Abstract
To improve bone regeneration around orthopedic biomaterials, researchers have attempted to combine growth factors on and in implants. Equally as exciting, greater bone growth has been demonstrated around nano-scaled materials (like helical rosette nanotubes or nanocrystalline hydroxyapatite) which mimic the geometry of the natural components of bone. To combine these two approaches, in this in vitro study, the ability of three short peptides (labeled for convenience: a or SNVILKKYRN, b or KPSSAPTQLN, and c or KAISVLYFDDS chosen from the larger bone morphogenetic protein-7 (BMP-7)) to promote osteoblast (bone-forming cells) functions were determined. Shorter peptides of BMP-7 are required for growth factor incorporation into nano-scale biomaterials due to their nanometer size. Results showed that of all the peptides, peptide b and the peptide combination a,b enhanced osteoblast density the most after five days compared to the controls (no growth factors). Furthermore, osteoblasts cultured with peptide b had a larger and more spread morphology than controls. In addition, peptide c and its combinations (a, c; b, c; and a, b, c) increased osteoblast calcium deposition after 14 and 21 days compared to the controls. Since, these peptides are much smaller than BMP-7, the results of this study provided peptides that can be easily chemically functionalized onto nano-scaled biomaterials to improve bone growth. Thus, the present study elucidated shorter peptides in BMP-7 more appropriate for inclusion in and on nano-materials to promote osteoblast proliferation (peptide b and the peptide combination a,b) and osteoblast deposition of calcium containing mineral (peptide c and the peptide combinations a,c; b,c; and a, b, c).
Keywords: bone morphogenetic protein-7, peptides, osteoblasts, orthopedics, nanotechnology
Introduction
Bone fracture is one of the most common bone complications. For adults, it may take 6 weeks to achieve a sufficiently mineralized bone fracture healing callus and 3 months to achieve 80% of the normal strength of healthy bone [1]. The long recovery time of bone fractures clearly bring significant pain and inconvenience to patients. It is continually hoped that orthopedic implant materials (or fixation devices) can provide solutions to improve the treatment of bone fractures [2]. Today, one of the most widely used implant materials is titanium. However, the use of conventional titanium (that is, micron-rough and nano-smooth) to provide quick bone regeneration as well as sustained, prolonged bone growth are lagging in satisfaction. As one of many examples, 5% to 10% of all hip implants composed of conventionally structured titanium show peri-implant bone loss, and eventually, result in failure over a 10-year period [3]. One reason that today’s implantation materials fail is due to a lack of promoting osteoblast (bone-forming cells) functions to form bone quickly juxtaposed to the implant [3].
Because nanophase materials (or materials with one dimension less than 100nm) are able to mimic the dimensions of constituent components of natural bone (for example, collagen and hydroxyapatite), they have great potential to be successful alternative implant materials to what is implanted today [4]. Moreover, to improve osteoblast adhesion, proliferation and long-term differentiation functions at orthopedic implantation sites, researchers have been immobilizing recombinant proteins or peptides onto nano-structured implants, such as nano-particulate metals or ceramics (i.e., surface-modified titanium [5] and hydroxyapatite [6]), and biologically-inspired naturally-derived nano-structured materials (i.e., collagen [7] and helical rosette nanotubes [8][9]).
In this manner, several bone-related growth factors (which are produced by bone cells and stored in the bone matrix) have been used to induce bone formation either through injection or direct implant surface modification. These include fibroblast growth factors, insulin-like growth factors I and II, platelet-derived growth factors, transforming growth factors-β and bone morphogenetic proteins [10~12]. However, the most potent inducers of bone formation utilized in and on today’s orthopedic implants are members of the bone morphogenetic protein (BMP) family [13]. For example, BMP-7 (or osteogenic protein-1) promotes the formation and regeneration of bone and cartilage [14~16]. However, BMPs have several hundred amino acids making them too large and complex to chemically functionalize onto nano-structured materials (such as particles and fibers). For example, helical rosette nanotubes (which are biomimetic, self-assembled functionalized DNA base-pairs) have a 1.1 nm-diameter inner channel, while BMP-7 has a diameter of approximately 2~3 nm [17]. This limitation has inhibited the use of BMPs in conjunction with the advantages of nano-materials to promote bone growth.
To use these growth factors in and on nano-materials, it is clearly important to identify smaller bioactive regions of BMPs. Some researchers have found that short peptides in BMPs also improve osteoblast growth. For example, a synthetic peptide (KIPKASSVPTELSAISTLYL) derived from the “knuckle epitope” in BMP-2 has increased alkaline phosphatase activity in osteoblasts [18]. In addition, Kirkwood et al. also found that some peptides (for example, AISVLYFDDSSNVILKKYRN) in BMP-7 encourage the mineralization process in osteoblasts [19]. By comparing BMP-2 and BMP-7 structures [17], one can ascertain that certain bioactive areas are similar in function (such as the BMP type II receptor) and amino acid sequence (Figure 1).
Figure 1.
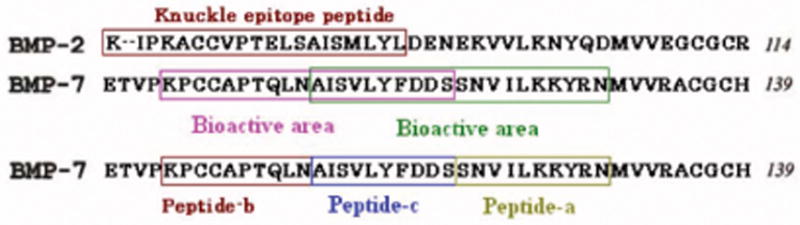
The comparison of the bioactive areas in BMP-2 and BMP-7.
Thus, in order to utilize the advantageous properties of BMPs in conjunction with nano-materials, the present study investigated the ability of three short peptides of BMP-7 (a or SNVILKKYRN, b or KPSSAPTQLN, and c or KAISVLYFDDS) to promote osteoblast functions in order to determine the best short peptides of BMP-7 that could eventually be functionalized onto nano-structured implant materials. Importantly, these shorter peptides have terminal K or S amino acids, making them easier to eventually functionalize on a particular nanomaterial of interest (helical rosette nanotubes [8, 9]). Results showed for the first time that some of these shorter BMP-7 peptides enhanced the proliferation (specifically, peptide b or KPSSAPTQLN and peptide combination a,b or SNVILKKYRN, KPSSAPTQLN) and calcium deposition by osteoblasts (specifically, peptide c or KAISVLYFDDS and its combination with peptide a or b mentioned above) compared to no peptide additive at all.
Materials and Methods
Peptide solution preparation
Synthetic peptide-a (SNVILKKYRN, 121~130 amino acid residue sequence of BMP-7), peptide-b (KPSSAPTQLN, 101~110 amino acid residue sequence of BMP-7) and peptide-c (KAISVLYFDDS, 110~120 amino acid residue sequence of BMP-7) were purchased from GenScript Corporation (NJ, USA), and had a purity of 97% (amino acid residues different from the sequence of BMP-7 are shown in bold). Peptide-a, b, c and their various combinations were dissolved separately in cell culture medium to a final concentration of 100μg/mL for most of the experiments (with the exception of elucidating a dose response in osteoblast alkaline phosphatase activity when concentrations ranging from 1 to 100μg/mL were created); when peptide combinations were used, equal amounts of the peptides were mixed. Human bone morphogenetic protein-7 was purchased from GenScript Corporation (NJ, USA). BMP-7 was dissolved to a final concentration of 200ng/mL (with the exception of elucidating a dose response in osteoblast alkaline phosphatase activity when concentrations ranging from 200ng/ml to 1 μg/mL were created).
Osteoblast culture
Human fetal osteoblasts (bone-forming cells; CRL-11372 American Type Culture Collection) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone)/F-12 Ham media supplemented with 10% fetal bovine serum (FBS; Hyclone) and 1% penicillin/streptomycin (P/S; Hyclone) under standard cell culture conditions (that is, a sterile, 37°C, humidified, 5% CO2/95% air environment). Cells at population numbers 6–10 were used in the experiments without further characterization.
Osteoblast adhesion
Osteoblasts were seeded at a concentration of 2500 cells/cm2 into wells of polystyrene 24-well plates in DMEM (with or without the peptides of interest to this study) supplemented with 10% FBS and 1% P/S and were incubated under standard cell culture conditions for 4 h. At that time, non-adherent cells were removed by rinsing with phosphate buffered saline and adherent cells were then fixed with formaldehyde (Fisher Scientific, Pittsburgh, PA) and stained with Hoechst 33258 dye (Sigma); the cell nuclei were, thus, visualized and counted under a fluorescence microscope (Axiovert 200M, Zeiss). Cell counts were expressed as the average number of cells in at least five random fields per square centimeter.
Osteoblast proliferation and morphologies
For proliferation studies, osteoblasts were seeded at 1000 cells/cm2 into wells of polystyrene 24-well plates in DMEM (with or without the peptides of interest to this study) under standard cell culture conditions (37°C, 5% CO2, 95% air humidification) for 1, 3 and 5 days. Medium (with and without the peptides of interest to this study) was changed every two days. At the end of each time period, cells were fixed with formaldehyde (Fisher Scientific, Pittsburgh, PA) and stained with 4′, 6- Diamidino-2- phenylindole (DAPI, Sigma); the cell nuclei were, thus, visualized and counted under a fluorescence microscope (Axiovert 200M, Zeiss). Cell counts were expressed as the average number of cells in at least five random fields per well. For the cells cultured for 1 and 3 days, they were also stained with rhodamine phalloidin (Invitrogen); the cell was, thus, visualized under a fluorescence microscope (Axiovert 200M, Zeiss).
Osteoblast long-term functions
For differentiation studies, osteoblasts were seeded at a density of 50,000 cells/cm2 into wells of polystyrene 24-well plates. Osteoblasts were cultured in DMEM supplemented with 10% FBS, 1% P/S, 50 μg/mL l-ascorbic acid (Sigma), and 10 mM β-glycerophosphate (Sigma) in the presence or absence of the peptides of interest to this study under standard cell culture conditions for 7, 14, and 21 days. Medium (with and without the peptides of interest to this study) was changed every two days. At the end of each time period, cells were lysed to determine total protein content, total collagen content and alkaline phosphatase activity via well-established commercially available kits (as described below). Calcium deposited by osteoblasts were dissolved in 0.6mol/L hydrochloric acid and tested for calcium concentration using a commercially available kit (as described below).
Total protein content
Total protein content in the cell lysates was determined using a commercial Better Bradford Assay Kit (Pierce Biotechnology), following manufacturer’s instructions. For this purpose, aliquots of each protein-containing, distilled water supernatant of cell lysates were mixed with the assay reagent and incubated for 10 min at room temperature. Light absorbance of these samples was measured at 595 nm on a Spectra MAX 190 spectrophotometer (Molecular Devices). Total protein synthesized by osteoblasts cultured with BMP-7 short peptides of interest to the present study was determined from a standard curve of absorbance versus known concentrations of albumin run in parallel with experimental samples.
Total collagen content
Cell lysates were prepared as described earlier. Aliquots of the distilled water supernatant were dried onto a microplate, mixed with 0.1% Sirius Red stain (Sigma), and incubated for 1 h at room temperature. The microplate was washed five times with 0.01M HCl (Mallinckrodt Technical) for 10 s per wash to remove the unbound stain. The collagen bound stain was then desorbed by washing with 0.1M NaOH for 5 min. The eluted stain was then mixed several times into a multichannel pipette and was placed into a second microplate. Absorbance was then read at 540 nm in a spectrophotometer (Spectra MAX 190, Molecular Devices). A standard curve was plotted as mg of collagen versus absorbance at 540 nm and collagen content was calculated from this curve.
Alkaline phosphatase activity
Alkaline phosphatase is an enzyme whose production signifies increased osteoblast differentiation [15]. An Alkaline Phosphatase Assay Kit (Upstate) was used to assay alkaline phosphatase activity in the cell lysates prepared as described earlier. For this purpose, aliquots of the distilled water supernatants of cell lysates were mixed with 5 μL NiCl2, 5 μL BSA and 5 μL phosphopeptide solution in the wells of a microplate. Then, the reaction was incubated for 15 min at 37°C. Alkaline phosphatase activity was detected by the addition of 100 μL Malachite Green solution. The assay was read with blank and standards at 650 nm using a spectrophotometer (Spectra MAX 190, Molecular Devices). Alkaline phosphatase activity was calculated by comparing absorbance values to a standard curve. Alkaline phosphatase activity values were normalized by total protein content and expressed as nmol alkaline phosphatase/μg protein.
Cellular calcium deposition
Most importantly, calcium deposition (an indicator of osteoblast differentiation) was also determined in this study. For this purpose, after the cells were lysed and removed, the substrates (Petri-dish) were treated with 0.6 mol/mL HCl at 37°C overnight. After the prescribed time period, the amount of calcium present in the acidic supernatant was quantified using a commercially available kit (Sigma) and following the manufacturer’s instructions. Light absorbance of the samples was measured at 575 nm using a spectrophotometer (Spectra MAX 190, Molecular Devices). Total calcium was calculated from standard curves of absorbance versus known concentrations of calcium standards (Sigma) run in parallel with the experimental samples. Calcium concentration values were normalized by total protein content and expressed as μg calcium/μg protein.
Statistical analysis
Statistics were performed using a one-tail T-Test for n=9, N=3 (triplicate experiments and repeated three separate times) for adhesion, proliferation, and long-term differentiation studies where p<0.10 was considered statistically significant.
Results
Osteoblast adhesion
Results showed that osteoblast adhesion cultured in the presence of the different peptides of interest to this study was not significantly different compared with controls (no additives) and each other (Figure 2).
Figure 2.
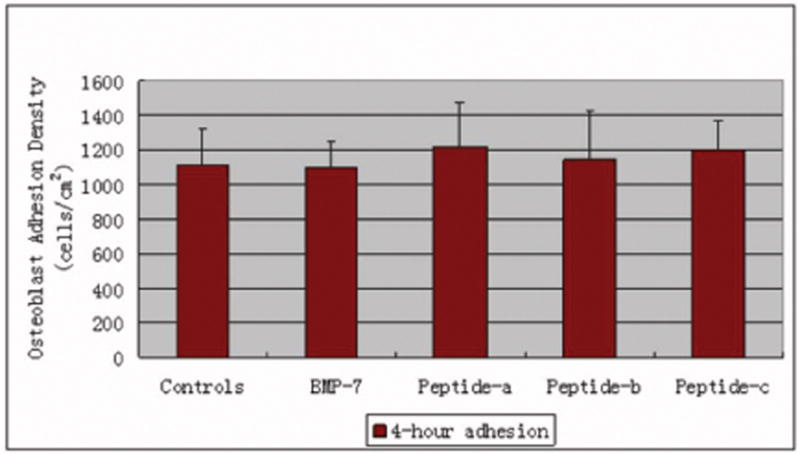
No significant difference in osteoblast adhesion density cultured in the presence of BMP-7 short peptides. Data are mean ± SEM (N=3).
Osteoblast proliferation
For proliferation studies, the number of osteoblasts increased when cultured in the presence of peptide b, c, BMP-7 and the combinations of peptide a, b; b, c; a, c; and a, b, c compared to controls (no additives) after 5 days (Figure 3). Most importantly, osteoblast density after 5 days in the presence of peptide b and the peptide combination a, b was the most, even higher than BMP-7 (although at a different concentration). Of all the peptides, osteoblast density after 5 days was the least in the presence of peptide a. Moreover, osteoblasts in the presence of peptide b after 1 and 3 days of culture were more spread and had larger average sizes than controls (no additives), as shown in Figures 4 and 5. However, osteoblast morphology in the presence of peptide a and c was similar with controls (no additives) after 1 and 3 days of culture (data not shown).
Figure 3.
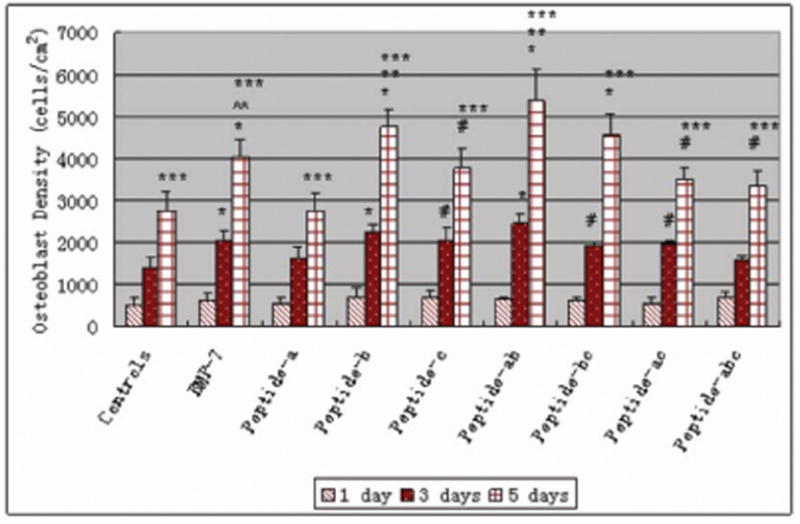
Increased number of osteoblasts cultured in the presence of BMP-7 short peptides b and a,b. Data are mean ± SEM (N=3). #p<0.05 and *p<0.01 compared to controls (no additives) at respective days. **p<0.05 compared to BMP-7 and peptide a at respective days. #p<0.01 compared to peptide a at respective days and ***p<0.01 compared to the same samples at 1 and 3 days.
Figure 4.
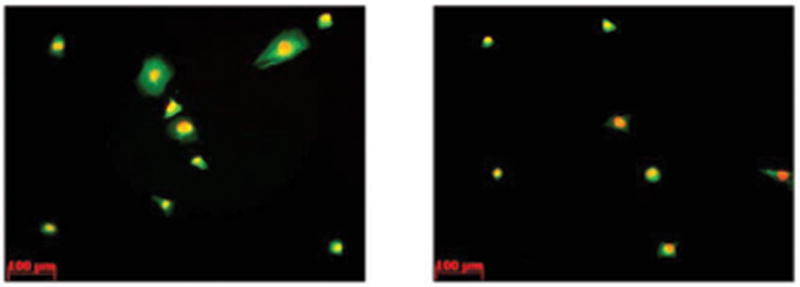
Osteoblast morphology cultured in (left) and not cultured in (right) the presence of peptide b after 1 day.
Figure 5.
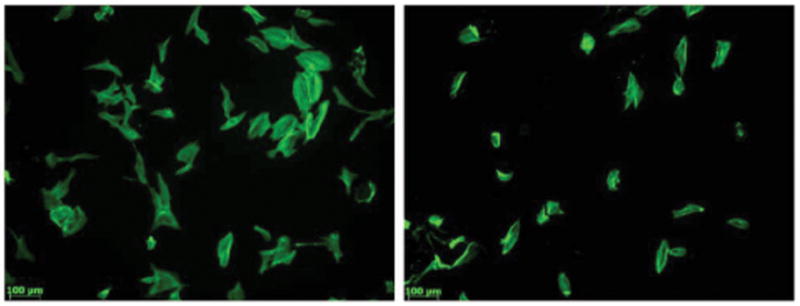
Osteoblast morphology cultured in (left) and cultured not in (right) the presence of peptide b after 3 days.
Osteoblast long-term functions
There were detectable amounts of total collagen synthesized by osteoblasts cultured with all additives after 7, 14 and 21 days of culture, as shown in Figure 6. Generally, total collagen synthesis by osteoblasts increased after 21 days when compared with that after 7 and 14 days for each condition of the study. After 21 days of culture, BMP-7, all the peptides and peptide combinations enhanced the total collagen content in osteoblasts compared to controls. Most importantly, peptide b and peptide c enhanced total collagen content in osteoblasts after 21 days of culture compared to BMP-7.
Figure 6.
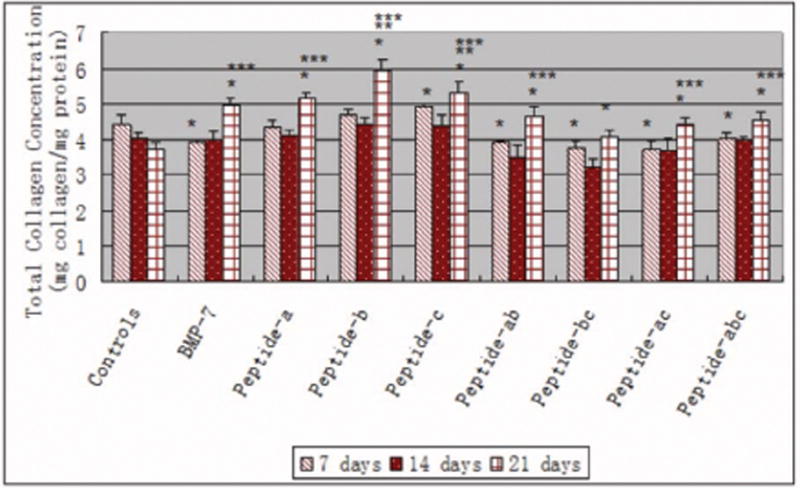
Total collagen concentration (per unit protein) in osteoblasts in the presence of BMP-7 short peptides and BMP-7. Data are mean ± SEM (N=3). *p<0.05 compared to controls (no additives) at respective days, **p<0.05 compared to BMP-7 at respective days, and ***p<0.05 compared to the same samples at 7 days and 14 days.
There were detectable amounts of total proteins synthesized by osteoblasts cultured with all additives after 7, 14 and 21 days of culture, as shown in Figure 7. The total protein content increased with longer time periods of culture for all of the peptides studied here. Moreover, for BMP-7, nearly all the peptides and peptide combinations (except peptide combination a, b, c) enhanced the total protein content in osteoblasts after 21 days of culture compared to controls. Importantly, BMP-7, peptide a and the peptide combination a, b were able to increase total protein content in osteoblasts after 14 days of culture compared to controls (no additives).
Figure 7.
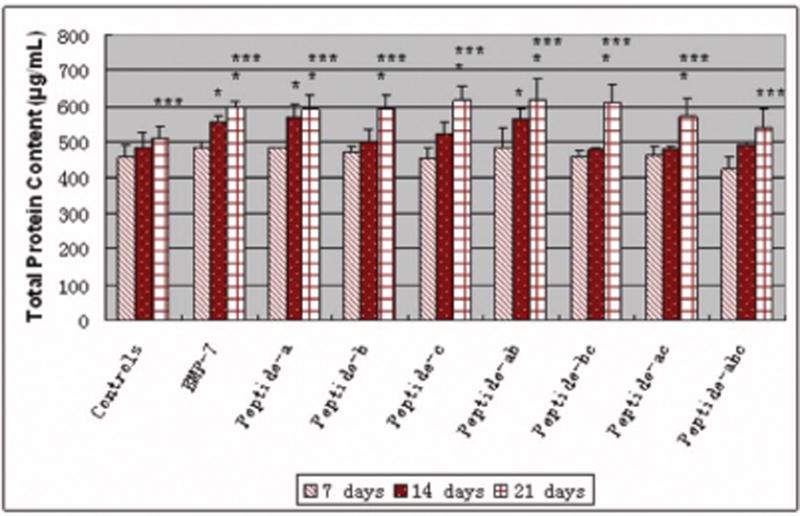
Similar total protein content in osteoblasts cultured in the presence of BMP-7 short peptides and BMP-7. Data are mean ± SEM (N=3). *p<0.05 compared to controls (no additives) at respective days and ***p<0.05 compared to the same samples at 7 and 14 days.
There were detectable amounts of alkaline phosphatase activity by osteoblasts cultured with all additives after 7, 14 and 21 days of culture, as shown in Figure 8. Generally, the amounts of alkaline phosphatase activity by osteoblasts were highest after 14 days when compared with the other time periods for BMP-7 short peptides. Peptide combinations a, c; b, c; and a, b, c increased alkaline phosphatase activity in osteoblasts after 14 days compared to controls and BMP-7. All peptide combinations increased alkaline phosphatase activity from osteoblasts after 21 days compared to controls and BMP-7.
Figure 8.
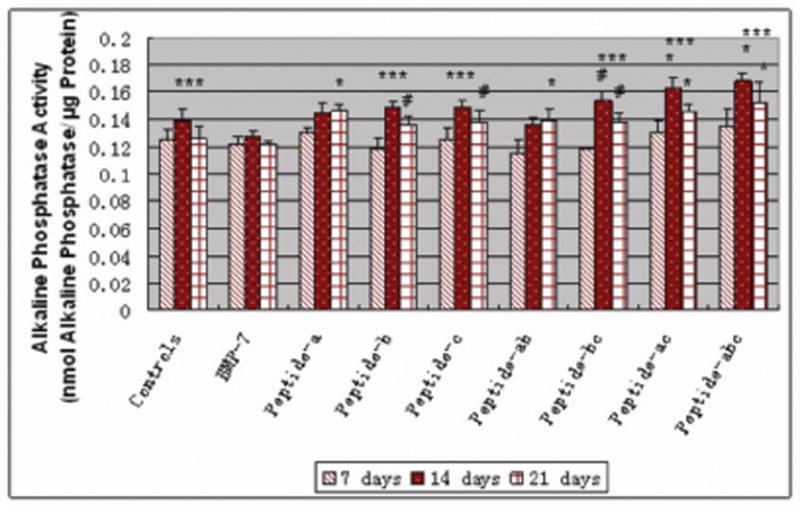
Increased alkaline phosphatase activity in osteoblasts (per unit protein) cultured in the presence of all BMP-7 short peptides. Data are mean ± SEM (N=3). #p<0.1 and *p<0.05 compared to controls (no additives) and BMP-7 at respective days. ***p<0.05 compared to the same samples at 7 and 21 days.
There were also detectable amounts of calcium deposited by osteoblasts cultured with all additives after 7, 14 and 21 days of culture, as shown in Figure 9. Calcium deposited by osteoblasts cultured with all peptides and peptide combinations increased after 21 days when compared to after either 7 or 14 days. Specifically, compared to controls and BMP-7, peptide c and all peptide combinations including peptide c increased calcium deposited by osteoblasts after 7, 14, and 21 days; peptide a and all peptide combinations including peptide a increased calcium deposited by osteoblasts after 21 days.
Figure 9.
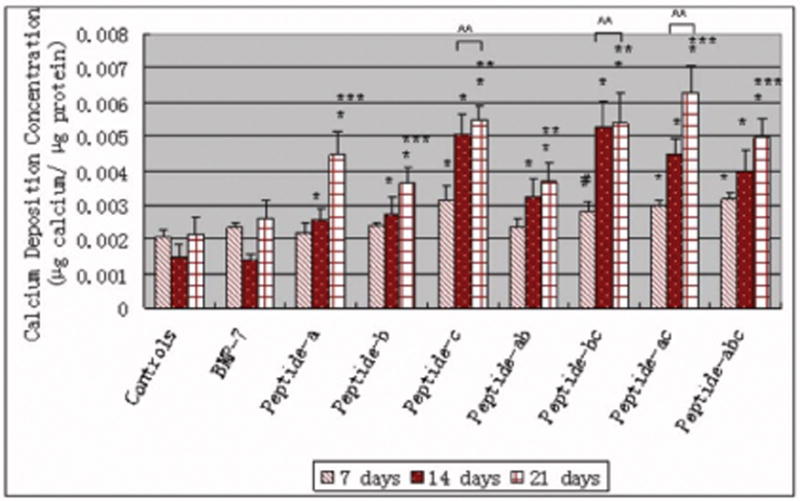
Greater calcium deposition by osteoblasts (per unit protein) in the presence of BMP-7 short peptide c and all peptide combinations with peptide c. Data are mean ± SEM (N=3). #p<0.1 and * p<0.05 compared to controls (no additives) and BMP-7 at respective days.^^p<0.05 compared to peptide a, peptide b and peptide combination a, b at respective days. **p<0.05 compared to the same samples at 7 days ***p<0.05 compared to the same samples at 7 and 14 days.
Lastly, to evaluate a dose-response, the amount of alkaline phosphatase synthesized by osteoblasts was found to increase in the presence of greater amounts of BMP-7 and all peptides of interest to this study (Figure 10). Especially, osteoblasts cultured with 1 μg/mL BMP-7 had the highest alkaline phosphatase activity compared with the controls and other additives.
Figure 10.
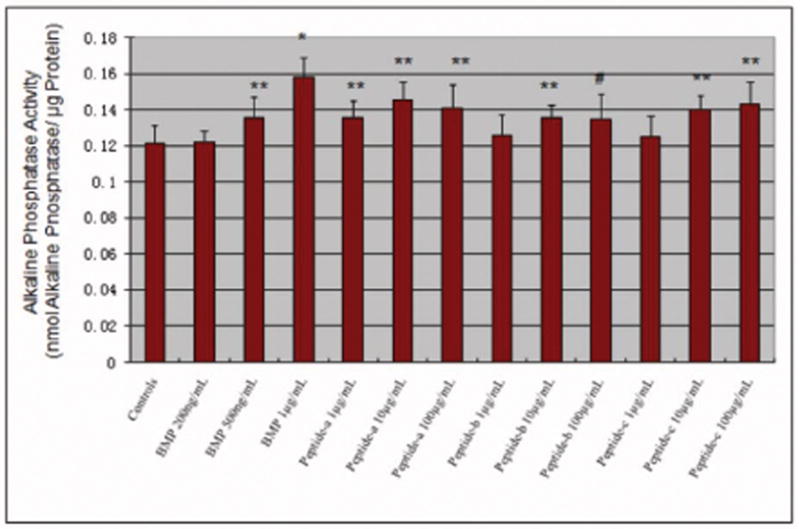
Alkaline phosphatase activity (after 21 days) in osteoblasts (per unit protein) in the presence of different concentrations of BMP-7 short peptides. Data are mean ± SEM (N=3). #p<0.1 compared to controls (no additives), *p<0.05 compared to lowest concentration of respective peptide, and **p<0.05 compared to the controls (no additives).
Discussion
In this study, the ability of three BMP-7 short peptides to improve osteoblast functions was tested. The osteoblasts in the presence of peptide b had a more well-spread morphology than the other treatment conditions. It is known that anchorage-dependent cells, like osteoblasts, must first adhere to a surface in order for subsequent cellular functions, such as proliferation and the deposition of calcium containing mineral [20]. Consistent with the morphology results, at early time points (3 and 5 days), peptide b and the peptide combination a, b was able to improve osteoblast proliferation the most. On the other hand, peptide c and all peptide combinations containing peptide c enhanced osteoblast calcium deposition after all time points of this study. Thus, all of these results suggest a role for BMP-7 short peptides to improve osteoblast functions from proliferation to calcium deposition.
In addition, the present study suggested a way of utilizing peptide combinations to achieve better functions from osteoblasts compared to individual peptides. For example, in osteoblast long-term function studies, peptide combinations a, c and a, b, c enhanced alkaline phosphatase activity of osteoblast after 14 days compared to controls, but neither peptide a nor peptide c were able to enhance osteoblast alkaline phosphatase activity after the same time period. In another experiment, the peptide combination a, c increased calcium deposition both after 7 or 14 days compared to peptide a alone. Furthermore, in the BMP-7 natural sequence, peptide a and peptide c are connected to each other, which suggests that even these two peptides when functionalized separately on nano-structured materials may complement osteoblast functions. Thus, as one example, in drug delivery applications employing nanometer particles, if it is difficult to impregnate the entire macromolecule with proteins due to their size, an alternative choice may be to impregnate several of these shorter segments of BMP-7.
In this manner, design strategies for creating biomimetic materials have been a key focus area in tissue engineering and drug delivery. Especially, compared to conventional materials, nanostructured materials (such as particles) have many unique advantages, such as long circulation times, high drug load efficiency and easy passage through cell membranes [22~24]. Thus, today’s drug release studies have focused on designing nano-materials for improved, prolonged release of drugs. For orthopedic studies, the TGF-β family of growth factors (including BMP-2 and 7) are the most widely used drugs to improve bone growth. However, in the traditional soaking method to load drugs into and onto biomimetic materials, most drugs are released after very short periods [25, 26]. By utilizing BMP-7 short peptides instead of the entire BMP protein, one can expect an easier ability to functionalize such groups onto the smaller nano-structured biomimetic materials for improved prolonged release. It is simply not feasible to functionalize growth factors larger than the size of nano-particles on nano-particles. For example, helical rosette nanotubes (HRNs) are novel biomimetic nano-materials which can increase osteoblast functions [8][9]. BMPs are too large to functionalize onto these nano-materials. Short peptides (especially those as studied here with terminal K or S amino acids) can be functionalized into the HRN inner channels by hydrogen bonds or onto HRN side chains with peptide bonds [27][28]. Importantly, results of this study can be used to improve the ability of these nanomaterials to regenerate bone. For example, because hydrogen bonds are weaker than peptide bonds, peptide b and peptide combinations containing peptide b can be functionalized into HRNs by hydrogen bonds and be quickly released to increase osteoblast proliferation. In contrast, peptide c and peptide combinations containing peptide c can be functionalized onto HRNs by peptide bonds to be slowly released to increase osteoblast long term functions, such as calcium deposition. In this way, BMP-7 short peptides can be more effectively used in conjuction with nano-materials to promote osteoblast functions.
It is important to note that in this light, BMP-7 increased total protein and collagen content from osteoblasts after 14 and 21 days compared to controls, but in terms of alkaline phosphatase activity and calcium deposition, BMP-7 seemed to have less bioactivity than the shorter peptides. The reason could be related to the concentration difference between these shorter peptides (100μg/mL) and BMP-7 (200ng/mL). As a separate experiment (Figure 10), it was found that when the BMP-7 concentration reached 1μg/mL, alkaline phosphatase activity in osteoblasts was higher than when osteoblasts were cultured with 100μg/mL of the shorter peptides. Because shorter peptides lack secondary structures important for BMP-7 bioactivity, the carbon backbone can easily rotate and change conformation. On the one hand, the interchangeable conformation lowers the efficiency of such peptides to match cell acceptors. As a result, shorter peptides need higher concentrations to reach equivalent bioactivities as lower concentrations of the entire BMP-7 macromolecule. However, because of the complex structure of BMP-7, it is easy to degrade or be damaged, especially, when it is functionalized into or onto nano-scaled biomaterials, thus, shorter peptides with simpler structures can be very stable and easier to control in experiments. In addition, many nanomaterials themselves (when not functionalized) have been shown to promote bone formation more so than micron structured materials [4, 6, 8, 9]. As a result, shorter peptides functionalized on nanostructured biomaterials can be widely used in the clinic in all settings to promote numerous orthopedic applications.
Lastly, researchers have known that BMPs form dimers and bind to BMP receptor type I and II on cell surfaces to initiate cellular activities [21]. Although the precise receptor-binding region in BMPs has not yet been clarified, some studies suggest that the region around the “knuckle epitope” (KIPKASSVPTELSAISTLYL) of BMP-2 binds the receptor type II complex with receptor type I on osteoblasts, to initiate signal transduction [18]. Comparing the structure of BMP-2 and BMP-7, peptide a, b and c is at a similar region in BMP-7 as the knuckle epitope in BMP-2 (Figure 11). This could be the reason why BMP-7 short peptides in this study were able to improve osteoblast functions as shown here for the first time.
Figure 11.
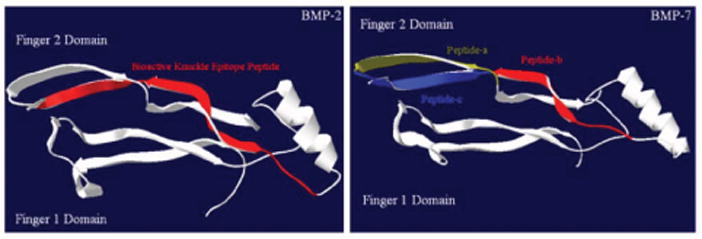
The comparison of BMP-2 (left) and BMP-7 (right) structures. (3-D files of BMPs obtained from the Research Collaboratory for Structural Bioinformatics (RCSB) protein data bank.)
Conclusions
Results of this study showed that peptide b (KPSSAPTQLN) and the peptide combination a,b (SNVILKKYRN, KPSSAPTQLN) from BMP-7 increased osteoblast proliferation, while peptide c (KAISVLYFDDS) and the peptide combinations containing peptide c from BMP-7 promoted osteoblast calcium deposition. These results indicate great potential for combining BMP-7 short peptides with nano-scaled biomaterials to develop improved materials for orthopedic applications.
Acknowledgments
This study was supported by a National Institutes of Health grant (No. 1R21AG027521).
References
- 1.Arthur HW, Harris Ham WR. The biochemistry and physiology of bone. New York: Academic Press; 1972. p. 337. [Google Scholar]
- 2.Slone RM, Heare MM, Vander RA, Montgomery WJ. Orthopedic fixation devices. Radiographics. 1991;11:823–847. doi: 10.1148/radiographics.11.5.1947319. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JM, Gristine AG, Hanson SR. Biomaterial Science. Academic Press, Inc.; San Diego: 1996. p. 165. [Google Scholar]
- 4.Balasundaram G, Webster TJ. A perspective on nanophase materials for orthopedic implant application. Journal of Materials Chemistry. 2006;16:3737–3745. [Google Scholar]
- 5.Simank HG, Stuber M, Frahm R, Helbig L, van Lenthe H, Müller R. The influence of surface coatings of dicalcium phosphate (DCPD) and growth and differentiation factor-5 (GDF-5) on the stability of titanium implants in vivo. Biomaterials. 2006;27:3988–3994. doi: 10.1016/j.biomaterials.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 6.Balasundaram G, Sato M, Webster TJ. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials. 2006;14:2798–2805. doi: 10.1016/j.biomaterials.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Gavenis K, Klee D, Pereira-Paz RM, Walter M, Mollenhauer J, Schneider U, Schmidt-Rohlfing B. BMP-7 loaded microspheres as a new delivery system for the cultivation of human chondrocytes in a collagen type-I gel. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2006;82B:275–283. doi: 10.1002/jbm.b.30731. [DOI] [PubMed] [Google Scholar]
- 8.Chun AL, Moralez JG, Fenniri H, Webster TJ. Helical rosette nanotubes: a biomimetic coating for orthopedics? Biomaterials. 2005;26:7304–7309. doi: 10.1016/j.biomaterials.2005.05.080. [DOI] [PubMed] [Google Scholar]
- 9.Chun AL, Moralez JG, Fenniri H, Webster TJ. Helical rosette nanotubes: A more effective. orthopaedic implant material Nanotechnology. 2004;15:s234–s239. [Google Scholar]
- 10.Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996;19:23s–37s. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 11.Lind M, Deleuran B, Thestrup-Pedersen K, Søballe K, Eriksen EF, Bünger C. Chemotaxis of human osteoblasts. Effects of osteotropic growth factors APMIS. 1995;103:140–146. [PubMed] [Google Scholar]
- 12.Wang JS. Basic fibroblast growth factor for stimulation of bone formation in osteoinductive or conductive implants. Acta Orthop Scand Suppl. 1996;269:1–33. doi: 10.3109/17453679609155229. [DOI] [PubMed] [Google Scholar]
- 13.Sailer HF, Kolb E. Application of purified bone morphogenetic protein (BMP) in cranio-maxillo-facial surgery. BMP in compromised surgical reconstructions using titanium implants. J Craniomaxillofac Surg. 1994;22:2–11. doi: 10.1016/s1010-5182(05)80289-6. [DOI] [PubMed] [Google Scholar]
- 14.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83A:151–158. [PMC free article] [PubMed] [Google Scholar]
- 15.Baylink DJ, Finkelman RD, Mohan S. Growth factors to stimulate bone formation. J Bone Mineral Res. 1993;8:s565–572. doi: 10.1002/jbmr.5650081326. [DOI] [PubMed] [Google Scholar]
- 16.Reddi AH, Cunningham NS. Initiation and promotion of bone differentiation by bone morphogenetic proteins. J Bone Mineral Res. 1993;8:s499–s502. doi: 10.1002/jbmr.5650081313. [DOI] [PubMed] [Google Scholar]
- 17.Scheufler C, Sebald W, Hulsmeyer M. Crystal structure of human bone morphogenetic protein-2 at 2.7 a resolution. J Mol Biol. 1999;287:103–115. doi: 10.1006/jmbi.1999.2590. [DOI] [PubMed] [Google Scholar]
- 18.Saito A, Suzuki Y, Ogata S, Ohtsuki C, Tanihara M. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochimica et Biophysica Acta. 2003;1651:60–67. doi: 10.1016/s1570-9639(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood K, Rheude B, Kim YJ, White K, Dee KC. In vitro mineralization studies with substrate-immobilized bone morphogenetic protein peptides. Journal of Oral implantology. 2000;29:57–65. doi: 10.1563/1548-1336(2003)029<0057:IVMSWS>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Dee KC, Rueger DC, Andersen TT, Bizios R. Conditions which promote mineralization at the bone-implant interface: a model in vitro study. Biomaterials. 1996;17:209–215. doi: 10.1016/0142-9612(96)85765-6. [DOI] [PubMed] [Google Scholar]
- 21.Massague J. TGF-β signal transduction. Ann Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Zhang ZP, Feng S. Nanoparticles of poly (lactide) - Tocopheryl polyethylene glycol succinate (PLA-TPGS) copolymers for protein drug delivery. Biomaterials. 2007;28:2041–2050. doi: 10.1016/j.biomaterials.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Vonarbourg A, Passirani C, Saulnier P, Benoit J. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials. 2006;27:4356–4373. doi: 10.1016/j.biomaterials.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 24.Yin H, Too HP, Chow GM. The effects of particle size and surface coating on the cytotoxicity of nickel ferrite. Biomaterials. 2006;26:5818–5826. doi: 10.1016/j.biomaterials.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 25.Rao KP. Recent developments of collagen-based materials for medical applications and drug delivery systems. J Biomater Sci Polymer Ed. 1995;7:623–645. doi: 10.1163/156856295x00526. [DOI] [PubMed] [Google Scholar]
- 26.Friess W. Collagen - biomaterial for drug delivery. European Journal of Pharmaceutics and Biopharmaceutics. 1998;45:113–136. doi: 10.1016/s0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 27.Fenniri H, Packiarajan M, Vidale KL, Sherman DM, Hallenga K, Wood KV, Stowell JG. Helical rosette nanotubes: Design, self-assembly, and characterization. J Am Chem Soc. 2001;123:3854–3855. doi: 10.1021/ja005886l. [DOI] [PubMed] [Google Scholar]
- 28.Fenniri H, Deng BL, Ribbe AE. Helical rosette nanotubes with tunable chiroptical properties. J Am Chem Soc. 2002;124:11064–11072. doi: 10.1021/ja026164s. [DOI] [PubMed] [Google Scholar]


