assessments*
| Visit no. | Men | Women | Total |
|---|---|---|---|
| 1 | 63 | 47 | 110 |
| 2 | 63 | 47 | 110 |
| 3 | 58 | 43 | 101 |
| 4 | 53 | 43 | 96 |
| 5 | 50 | 41 | 91 |
| 6 | 48 | 41 | 89 |
| 7 | 45 | 37 | 82 |
| 8 | 35 | 31 | 66 |
| 9 | 20 | 14 | 34 |
| 10 | 1 | 3 | 4 |
Data collection is ongoing, thus only a small portion of participants have the last visit. Frequency data are based on the number of visits up to the time that data analysis for this study began.
Table 3.
Antidepressant use among study participants
SSRI = selective serotonin reuptake inhibitor; TCA = tricyclic antidepressant.
One participant changed medications twice; thus, the numbers in the table do not sum to 12.
Bupropion was the only atypical antidepressant used among participants in this study.
The results of mixed-effects regression analyses of frontal and temporal regions, adjusted for intracranial volume and sex, are shown in Table 4. Results of exploratory analyses are presented in the supplementary tables (available in Appendix 2 at www.cma.ca/jpn). Significant cross-sectional and longitudinal results for frontal and temporal ROIs are shown in Figure 2 and Figure 3, respectively. In our models, significant CES-D effects reflect a cross-sectional difference in brain volumes as a function of mean CES-D score. That is, CES-D effects suggest that individuals with more chronic depressive symptoms have either smaller or larger brain volumes than individuals with less severe depressive symptoms. Significant baseline age by CES-D effects indicate that the cross-sectional relation between mean depressive symptoms and brain volumes varies with age of entry into the study. Significant CES-D by interval interactions suggest that changes in brain volumes over time differ as a function of severity of depressive symptoms.
Table 4.
Regression coefficients in the final mixed-effects regression models for CES-D total score*
| Brain region | Baseline age | Interval | CES-D | Baseline age × CES-D | CES-D × interval |
|---|---|---|---|---|---|
| Frontal lobes | |||||
| Left grey matter | −145.2† | −700.5‡ | 69.6 | — | — |
| Right grey matter | −146.0† | −301.9‡ | −23.9 | — | — |
| Left white matter | −366.1‡ | −360.4‡ | 235.5 | — | −32.6† |
| Right white matter | −413.1‡ | −1362.1‡ | 407.5‡ | — | — |
| Temporal lobes | |||||
| Left grey matter | −98.6 | −347.0‡ | 1713.6† | −24.4† | — |
| Right grey matter | −208.1‡ | −73.3 | −62.6 | — | — |
| Left white matter | −264.2‡ | −559.5‡ | 81.6 | — | — |
| Right white matter | −259.1‡ | −1043.9‡ | 204.8† | — | — |
| Cingulate gyrus | −76.4 | −208.2‡ | 846.1 | −12.8§ | — |
| Orbitofrontal cortex | 22.1 | −79.6‡ | 743.9 | −11.3† | — |
| Hippocampus | |||||
| Left | −2.7 | −10.7‡ | 2.8 | — | — |
| Right | −1.8 | −12.9‡ | 4.7 | — | — |
CES-D = Center for Epidemiologic Studies Depression Scale.32
We entered intracranial volume and sex as covariates in all analyses. Intracranial volume was significant in all models (all p < 0.001). Sex effects (smaller volumes in men) were significant for right frontal grey matter (p = 0.032) and cingulate gyrus (p = 0.008) volumes. For left frontal white matter, we added baseline volume (p < 0.001) as a covariate because we found individuals with higher depressive symptom scores to have larger volumes at baseline. Blanks indicate variables that were dropped from the model by backward elimination.
Significance at p < 0.05.
Significance at p < 0.01.
p = 0.054.
Fig. 2.
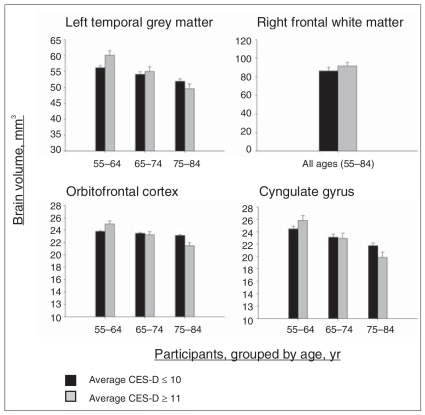
Cross-sectional associations between mean depressive symptoms and brain volumes. These graphs depict brain regions for which statistical analyses revealed significant Center for Epidemiologic Studies Depression Scale (CES-D) and CES-D × baseline age effects, as shown in Table 4. Values reflect estimated scores computed from parameter estimates of the individual mixed-effects regression equations, including all main effects and significant interactions. Although depressive symptoms and age were continuous variables in all analyses, depressive symptoms and age groupings are depicted in the figure for ease of display. To show maximal differences, depressive symptoms groups are based on a cutoff at the 10th percentile of CES-D scores in our sample. Solid bar = average CES-D scores ≤ 10; striped bar = CES-D scores ≥ 11.
Fig. 3.
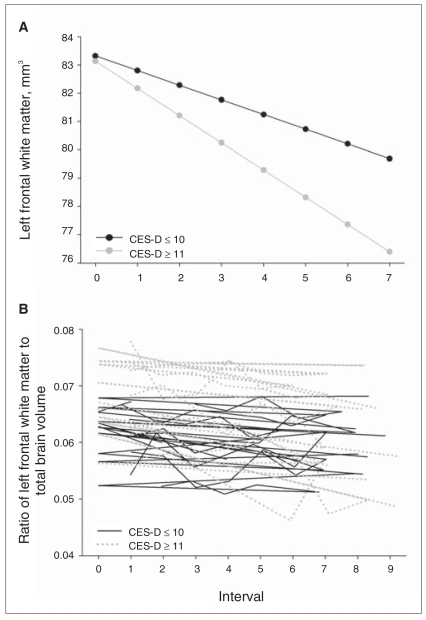
Longitudinal association between mean depressive symptoms and left frontal white matter. In Table 4, this relation is reflected in the significant Center for Epidemiologic Studies Depression Scale (CES-D) by interval effect. (A) Values reflect estimated scores computed from parameter estimates of the individual mixed-effects regression equation, including all main effects and significant interactions. (B) Values represent the ratio of raw left frontal white matter volumes to total intracranial volume for a random selection of 10 participants with lower CES-D means and 10 participants with higher CES-D means. Although depressive symptoms were a continuous variable in all analyses, depressive symptoms groupings are depicted in the figure for ease of display. To show maximal differences, depressive symptoms groups are based on a cutoff at the 10th percentile of CES-D scores in our sample. Solid line = average CES-D scores ≤ 10; dotted line = CES-D scores ≥ 11.
Overall, we observed the expected longitudinal decline in brain volumes across the range of depressive symptom scores, reflected in significant interval effects. We observed baseline age effects, reflecting cross-sectional age differences, primarily for ventricular volume and for frontal, parietal and temporal white matter volumes.
Regional brain volumes
Frontal lobes
A baseline age by CES-D effect for the orbitofrontal cortex revealed that higher average depressive symptoms were significantly associated with smaller volumes in that region at older, but not younger, baseline ages (F1–669 = 3.88, p = 0.049, effect size = −We observed a similar age by depressive symptoms interaction for the cingulate gyrus, although the effect was marginally significant (F1–669 = 3.72, p = 0.05, effect size = −1.93). We observed larger volumes as a function of higher average depressive symptoms, reflected in a significant CES-D effect, in right frontal white matter (F1–671 = 7.02, p = 0.008, effect size = 2.65). A significant interaction between mean CES-D and interval for left frontal white matter (F1–669 = 5.77, p = 0.016, effect size = −2.41) reflected a faster rate of volume decline in individuals with higher average depressive symptoms; however, similar to right frontal white matter, baseline volumes were larger as a function of higher average depressive symptoms. When baseline volume was added to the model as a covariate to account for volume differences at baseline, the longitudinal association between higher mean depressive symptoms and volume decline in left frontal white matter remained (F1–669 = 5.82, p = 0.013, effect size = −2.48).
Analyses revealed a sex effect for right frontal grey matter (F1–106 = 4.75, p = 0.032) and cingulate gyrus (F1–106 = 7.20, p = 0.008) volumes, reflecting smaller ICV-adjusted volumes in men compared with women. However, significant CES-D by sex interactions were not observed.
Temporal lobes
Higher average depressive symptoms were associated with larger grey matter volumes in left temporal regions (F1–669 = 5.38, p = 0.021, effect size = 2.32) and white matter volumes in right temporal regions (F1–671 = 3.87, p = 0.049, effect size = 1.97). However, a CES-D × age interaction for left temporal grey matter revealed that increased depressive symptoms were associated with smaller volumes at older ages, though not at younger ages (F1–669 = 5.32, p = 0.021, effect size = −2.31). Depressive symptoms were not associated with hippocampus volumes, and there were no significant effects of CES-D on rates of longitudinal volume decline in temporal regions. We observed no significant CES-D × sex interactions for temporal regions.
Secondary analyses with the CES-D depressed mood subscale
In general, we confirmed significant effects for lobar volumes when we performed analyses using the CES-D depressed mood subscale rather than the CES-D total score. The age by CES-D effect, which was marginally significant in the total CES-D analysis for the cingulate gyrus, was significant in the depressed mood subscale analysis (F1–103 = 4.97, p = 0.028, effect size = −2.23). For the orbitofrontal cortex, the age × CES-D effect failed to reach significance (p = 0.05) but showed a similar pattern as the CES-D total score. In addition to confirming effects found in the total CES-D analyses, depressed mood subscale analyses revealed depressed mood × sex effects for left frontal white matter (F1–104 = 6.58, p = 0.007, effect size = 2.73) and the cingulate gyrus (F1–103 = 6.15, p = 0.015, effect size = 2.48). We found smaller volumes in women with higher depressed mood subscale scores, whereas we observed larger volumes in men with higher depressed mood subscale scores. Results of these analyses are summarized in Table 5.
Table 5.
Regression coefficients in the final mixed-effects regression models for the depressed mood subscale for selected regions*
| Brain region | Baseline age | Interval | Depressed mood | Baseline age × depressed mood | Depressed mood × interval | Depressed mood × sex |
|---|---|---|---|---|---|---|
| Frontal white matter | ||||||
| Left | −369.2† | −412.4† | 84.6 | — | −103.8† | 2436.1† |
| Right | −400.9† | −1344.3† | 1215.7‡ | — | — | — |
| Left temporal gray matter | −154.8† | −345.5† | 4288.6‡ | −62.9‡ | — | — |
| Right temporal white matter | −252.5† | −1034.8† | 482.1 | — | — | — |
| Cingulate gyrus | −100.9† | −209.9† | 2443.8 | −43.2‡ | — | 737.6‡ |
| Orbitofrontal cortex | −3.1 | −81.3† | 2104.1 | −30.6§ | — | — |
Intracranial volume and sex were entered as covariates in all analyses. Intracranial volume was significant in all models (all p < 0.001). The sex effect (smaller volumes in men) was significant for left frontal white matter (p = 0.004) and the cingulate gyrus (p < 0.001). Blanks indicate variables that were dropped from the model by backward elimination.
Significance at p < 0.05.
Significance at p < 0.01.
p = 0.054.
Discussion
To our knowledge, this study provides the first examination of depressive symptoms and regional brain volumes within the context of longitudinal volume changes in older adults. Consistent with predictions, we observed both cross-sectional and longitudinal associations between depressive symptoms and frontal and temporal brain volumes. Higher average depressive symptoms, which may index more chronic symptoms, showed significant cross-sectional associations with smaller frontal and temporal regional volumes, including left temporal grey matter, the cingulate gyrus and orbitofrontal cortex, though effects were only observed at older ages. Furthermore, we observed longitudinal associations between depressive sypmtoms and left frontal white matter across the range of ages in our sample, with a faster rate of volume decline associated with higher depressive symptoms. In contrast to these findings, depressive symptoms were not associated with smaller hippocampus volumes or longitudinal temporal lobe volume decline.
These results are consistent with frontolimbic theories of depression43–45 and with previous investigations demonstrating associations between subthreshold depressive symptoms and smaller prefrontal volumes,8,9,27 but not hippocampus volumes.27 Our findings also extend previous results by documenting the impact of age on the association between depressive symptoms and brain volumes, and by demonstrating an association between depressive symptoms and longitudinal volume decline in left frontal white matter. The observation that age modifies the association between depressive symptoms and brain volumes is consistent with previous neuro-imaging studies that have found interactive effects of age and depression on cerebral blood flow and metabolism.46,47 These results also parallel the finding that the adverse impact of depression on some cognitive functions increases with advancing age.48,49 Perhaps older adults, who are already vulnerable to neural and cognitive changes as a function of advancing age, are particularly susceptible to the effects of depressive symptoms.
Somewhat surprisingly, the predicted acceleration of longitudinal volume decline in individuals with higher depressive symptoms was only observed for left frontal white matter. Longitudinal stability of hippocampus50 and orbitofrontal cortex51 volumes have been demonstrated in young adults with clinical depression over 1-year and 3-month follow-up, respectively. Nonetheless, we expected to find a faster rate of longitudinal decline in association with depressive symptoms owing to the older age of our participants and the longer follow-up interval. Additional longitudinal studies are needed to test the possibility that clinical depression is associated with volume decline that is greater in magnitude and occurs over a shorter time interval compared with subthreshold depressive symptoms. Our finding of longitudinal effects for left frontal white matter in the absence of significant effects for other brain regions suggests that frontal regions are particularly susceptible to subthreshold depressive symptoms. Furthermore, our finding of cross-sectional associations between sub-threshold depressive symptoms and frontal lobe structures, but not the hippocampus, suggests that frontal regions may be more vulnerable to subthreshold depressive symptoms than the hippocampus and possibly other temporal structures.
Higher depressive symptoms were associated with enlarged volumes in frontal white matter, right temporal white matter and left temporal grey matter. Although previous studies have shown an association between depressive symptoms and increased white matter lesions,29,52,53 our finding of enlarged white matter volumes is not attributable to the presence of white matter lesions in individuals with higher average depressive symptoms because white matter hyperintensity ratings available for this sample54 did not predict either depressive symptoms or regional brain volumes in supplemental analyses in a subset of the sample. Although volume enlargement has not been previously reported in subthreshold depression, previous studies have documented increased regional volumes in patients with major depression. For example, compared with controls, patients with major depression were found to have enlarged amygdala55,56 and corpus callosum57 volumes. Moreover, the number of prior episodes of depression has been associated with increased volume of the globus pallidus.58 Additional research is needed to elucidate the clinical significance of regional volume enlargement in threshold and subthreshold depressive disorders.
Given the extensive literature linking late-life depression to dementia59 and implicating hippocampal atrophy in Alzheimer disease,60 the finding of hippocampus volume reduction in some studies of late-life depression may be attributed to incipient dementia. We excluded individuals in whom dementia or mild cognitive impairment developed at any point during a 9-year follow-up period, which allowed us to more confidently attribute the observed effects to depressive symptoms rather than the prodromal phase of dementia. Furthermore, the lack of association between depressive symptoms and hippocampus volumes in our sample suggests that observed associations for other brain regions are more likely due to depressive symptoms than incipient dementia.
Associations between depressive symptoms and brain volumes in geriatric samples may also be confounded by physical symptoms and comorbid medical conditions such as cerebrovascular disease in older adults. We not only excluded individuals with cerebrovascular disease at baseline, but the longitudinal nature of the study also allowed us to exclude data for participants in whom cerebrovascular disease was diagnosed during the study interval. Our inclusion of data for such individuals before the diagnosis does not appear to have significantly impacted our results because exploratory analyses in which we eliminated prediagnosis data from the sample yielded results that did not substantially differ from the reported results. Moreover, secondary analyses with the depressed mood subscale of the CES-D yielded similar results to the CES-D total symptom score, providing confidence that our findings reflect associations with negative affect rather than physical symptoms. Sex differences in the association of depressed mood subscales scores with left frontal white matter and cingulate gyrus volumes are consistent with previous research documenting sex differences in the relations between depressive symptoms, brain volumes and cognitive impairment in the elderly.27,61,62
Limitations
The measurement of brain volumes in our study was based on a semiautomated approach. Given the number of scans in this longitudinal study (783 scans in the present sample), this approach renders MRI data analysis more feasible than manual tracings. This approach is well validated37,38 and has been shown to accurately segment grey and white matter and cerebrospinal fluid compartments with error rates of less than 2%–3% when tested against our realistic digital brain phantom.37 Moreover, reliability of labelling for the ROIs in the present study exceeds 0.84. Nonetheless, the semiautomated approach is not without limitation. Labelling is limited by registration accuracy, as well as by changes in grey matter–white matter contrast with age. These limitations may result in increased noise. Our finding of significant associations of brain volumes with depressive symptoms despite this increased noise attests to the strength of our results. An additional limitation of this method is that white matter signal hypointensities on T1-weighted MRI scans are segmented as grey matter, leading to a small overestimation of grey and underestimation of white matter. However, based on manual tracing, we have found that these signal abnormalities account for less than 1% of brain volume even in individuals with extensive white matter findings. These limitations do not outweigh the aforementioned strengths of the automated approach.
Early- and late-onset depression may have different etiological contributors and associations with brain pathology. However, information regarding the age at onset of depressive symptoms was not available for the current study and is a potential limitation. The restricted age range of our sample (i.e., age 56–85 at baseline) is an additional limitation given our interest in examining whether age modified the effects of depressive symptoms on brain volumes. Nonetheless, we found that older age was associated with an adverse impact of depressive symptoms on brain volumes, even within our limited age range. The potential for inflated type-I error is a further limitation and suggests that statistically less pronounced differences should be interpreted with caution. However, since to our knowledge this is the first study that explores the longitudinal association between depressive symptoms and volume decline in older adults, we tolerated greater type-I error rather than miss a true positive finding.41 Moreover, the large effect sizes observed for the effects would suggest that the differences are indeed meaningful rather than spurious findings.42
Our sample was not population-based, and most participants were highly educated and white, limiting the generalizability of our findings. However, the relative homogeneity of the sample may also have been a strength of this study because it diminished possible effects of demographic variables as confounds. These limitations should be viewed in the context of the strengths of the study, such as the large sample size, relatively long follow-up period with prospective assessments and well characterized nature of the sample.
Our results provide evidence that depressive symptoms, even at subthreshold levels, are associated with volume reduction in frontal and temporal brain regions, particularly with advancing age. Future longitudinal studies investigating the temporal relation between brain pathology and depressive symptoms over longer follow-up intervals and a wider age range are critical to elucidate whether structural and functional brain changes are the etiology or a consequence of depression.
Acknowledgements
The National Institute on Aging Intramural Research Program of the National Institutes of Health supported this research. The authors wish to thank Yang An, MS, for assistance with statistical analyses.
Footnotes
Competing interests: None declared.
Contributors: Drs. Dotson and Resnick designed the study. Dr. Dotson wrote the article, which Drs. Davatzikos, Kraut and Resnick reviewed. All authors analyzed data and provided final approval for publication.
The National Institute on Aging Intramural Research Program of the National Institutes of Health supported this research.
References
- 1.Beck DA, Koenig HG. Minor depression: a review of the literature. Int J Psychiatry Med. 1996;26:177–209. doi: 10.2190/AC30-P715-Y4TD-J7D2. [DOI] [PubMed] [Google Scholar]
- 2.Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58:249–65. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- 3.Girling DM, Huppert FA, Brayne C, et al. Depressive symptoms in the very elderly–their prevalence and significance. Int J Geriatr Psychiatry. 1995;10:497–504. [Google Scholar]
- 4.Lavretsky H, Kumar A. Clinically significant non-major depression: old concepts, new insights. Am J Geriatr Psychiatry. 2002;10:239–55. [PubMed] [Google Scholar]
- 5.Lyness JM, King DA, Cox C, et al. The importance of subsyndromal depression in older primary care patients: prevalence and associated functional disability. J Am Geriatr Soc. 1999;47:647–52. doi: 10.1111/j.1532-5415.1999.tb01584.x. [DOI] [PubMed] [Google Scholar]
- 6.Almeida OP, Burton EJ, Ferrier N, et al. Depression with late onset is associated with right frontal lobe atrophy. Psychol Med. 2003;33:675–81. doi: 10.1017/s003329170300758x. [DOI] [PubMed] [Google Scholar]
- 7.Ballmaier M, Toga AW, Blanton RE, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161:99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Jin Z, Bilker W, et al. Late-onset minor and major depression: early evidence for common neuroanatomical substrates detected by using MRI. Proc Natl Acad Sci U S A. 1998;95:7654–8. doi: 10.1073/pnas.95.13.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar A, Schweizer E, Jin Z, et al. Neuroanatomical substrates of late-life minor depression. A quantitative magnetic resonance imaging study. Arch Neurol. 1997;54:613–7. doi: 10.1001/archneur.1997.00550170085018. [DOI] [PubMed] [Google Scholar]
- 10.Awata S, Ito H, Konno M, et al. Regional cerebral blood flow abnormalities in late-life depression: relation to refractoriness and chronification. Psychiatry Clin Neurosci. 1998;52:97–105. doi: 10.1111/j.1440-1819.1998.tb00980.x. [DOI] [PubMed] [Google Scholar]
- 11.de Asis JM, Stern E, Alexopoulos GS, et al. Hippocampal and anterior cingulate activation deficits in patients with geriatric depression. Am J Psychiatry. 2001;158:1321–3. doi: 10.1176/appi.ajp.158.8.1321. [DOI] [PubMed] [Google Scholar]
- 12.Nobler MS, Roose SP, Prohovnik I, et al. Regional cerebral blood flow in mood disorders, V.: effects of antidepressant medication in late-life depression. Am J Geriatr Psychiatry. 2000;8:289–96. [PubMed] [Google Scholar]
- 13.Andreescu C, Butters MA, Begley A, et al. Gray matter changes in late life depression–a structural MRI analysis. Neuropsychopharmacology. 2008;33:2566–72. doi: 10.1038/sj.npp.1301655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballmaier M, Sowell ER, Thompson PM, et al. Mapping brain size and cortical gray matter changes in elderly depression. Biol Psychiatry. 2004;55:382–9. doi: 10.1016/j.biopsych.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Lai T, Payne ME, Byrum CE, et al. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48:971–5. doi: 10.1016/s0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Payne ME, Steffens DC, et al. Subcortical lesion severity and orbitofrontal cortex volume in geriatric depression. Biol Psychiatry. 2003;54:529–33. doi: 10.1016/s0006-3223(03)00063-5. [DOI] [PubMed] [Google Scholar]
- 17.Taylor WD, Macfall JR, Payne ME, et al. Orbitofrontal cortex volume in late life depression: influence of hyperintense lesions and genetic polymorphisms. Psychol Med. 2007;37:1763–73. doi: 10.1017/S0033291707000128. [DOI] [PubMed] [Google Scholar]
- 18.Bell-McGinty S, Butters MA, Meltzer CC, et al. Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am J Psychiatry. 2002;159:1424–7. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- 19.Caetano SC, Hatch JP, Brambilla P, et al. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res. 2004;132:141–7. doi: 10.1016/j.pscychresns.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Hickie I, Naismith S, Ward PB, et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd AJ, Ferrier IN, Barber R, et al. Hippocampal volume change in depression: late- and early-onset illness compared. Br J Psychiatry. 2004;184:488–95. doi: 10.1192/bjp.184.6.488. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien JT, Lloyd A, McKeith I, et al. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161:2081–90. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 23.Sheline YI, Wang PW, Gado MH, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–13. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steffens DC, Byrum CE, McQuoid DR, et al. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–9. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg DL, Payne ME, MacFall JR, et al. Hippocampal volumes and depression subtypes. Psychiatry Res. 2008;163:126–32. doi: 10.1016/j.pscychresns.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Bilker W, Lavretsky H, et al. Volumetric asymmetries in late-onset mood disorders: an attenuation of frontal asymmetry with depression severity. Psychiatry Res. 2000;100:41–7. doi: 10.1016/s0925-4927(00)00067-6. [DOI] [PubMed] [Google Scholar]
- 27.Taki Y, Kinomura S, Awata S, et al. Male elderly subthreshold depression patients have smaller volume of medial part of prefrontal cortex and precentral gyrus compared with age-matched normal subjects: a voxel-based morphometry. J Affect Disord. 2005;88:313–20. doi: 10.1016/j.jad.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Chen PS, McQuoid DR, Payne ME, et al. White matter and subcortical gray matter lesion volume changes and late-life depression outcome: a 4-year magnetic resonance imaging study. Int Psychogeriatr. 2006;18:445–56. doi: 10.1017/S1041610205002796. [DOI] [PubMed] [Google Scholar]
- 29.Nebes RD, Reynolds CF, III, Boada F, et al. Longitudinal increase in the volume of white matter hyperintensities in late-onset depression. Int J Geriatr Psychiatry. 2002;17:526–30. doi: 10.1002/gps.635. [DOI] [PubMed] [Google Scholar]
- 30.Resnick SM, Goldszal AF, Davatzikos C, et al. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10:464–72. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- 31.Kawas C, Gray S, Brookmeyer R, et al. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–7. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 32.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 33.Beekman AT, Deeg DJ, Van Limbeek J, et al. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in the Netherlands. Psychol Med. 1997;27:231–5. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- 34.Haringsma R, Engels GI, Beekman AT, et al. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004;19:558–63. doi: 10.1002/gps.1130. [DOI] [PubMed] [Google Scholar]
- 35.Gatz M, Hurwicz ML. Are old people more depressed? Cross-sectional data on Center for Epidemiological Studies Depression Scale factors. Psychol Aging. 1990;5:284–90. doi: 10.1037//0882-7974.5.2.284. [DOI] [PubMed] [Google Scholar]
- 36.Hertzog C, van Alstine J, Usala PD, et al. Measurement properties of the Center for Epidemiological Studies Depression Scale (CES-D) in older populations. Psychol Assess. 1990;2:64–72. [Google Scholar]
- 37.Goldszal AF, Davatzikos C, Pham DL, et al. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998;22:827–37. doi: 10.1097/00004728-199809000-00030. [DOI] [PubMed] [Google Scholar]
- 38.Resnick SM, Pham DL, Kraut MA, et al. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging. 2002;21:1421–39. doi: 10.1109/TMI.2002.803111. [DOI] [PubMed] [Google Scholar]
- 40.Morrell CH, Pearson JD, Brant LJ. Linear transformations of linear mixed-effects models. Am Stat. 1997;51:338–43. [Google Scholar]
- 41.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–8. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garamszegi LZ. Comparing effect sizes across variables: generalization without the need for Bonferroni correction. Behav Ecol. 2006;17:682–7. [Google Scholar]
- 43.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 44.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 45.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–54. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 46.Konarski JZ, Kennedy SH, McIntyre RS, et al. Relationship between regional brain metabolism, illness severity and age in depressed subjects. Psychiatry Res. 2007;155:203–10. doi: 10.1016/j.pscychresns.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Sackeim HA, Prohovnik I, Moeller JR, et al. Regional cerebral blood flow in mood disorders. I. Comparison of major depressives and normal controls at rest. Arch Gen Psychiatry. 1990;47:60–70. doi: 10.1001/archpsyc.1990.01810130062009. [DOI] [PubMed] [Google Scholar]
- 48.Dotson VM, Resnick SM, Zonderman AB. Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. Am J Geriatr Psychiatry. 2008;16:318–30. doi: 10.1097/JGP.0b013e3181662a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119–26. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- 50.Frodl T, Meisenzahl EM, Zetzsche T, et al. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. J Clin Psychiatry. 2004;65:492–9. doi: 10.4088/jcp.v65n0407. [DOI] [PubMed] [Google Scholar]
- 51.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 52.Krishnan MS, O’Brien JT, Firbank MJ, et al. Relationship between periventricular and deep white matter lesions and depressive symptoms in older people. The LADrosoph Inf Serv Study. Int J Geriatr Psychiatry. 2006;21:983–9. doi: 10.1002/gps.1596. [DOI] [PubMed] [Google Scholar]
- 53.Teodorczuk A, O’Brien JT, Firbank MJ, et al. White matter changes and late-life depressive symptoms: longitudinal study. Br J Psychiatry. 2007;191:212–7. doi: 10.1192/bjp.bp.107.036756. [DOI] [PubMed] [Google Scholar]
- 54.Kraut MA, Beason-Held LL, Elkins WD, et al. The impact of magnetic resonance imaging-detected white matter hyperintensities on longitudinal changes in regional cerebral blood flow. J Cereb Blood Flow Metab. 2008;28:190–7. doi: 10.1038/sj.jcbfm.9600512. [DOI] [PubMed] [Google Scholar]
- 55.Frodl T, Meisenzahl E, Zetzsche T, et al. Enlargement of the amyg-dala in patients with a first episode of major depression. Biol Psychiatry. 2002;51:708–14. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- 56.Frodl T, Meisenzahl EM, Zetzsche T, et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. 2003;53:338–44. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- 57.Lacerda AL, Brambilla P, Sassi RB, et al. Anatomical MRI study of corpus callosum in unipolar depression. J Psychiatr Res. 2005;39:347–54. doi: 10.1016/j.jpsychires.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Lacerda AL, Nicoletti MA, Brambilla P, et al. Anatomical MRI study of basal ganglia in major depressive disorder. Psychiatry Res. 2003;124:129–40. doi: 10.1016/s0925-4927(03)00123-9. [DOI] [PubMed] [Google Scholar]
- 59.Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and meta-regression analysis. Arch Gen Psychiatry. 2006;63:530–8. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kantarci K, Jack CR., Jr Neuroimaging in Alzheimer disease: an evidence-based review. Neuroimaging Clin N Am. 2003;13:197–209. doi: 10.1016/s1052-5149(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 61.Dal Forno G, Palermo MT, Donohue JE, et al. Depressive symptoms, sex, and risk for Alzheimer’s disease. Ann Neurol. 2005;57:381–7. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- 62.Hastings RS, Parsey RV, Oquendo MA, et al. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–9. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]