Abstract
We have been investigating thermoresponsive hydrogel nanoparticle composite networks to develop photopolymerized hydrogels in order to deliver drugs for prevention of restenosis after angioplasty. These composite systems can form a gel under physiological conditions and release drugs in response to temperature changes. Our novel system consisting of poly(N-isopropylacrylamide) (PNIPA) thermoresponsive nanoparticles, photo cross linker poly(ethylene glycol) diacrylate (PEGDA), and UV photoinitiator, 2-hydroxy-1-[4-(2-hydroxyethoxy) phenyl]-2-methyl-1-propanone-1-one (Irgacure 2959), would be photopolymerized in situ in the presence of UV light. The focus of this study was to evaluate the effects of a photoinitiator and UV exposure on human aortic smooth muscle cells (HASMCs). We found that exposure to UV light did not significantly affect cellular survival within doses required for photopolymerization. The photoinitiator was cytocompatible at low concentrations (≤ 0.015% w/v); however, cytotoxicity increased with increasing photoinitiator concentrations. In addition, free radicals formed in the presence of a photoinitiator and UV light caused significant levels of cell death. An antioxidant (free radical scavenger), ascorbic acid, added to the cell media significantly improved relative cell survival, but increased the hydrogel gelation time. Finally, HASMC survival when exposed to all potential cytotoxic components was also evaluated by exposing HASMCs to media incubated with our composite hydrogels. In summary, our studies show that the photoinitiator and free radicals are responsible for the cytotoxicity on HASMCs, and the addition of antioxidants can significantly reduce these harmful effects.
Keywords: cytotoxicity, Irgacure 2959, UV, human aortic smooth muscle cells, thermoresponsive nanoparticles, hydrogels, photopolymerization
Introduction
Our long term goal is to develop an in situ photopolymerized thermoresponsive hydrogel nanoparticle composite system to aid in the prevention of restenosis after angioplasty. Coronary balloon angioplasty involves clearing the blocked artery by inflating a balloon and compressing the plaque against the arterial wall, commonly resulting in damage to the endothelial layer. Restenosis, the re-narrowing of the treated artery, is caused by a major loss of the endothelial cell population (a natural vascular barrier), resultant smooth muscle cell (SMC) migration, and subsequent SMC proliferation at the injured arterial wall site.1-3 Our system, consisting of poly(N-isopropylacrylamide) (PNIPA) thermoresponsive nanoparticles, photo cross-linker poly(ethylene glycol) diacrylate (PEGDA), and UV photoinitiator 2-hydroxy-1-[4-(2-hydroxyethoxy) phenyl]-2-methyl-1-propanone (Irgacure 2959), would be photopolymerized at the injured wall with exposure to UV light following angioplasty for local drug delivery.
The advantage of our hydrogel nanoparticle composite system is that it would provide both local and stimuli-responsive drug delivery capable of releasing a drug in response to temperature changes.4 The drug would be selected based on its ability to prevent further human aortic smooth muscle cell (HASMC) migration and proliferation, major causes of restenosis. In addition to releasing the drug in a temperature responsive manner, the hydrogel film would also act as a protective barrier against the recruitment of blood cells such as platelets and leukocytes, major causes of thrombosis and inflammation at the damaged arterial wall.5 Another advantage of our system would be the ability to quickly photopolymerize at the injured arterial wall after angioplasty. Photopolymerization of hydrogels has been investigated extensively for use in various biomedical applications, including drug delivery 6-10 and tissue engineering.11-19 Upon exposure to UV light, photopolymerization allows rapid conversion of a liquid monomer or macromer solution into a gel in situ.20,21 Other advantages of photopolymerized hydrogels include spatial and temporal control of reaction kinetics, fast curing rates to provide rapid polymerization, and effective control over cross-linking density, thereby governing the release rate.22-24 These advantages make photopolymerized hydrogels extremely desirable as systems for smart local drug delivery.21
The essential components needed to form our photopolymerized composite hydrogel network are the photo cross-linker, photoinitiator, and UV irradiation. Poly(ethylene glycol) (PEG) functionalized with diacrylate group (PEGDA) was chosen as the photo cross-linker, as it cross-links quickly in the presence of UV light and a photoinitiator to form hydrogels.4,21 Additionally, PEGDA is considered biocompatible and nontoxic as it is a derivative of PEG.25 When photoinitiator molecules are exposed to specific wavelengths of visible or UV light, they dissociate into free radicals, which initiates the polymerization reaction.20,21 The UV photoinitiator Irgacure 2959 was selected based on the results of previous studies where Irgacure 2959 was found to be the most cytocompatible UV photoinitiator compared to other photoinitiators for different cell types.20,22,26 However, one such study on the effect of Irgacure 2959 also noted that different cell types displayed different sensitivities to the same concentration of this photoinitiator.22 Therefore, it is essential to determine the sensitivity of HASMCs specifically to Irgacure 2959.
The use of UV light and photoinitiator molecules makes it necessary to determine the compatibility of these systems before their use in biomedical applications. The cells can undergo cellular damage during photopolymerization as a result of exposure to photoinitiator molecules, reactive macromers, and free radicals.20 For our system, inhibiting HASMC migration and proliferation is necessary to prevent restenosis. However, the photoinitiating system must not have a deleterious effect on the existing HASMC population. Thus, it is critical to evaluate the biocompatibility of our system on HASMCs and minimize the cytotoxicity of its components. Therefore, the aim of this paper is to evaluate the cytotoxic effects of the composite system components on the HASMCs. First, these cells were exposed to different photoinitiator concentrations with or without UV light exposure for various periods. Cell survival was then determined by MTS assays. Ascorbic acid, an antioxidant, was also tested for its efficiency in reducing the cytotoxicity of free radicals. In addition, studies were performed to evaluate the effect of antioxidant addition on the gelation time of our composite hydrogels. Finally, media was incubated with our composite hydrogels for 8 hours and cell survival was determined after HASMCs were incubated with this media for 3 days.
Materials and Methods
Materials
Chemicals, if not specified, were purchased from Sigma-Aldrich (St. Louis, MO), including N-isopropylacrylamide (NIPA), N, N’-methylenebisacrylamide (BIS), potassium persulfate (KPS), and sodium dodecyl sulfate (SDS).
Human Aortic Smooth Muscle Cell (HASMC) Culture
Human aortic smooth muscle cells (Cascade Biologics, Portland, OR) were cultured in complete medium consisting of Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA). Upon 80-90% confluency, the cells were passaged or used for experiments. For all experiments, the cells were seeded in 24-well plates (Corning Inc., Corning, NY) at a density of 7000 cells per well. Following seeding, the cells were incubated at 37°C and 5% CO2 in a humid environment for 2 days to allow cellular attachment and growth. After 2 days, the HASMCs were exposed to varying concentrations of the photoinitiator Irgacure 2959 (Ciba Speciality Chemicals, Tarrytown, NY) and/or UV exposure.
Preparation of Photopolymerized Thermoresponsive Hydrogels
PNIPA nanoparticles were first prepared using the previously described method.4,27,28 Photopolymerized hydrogels were then produced using the method outlined by Ramanan et al.4 Briefly, PNIPA nanoparticles (20% by weight of PEGDA) were added to a solution containing PEGDA (3400 MW) with a final PEGDA concentration of 100 mg/ml. The UV photoinitiator, Irgacure 2959, was then added at a final concentration of 0.015% (w/v). 200 μl of this solution was added to a 48-well plate and exposed to long-wave, 365 nm UV light at about 10 mW/cm2 for five minutes to form the composite hydrogels.
Effects of UV Exposure Durations
To evaluate the effects of UV exposure on cell survival, cells were seeded and cultured as described above. The cells were then exposed to varying durations (1, 3, and 5 minutes) of long wave, 365 nm UV light (Model B-100AP/R, UVP) at about 10 mW/cm2. These durations of UV exposure were chosen because they are sufficient to photopolymerize the hydrogels. Cells not exposed to UV light served as the control. Following exposure, the cells were incubated for another 3 days before quantifying the cell survival.
Effects of Photoinitiator Concentrations
To evaluate the cytotoxic effects of photoinitiator concentrations on HASMCs, the cells were seeded and cultured as described above. Irgacure 2959 was directly dissolved in complete media to obtain final concentrations of 0.01%, 0.02%, 0.04%, 0.08%, and 0.16% (w/v). These photoinitiator concentrations were well within the range required for photopolymerization in a short period of time. The photoinitiator solutions were carefully protected from exposure to light to preserve their activity. These solutions were then sterilized using 0.2 μm syringe filters before they were added to the HASMCs. The control wells consisted of cells incubated with photoinitiator-free complete media. After the addition of the photoinitiator, the cells were incubated for 3 days and then cell survival was determined using MTS assays.
Combined Effects of Photoinitiator and UV Exposure
The cellular damage due to the combined effects of photoinitiator and UV exposure was evaluated using the previously described method.22 Briefly, the HASMCs were seeded onto 24-well plates and allowed to grow for 2 days. Irgacure 2959 solutions with final concentrations of 0.01%, 0.015%, 0.04%, and 0.08% (w/v) in complete media were prepared. After adding these solutions, cells were incubated for 30 minutes to allow for the mixing of the photoinitiator. The well plates were then exposed to 1, 3, and 5 minutes of UV light. Wells containing cells not exposed to either UV light or photoinitiator solution served as controls for this experiment. Cell samples were incubated for 3 days before analyzing the cell survival.
Effect of Antioxidants
An additional study was performed to evaluate the efficiency of the antioxidant, ascorbic acid, in scavenging the free radicals in an effort to increase cell survival. Ascorbic acid was chosen based on the observation by Williams et al. that ascorbic acid present in bovine chondrocyte specific media may be responsible for reducing the cytotoxic effects of Irgacure 2959.22 HASMCs were exposed to 0.15% (w/v) solution of Irgacure 2959 in complete media supplemented with varying concentrations of ascorbic acid (0-200mg/L). After incubating for 30 minutes, the cells were exposed to 5 minutes of UV light and cell survival was quantified after 3 days.
To evaluate the effect of added antioxidants on the gelation time, the gelation times (i.e. the time required for the materials to form a gel) were determined using three methods on 96-well plates. For this study, 50 mg/L of ascorbic acid was added to the hydrogel precursor solution, containing 0.15% (w/v) Irgacure 2959. In the first method, the gels were exposed to the UV light, and viscosities of the gel solution were observed.29 The end point was demonstrated when we were able to pick up the gel with the pippet tip. In the second method, a stir bar was placed in a well containing the hydrogel solution, and the gelation time was defined as a time required for the stir bar to stop stirring.30 In the last method, UV-Vis spectrophotometer was used in order to monitor for the change in the intensity of the gelation solution from 340 nm to 1020 nm wavelength to choose the optimal wavelength. The highest change in the intensity was observed at 610 nm. Then, the solution was monitored at that this specific wavelength (610 nm) over a time course. The result was graphed as function of time, and the gelation time was defined as the time that had the highest changes in the absorbance intensities.
Effect of Photopolymerized Hydrogels
Finally, a study was performed to evaluate the cytotoxic effects of the photopolymerized composite system on the HASMC population. This was done to evaluate how the whole composite hydrogel system affected cell survival. Here, hydrogels (n=4) were photopolymerized (as described above) with 0.015% and 0.15% (w/v) Irgacure 2959 concentrations. HASMC media was then incubated with the hydrogels for 8 hours to allow the leaching of all potential cytotoxic components from our hydrogels into the cell media. After 8 hours, this media was added to the HASMCs which had been grown for two days. For the controls, fresh media (not incubated with hydrogels) was added to the cells. After 3 days of incubation, the cell survival was evaluated using MTS assays.
Cell Survival Using MTS Assays
After the experiments, the cell survival was quantified using the MTS assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega) following the manufacturer’s instructions. The cells were incubated with the MTS reagent for four hours, after which 200 μl of the solution was transferred to 96-well plates and absorbance was read at 490 nm using a microplate reader (VMax, Molecular Devices). Relative cell survival was obtained by dividing the absorbance reading of a cell sample by the mean absorbance value of the control.
Statistical Analysis
Analysis of the results was performed using ANOVA and t-tests with p < 0.05 (StatView 5.0 software, SAS Institute). Post-hoc comparisons were made using the Fisher’s least significant differences (LSD). For each study, four samples were tested (n = 4) and all the results are given as mean ± SD.
Results
Effect of UV Exposure Durations
In our study, cells were exposed to UV light in the absence of photoinitiator molecules to determine the effects of UV exposure time only. The HASMCs were exposed to 1, 3, and 5 minutes of long-wave, 365 nm UV light at 10 mW/cm2, which is enough to photopolymerize the hydrogels. Following exposure, cells were cultured for 3 days to evaluate the cytotoxic effects of the UV light. Cells not exposed to ultraviolet light served as the controls and were used to determine the relative cell survival rate. The cell survival for controls was determined to be 1 ± 0.09. The effects of varying durations of UV exposure on the HASMC survival are shown in Figure 1. Exposure of HASMCs to 1, 3, and 5 minutes did not show any statistically significant decrease in cell survival and the relative cell survival rates were 0.96 ± 0.05, 0.97 ± 0.04, and 1.07 ± 0.02, respectively.
Figure 1.
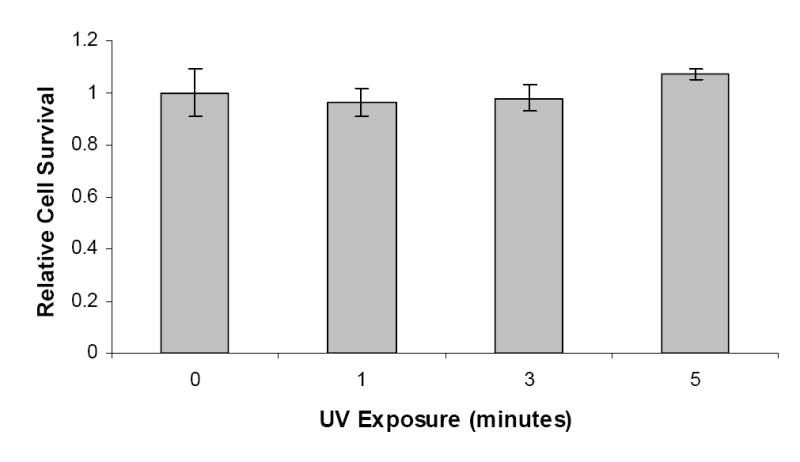
The effects of varying durations (0, 1, 3, and 5 minutes) of long wave, 365 nm UV light exposure at about 10 mW/cm2 on the survival of human aortic smooth muscle cells, in the absence of a UV photoinitiator. Controls were cells not exposed to UV light (0 minutes of exposure).
Effect of Photoinitiator Concentrations
HASMCs were incubated with various photoinitiator concentrations (0.01%, 0.02%, 0.04%, 0.08%, and 0.016% (w/v) Irgacure 2959) to evaluate the cytotoxicity of initiator molecules. The controls, consisting of wells exposed to photoinitiator-free media, were used to determine relative cell survival. The cell survival for the controls was determined to be 1 ± 0.09. The cytotoxic effects of the varying photoinitiator solutions are shown in Figure 2. Irgacure 2959 did not show a significant decrease in cell survival when HASMCs were exposed to 0.01% (w/v) photoinitiator solution, and relative cell survival was determined to be 1.03 ± 0.10 (n = 4). However, upon increasing the photoinitiator concentrations above 0.02% (w/v), a statistically significant decrease was noticed in the relative cell survival. Varying the photoinitiator concentration from 0.02% (w/v) to 0.16% (w/v) was found to progressively decrease the relative cell survival from 0.85 ± 0.09 to 0.25 ± 0.01, respectively.
Figure 2.
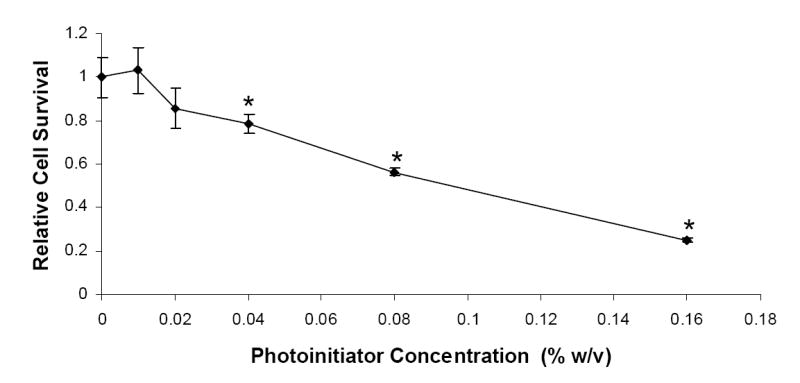
The effects of photoinitiator (Irgacure 2959) concentrations on the survival of human aortic smooth muscle cells, in absence of UV light.
Combined Effects of Photoinitiator and UV Exposure
It is essential to evaluate cytotoxic effects of the free radicals formed when photoinitiator molecules are exposed to UV light. For this study, the HASMCs were treated with photoinitiator solutions (0.01%, 0.015%, 0.04%, and 0.08% w/v) followed by exposure to UV light (1, 3, and 5 minutes). Relative cell survival, shown in Figure 3, was calculated using the control wells, which were not exposed to the photoinitiator solution or UV light. The cell survival for the controls was determined to be 1 ± 0.09. For 0.01% and 0.015% (w/v) of photoinitiator concentrations, the HASMCs did not show any statistically significant decrease in relative cell survival for 1, 3, and 5 minutes exposure (n = 4). At 0.04% (w/v) concentration of Irgacure 2959 in complete media, a significant decrease was observed at 1, 3, and 5 minutes of UV exposure, with relative cell survival values at 0.76 ± 0.05, 0.74 ± 0.04, and 0.67 ± 0.05 of the control samples, respectively. Finally, the relative cell survival rates were significantly reduced at 0.08% (w/v) photoinitiator concentration, ranging between 0.54 ± 0.04 and 0.45 ± 0.03 for the three durations of UV exposure.
Figure 3.
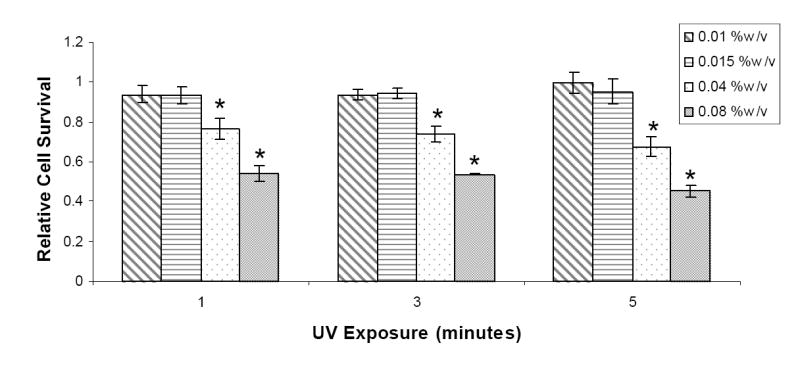
The combined effects of photoinitiator concentrations at four concentrations 0.01%, 0.015%, 0.04%, and 0.08% (w/v)) and UV exposure (with three durations 1, 3, and 5 minutes) on the survival of human aortic smooth muscle cells.
Effect of Antioxidant
To explore methods of reducing cytotoxicity due to the released free radicals, a study was carried out to evaluate the efficiency of ascorbic acid, an antioxidant, as a scavenger of free radicals in reducing cellular damage caused by free radicals. HASMCs were incubated in photoinitiator-media solutions supplemented with various concentrations of ascorbic acid. It was observed that even at low concentrations (50 mg/L), ascorbic acid was able to significantly improve the relative cell survival rates compared to samples without ascorbic acid (Figure 4). In addition, a parallel study was carried out to study the effect of antioxidant addition on the gelation time of the hydrogel. Hydrogels with 50 mg/L of ascorbic acid required more time to form when compared to hydrogels with no antioxidants, with all other conditions remaining constant (Table 1).
Figure 4.
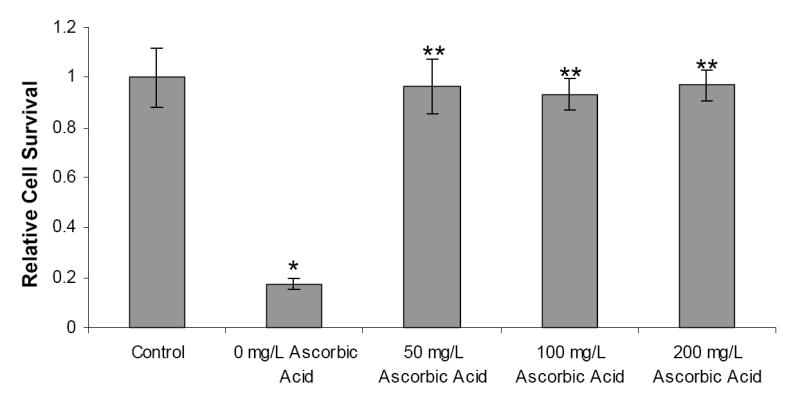
The effects of ascorbic acid on HASMC cell survival when exposed to 0.15% (w/v) Irgacure 2959 and 5 minutes of UV exposure. Controls were not exposed to the photoinitiator and UV light. * = Significant Difference (p<0.05) ** = significant difference (p<0.05) with respect to 0mg/L ascorbic acid concentration.
Table 1.
Effects of antioxidants (e.g. Ascorbic Acid) on the gelation time.
| Samples | Gelation Time (Minutes)
|
||
|---|---|---|---|
| Observation | Stirring | Spectrophotometer | |
| Without Ascorbic Acid | 3.44 ± 0.230 | 3.59 ± 0.290 | 4.00 ± 0.009 |
| With Ascorbic Acid* | 4.05 ± 0.100* | 4.20 ± 0.060* | 4.42 ± 0.016* |
significantly difference compared to samples without ascorbic acid, p < 0.05
Effect of Photopolymerized Hydrogels
An additional study was performed to evaluate the cytotoxic effects of the photopolymerized composite hydrogels. Hydrogels were photopolymerized with 0.015% and 0.15% (w/v) Irgacure 2959 concentrations and then incubated with HASMC media for 8 hours. This media was then added to the seeded HASMCs, and the relative cell survival rates for cells incubated with hydrogel media for 3 days are shown in Figure 5. Controls exhibited a relative cell survival rate of 1 ± 0.04. For hydrogels with 0.015% (w/v) Irgacure 2959, relative cell survival of HASMCs did not show any significant decrease (0.93 ± 0.04). However, for HASMCs incubated with media from hydrogels photopolymerized with 0.15% (w/v) Irgacure 2959, relative cell survival was significantly decreased compared to the controls (0.48 ± 0.19 of controls).
Figure 5.
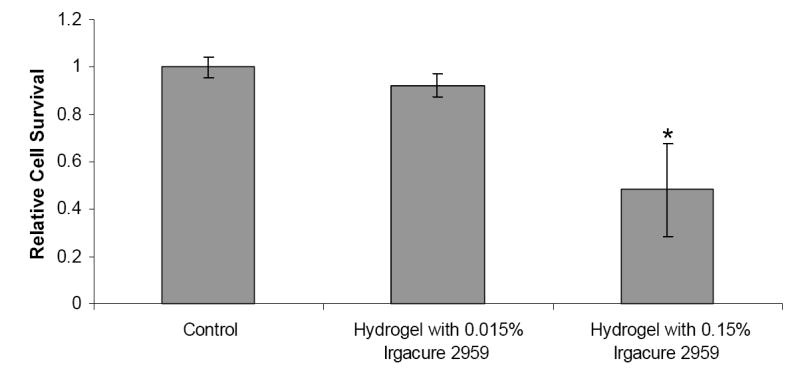
The effects of Photopolymerized Composite Hydrogels on HASMC cell survival. Controls were not exposed to the photopolymerized hydrogels. * = Significant Difference (p<0.05).
Discussion
Photopolymerizable hydrogel systems have been used in several applications including drug delivery and tissue engineering. The ability to rapidly form a hydrogel in situ using photopolymerization makes this system highly desirable for biomedical applications.4 In our particular application, we are developing a thermoresponsive nanoparticle composite hydrogel to aid in preventing restenosis. Following angioplasty, the hydrogel would be photopolymerized at the site of the injured arterial wall to release drugs that inhibit restenosis. It is critical that the drug delivery system components not cause additional damage to the surrounding cells and tissues. Hence, the aim of this paper was to evaluate the cytocompatibility of the components of the photoinitiating system, including UV light and the photoinitiator. HASMCs, normally present at an injured site, were chosen for the evaluation of the cytotoxic effects of the system components.
Short-time exposure to UV light did not cause significant cytotoxicity as shown in our study (Figure 1) and in previous studies from other investigators. Our study, evaluating the cytotoxicity of varying times of UV exposure, showed that UV light did not cause any significant decrease in HASMC survival (1.07 ± 0.02) after 5 minutes of exposure. Similar studies by others conducted on NIH/3T3 fibroblasts cells have shown relative cell survival rates of 1.08 ± 0.03 even after 10 minutes of UV exposure.20 Williams et al. performed an extensive study evaluating the effects of UV exposure on six different cell types, including human fetal osteoblasts, bovine chondrocytes, rabbit corneal epithelial cells, human mesenchymal stem cells, goat mesenchymal stem cells, and human embryonic germ cells, and found that short UV exposure (5 minutes) did not alter cell survival significantly.22 These results, combined with our data, confirm that while its cytotoxic effects may vary slightly among cell types, UV exposure does not significantly contribute to cell death at conditions (short-time exposure) required for photopolymerization.
Our second study evaluated the effects of different photoinitiator concentrations on the survival of HASMCs (Figure 2). It was important to evaluate the cytocompatibility of Irgacure 2959 as HASMCs would be exposed to the initiator molecules prior to polymerization. Additionally, it is possible that some undissociated initiator molecules could harm the cells after polymerization.20 Several groups have investigated the cytotoxicity of different, commercially available, visible light and UV photoinitiators.12,26,31 Bryant et al. investigated the cytocompatibility of four UV photoinitiators on NIH/3T3 fibroblast cells and found Irgacure 2959 to be the most cytocompatible UV photoinitiator that does not affect fibroblast survival at concentrations ≤ 0.05% (w/w). These studies also observed positive results on chondrocytes.20 Williams et al. studied the cytocompatibility of three UV photoinitiators with six different cell lines and found Irgacure 2959 to be the most cytocompatible amongst these six cell lines with different cell types reacting differently to the same concentration of a single photoinitiator.22 Based on the results from these studies, we selected Irgacure 2959 as a photoinitiator for our composite hydrogel system, and thus, it was important for us to evaluate the cytocompatibility of Irgacure 2959 specific to HASMCs. In our study, HASMCs showed a higher sensitivity to Irgacure 2959 compared to different cell types studied by other groups. Our results showed that below 0.02% (w/v) concentration, there was no significant decrease in cell survival. At concentrations greater than 0.02% (w/v), the cytotoxicity of Irgacure 2959 increased with increasing photoinitiator concentrations and significantly affected cell survival. It is also important to note that even low photoinitiator concentrations (0.015%) can photopolymerize the hydrogels within short periods of UV exposure (5 minutes). Additionally, the HASMCs were exposed to Irgacure 2959 for 3 days whereas, in vivo, the cells would be exposed to the photoinitiators for a shorter period.
The cellular damage caused by photopolymerization was also evaluated by studying the combined effects of photoinitiator molecules and UV light (Figure 3). It was essential to perform this study as initiator molecules dissociate into free radicals upon exposure to UV light, and free radicals can damage cellular membranes.20 In our studies, at lower photoinitiator concentrations (0.01% and 0.015% w/v) and UV exposure times (1, 3, and 5 minutes), cell survival rates were not statistically different from the controls. However, at 0.04% and 0.08% (w/v) photoinitiator concentrations, a significant decrease was noted in cell survival rates for all durations of UV exposure. For high photoinitiator concentrations, the increased cell death might be attributed to the free radicals released during photopolymerization. Furthermore, cells may also be exposed to a toxic environment consisting of initiator by-products and undissociated initiator molecules in addition to generated free radicals following polymerization.20
To reduce the cytotoxic effects, we tried scavenging the free radicals released during the photopolymerization process. Williams et al. reported that the presence of antioxidant ascorbic acid in the culture media for bovine chondrocytes might have reduced the sensitivity of these cells to the toxic effects of Irgacure 2959.22 In our study we evaluated the ability of ascorbic acid to scavenge free radicals and reduce photoinitiator toxicity, and it was found that ascorbic acid, even at low concentrations (50 mg/L), significantly increased cell survival. It was previously reported that different cell types might respond differently to a single photoinitiator due to variations in their expression of antioxidant enzymes, 32 receptors for antioxidant enzymes, 33,34 and the addition of antioxidants to their culture media.35,36 Our results confirm these findings as we were able to significantly alter cell survival rates by adding an antioxidant (Figure 4). However, it is important to note that free radicals are critical to the polymerization process. Hence, it was necessary to determine whether the presence of ascorbic acid altered the gelation time of the hydrogel. Our studies showed that upon addition of 50 mg/L concentration of antioxidant ascorbic acid, the time required for gelation was increased compared to the gelation time in the absence of an antioxidant. Although the addition of ascorbic acid significantly improved cell survival, its increased gelation time may result in increased photoinitiator and UV exposure, making ascorbic acid unattractive for use in our system.
Finally, we tried to evaluate the cytotoxic effects of the photopolymerized composite hydrogels as a whole. During photopolymerization, the reactive macromers would react with the free radicals, thereby possibly reducing the harmful effects of the radicals on the cells.22 Thus, it was essential to perform this study to determine whether there was a potential cytotoxic effect of all components in our drug delivery system on SMCs. This study was also essential to evaluate the cytotoxicity of the PEGDA and PNIPA polymers, which are an integral part of our system. Our results showed that there was no significant decrease in cell survival when hydrogels were photopolymerized with 0.015% (w/v) Irgacure 2959, which our earlier results had also shown to be biocompatible. For hydrogels photopolymerized with 0.15% (w/v) Irgacure 2959, the cell survival decreased significantly compared to the controls. It is important to note from our photoinitiator study, relative cell survival for HASMCs at 0.16% (w/v) Irgacure 2959 (but without polymers) was 0.25 ± 0.01. However, from Figure 5 we can see that relative cell survival for HASMCs exposed to media incubated hydrogels photopolymerized with 0.15% (w/v) Irgacure 2959 was 0.48 ± 0.19. Therefore, these results may be used to conclude that the reactive PEGDA macromers indeed react with the free radicals and reduce the system cytotoxicity.
Conclusion
The focus of this study was to investigate the cytotoxicity of the photoinitiating components of our composite hydrogel system on human aortic smooth muscle cells. Studies conducted to evaluate the effects of UV dose, photoinitiator concentrations, and combined effects conclusively showed that the photoinitiator and free radicals were the most cytotoxic components. At the same time, it was found that UV light did not significantly affect cell survival. Additionally, it was shown that ascorbic acid could significantly increase cell survival, but it also increased the gelation times of the hydrogel, potentially inducing cellular damage due to prolonged exposure times. Finally, relative cell survival was evaluated after exposure of HASMCs to media incubated with our composite hydrogels. Future work will involve testing other antioxidants or strategies to minimize cytotoxicity and optimizing our composite hydrogel system by altering various components in our system such as molecular weights of PEG and concentrations of nanoparticles.
Acknowledgments
This work is supported by the New Faculty Start-up and Seed-grant funds provided by the Department of Bioengineering, the College of Engineering, and the University of Texas at Arlington. We would also like to acknowledge the financial support from the AHA Scientist Development Award 0735270N and NIH grants HL082644 and HL091232 (K.N.).
References
- 1.Rivard A, Andrâes V. Vascular smooth muscle cell proliferation in the pathogenesis of atherosclerotic cardiovascular diseases. Histology and histopathology. 2000;15(2):557–71. doi: 10.14670/HH-15.557. [DOI] [PubMed] [Google Scholar]
- 2.Indolfi C, Coppola C, Torella D, Arcucci O, Chiariello M. Gene therapy for restenosis after balloon angioplasty and stenting. Cardiology in review. 1999;7(6):324–31. doi: 10.1097/00045415-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Ip JH, Fuster V, Badimon L, Badimon J, Taubman MB, Chesebro JH. Syndromes of accelerated atherosclerosis: role of vascular injury and smooth muscle cell proliferation. Journal of the American College of Cardiology. 1990;15(7):1667–87. doi: 10.1016/0735-1097(90)92845-s. [DOI] [PubMed] [Google Scholar]
- 4.Ramanan RM, Chellamuthu P, Tang L, Nguyen KT. Development of a temperature-sensitive composite hydrogel for drug delivery applications. Biotechnol Prog. 2006;22(1):118–25. doi: 10.1021/bp0501367. [DOI] [PubMed] [Google Scholar]
- 5.Hill-West JL, Chowdhury SM, Slepian MJ, Hubbell JA. Inhibition of thrombosis and intimal thickening by in situ photopolymerization of thin hydrogel barriers. Proc Natl Acad Sci U S A. 1994;91(13):5967–71. doi: 10.1073/pnas.91.13.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An Y, Hubbell JA. Intraarterial protein delivery via intimally adherent bilayer hydrogels. J Control Release. 2000;64:205–15. doi: 10.1016/s0168-3659(99)00143-1. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury SM, Hubbell JA. Adhesion prevention with ancrod released via a tissue-adherent hydrogel. J Surg Res. 1996;61(1):58–64. doi: 10.1006/jsre.1996.0081. [DOI] [PubMed] [Google Scholar]
- 8.Lu S, Anseth KS. Photopolymerization of multilaminated poly(HEMA) hydrogels for controlled release. J Control Release. 1999;57(3):291–300. doi: 10.1016/s0168-3659(98)00125-4. [DOI] [PubMed] [Google Scholar]
- 9.Peppas NA, Keys KB, Torres-Lugo M, Lowman AM. Poly(ethylene glycol)-containing hydrogels in drug delivery. J Control Release. 1999;62(1-2):81–7. doi: 10.1016/s0168-3659(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 10.Scott RA, Peppas NA. Highly crosslinked, PEG-containing copolymers for sustained solute delivery. Biomaterials. 1999;20(15):1371–80. doi: 10.1016/s0142-9612(99)00040-x. [DOI] [PubMed] [Google Scholar]
- 11.Cruise GM, Hegre OD, Lamberti FV, Hager SR, Hill R, Scharp DS, Hubbell JA. In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes. Cell Transplant. 1999;8(3):293–306. doi: 10.1177/096368979900800310. [DOI] [PubMed] [Google Scholar]
- 12.Cruise GM, Hegre OD, Scharp DS, Hubbell JA. A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets. Biotechnol Bioeng. 1998;57(6):655–65. doi: 10.1002/(sici)1097-0290(19980320)57:6<655::aid-bit3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Hill RS, Cruise GM, Hager SR, Lamberti FV, Yu X, Garufis CL, Yu Y, Mundwiler KE, Cole JF, Hubbell JA, et al. Immunoisolation of adult porcine islets for the treatment of diabetes mellitus. The use of photopolymerizable polyethylene glycol in the conformal coating of mass-isolated porcine islets. Ann N Y Acad Sci. 1997;831:332–43. doi: 10.1111/j.1749-6632.1997.tb52208.x. [DOI] [PubMed] [Google Scholar]
- 14.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51(2):164–71. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R. Transdermal photopolymerization for minimally invasive implantation. Proc Natl Acad Sci U S A. 1999;96(6):3104–7. doi: 10.1073/pnas.96.6.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Yaremchuk M, Langer R. Transdermal photopolymerization of poly(ethylene oxide)-based injectable hydrogels for tissue-engineered cartilage. Plast Reconstr Surg. 1999;104(4):1014–22. doi: 10.1097/00006534-199909040-00017. [DOI] [PubMed] [Google Scholar]
- 17.Sawhney AS, Pathak CP, Hubbell JA. Interfacial photopolymerization of poly(ethylene glycol)-based hydrogels upon alginate-poly(l-lysine) microcapsules for enhanced biocompatibility. Biomaterials. 1993;14(13):1008–16. doi: 10.1016/0142-9612(93)90194-7. [DOI] [PubMed] [Google Scholar]
- 18.Cruise GM, Scharp DS, Hubbell JA. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials. 1998;19(14):1287–94. doi: 10.1016/s0142-9612(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 19.Pathak CP, Sawhney AS, et al. Rapid photopolymerization of immunoprotective gels in contact with cells and tissue. J Am Chem Soc. 1992;114:8311–2. [Google Scholar]
- 20.Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed. 2000;11(5):439–57. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23(22):4307–14. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 22.Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26(11):1211–8. doi: 10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Quick DJ, Anseth KS. DNA delivery from photocrosslinked PEG hydrogels: encapsulation efficiency, release profiles, and DNA quality. J Control Release. 2004;96(2):341–51. doi: 10.1016/j.jconrel.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Quick DJ, Macdonald KK, Anseth KS. Delivering DNA from photocrosslinked, surface eroding polyanhydrides. J Control Release. 2004;97(2):333–43. doi: 10.1016/j.jconrel.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Bhadra D, Bhadra S, Jain P, Jain NK. Pegnology: a review of PEG-ylated systems. Pharmazie. 2002;57(1):5–29. [PubMed] [Google Scholar]
- 26.Atsumi T, Murata J, Kamiyanagi I, Fujisawa S, Ueha T. Cytotoxicity of photosensitizers camphorquinone and 9-fluorenone with visible light irradiation on a human submandibular-duct cell line in vitro. Arch Oral Biol. 1998;43(1):73–81. doi: 10.1016/s0003-9969(97)00073-3. [DOI] [PubMed] [Google Scholar]
- 27.Wu JY, Liu SQ, Heng PW, Yang YY. Evaluating proteins release from, and their interactions with, thermosensitive poly (N-isopropylacrylamide) hydrogels. J Control Release. 2005;102(2):361–72. doi: 10.1016/j.jconrel.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Gao J, Hu Z. Optical properties of N-isopropylacrylamide microgel spheres in water. Langmuir. 2002;18:1360–67. [Google Scholar]
- 29.Ono K, Saito Y, Yura H, Ishikawa K, Kurita A, Akaike T, Ishihara M. Photocrosslinkable chitosan as a biological adhesive. J Biomed Mater Res. 2000;49(2):289–95. doi: 10.1002/(sici)1097-4636(200002)49:2<289::aid-jbm18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 30.Murakami Y, Yokoyama M, Okano T, Nishida H, Tomizawa Y, Endo M, Kurosawa H. A novel synthetic tissue-adhesive hydrogel using a crosslinkable polymeric micelle. J Biomed Mater Res A. 2007;80(2):421–7. doi: 10.1002/jbm.a.30911. [DOI] [PubMed] [Google Scholar]
- 31.Hanks CT, Strawn SE, Wataha JC, Craig RG. Cytotoxic effects of resin components on cultured mammalian fibroblasts. J Dent Res. 1991;70(11):1450–5. doi: 10.1177/00220345910700111201. [DOI] [PubMed] [Google Scholar]
- 32.Manevich Y, Sweitzer T, Pak JH, Feinstein SI, Muzykantov V, Fisher AB. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci U S A. 2002;99(18):11599–604. doi: 10.1073/pnas.182384499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naji L, Carrillo-Vico A, Guerrero JM, Calvo JR. Expression of membrane and nuclear melatonin receptors in mouse peripheral organs. Life Sci. 2004;74(18):2227–36. doi: 10.1016/j.lfs.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36(1):1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 35.Bonorden WR, Pariza MW. Antioxidant nutrients and protection from free radicals. New York: Raven Press; 1994. [Google Scholar]
- 36.Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18(10):872–9. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]


