Abstract
Background
Distinct Crohn’s disease (CD) phenotypes correlate with antibody reactivity to microbial antigens. We examined the association between antibody response to two new flagellins called A4-Fla2 and Fla-X, anti-Saccharomyces cerevisiae antibodies (ASCA), anti-neutrophil cytoplasmic antibodies (p-ANCA), anti-pancreas antibodies (PAB), NOD2 mutations, and clinical CD phenotypes (according to Vienna criteria).
Methods
All above mentioned antibodies as well as NOD2 mutations (R702W, G908R, and L1007fsinsC) were determined in 252 CD patients, 53 with ulcerative colitis (UC), and 43 healthy controls (HC) and correlated with clinical data.
Results
A seroreactivity for A4-Fla2/Fla-X/ASCA/p-ANCA/PAB (in percent) was found in 59/57/62/12/22 of CD patients, 6/6/4/51/0 of UC patients, and 0/2/5/0/0 of healthy controls. CD behaviour: 37% B1, 36% B2, and 27% B3. In multivariate logistic regression, antibodies to A4-Fla2, Fla-X, and ASCA were significantly associated with stricturing phenotype (P=0.027, P=0.041, P<0.001), negative associations were found with inflammatory phenotype (P=0.001, P=0.005, P<0.001). Antibodies to A4-Fla2, Fla-X, ASCA, and NOD2 mutations significantly associated with small bowel disease (P=0.013, P=0.01, P<0.001, P=0.04) whereas ASCA were correlated with fistulizing disease (P=0.007), and small bowel surgery (P=0.009). Multiple antibody responses against microbial antigens were associated with stricturing (P<0.001), fistulizing disease (P=0.002), and small bowel surgery (P=0.002).
Conclusions
Anti-flagellin antibodies and ASCA are strongly associated with complicated CD phentoypes. CD patients with serum reactivity against multiple microbes have the greatest frequency of strictures, perforations, and small bowel surgery. Further prospective longitudinal studies are needed to show that antibody-based risk stratification improves the clinical outcome of CD patients.
Keywords: Anti-Saccharomyces cerevisiae antibodies (ASCA), Anti-pancreas antibodies (PAB), Anti-flagellin antibodies, Complicated CD phenotype, NOD2 mutations
Introduction
Chronic intestinal inflammation in inflammatory bowel disease (IBD) results from an aberrant mucosal immune response to the microbiota of the gastrointestinal tract in genetically susceptible individuals.1 The luminal flora is essential to perpetuate the inflammatory process. In IBD models, mice develop colitis only in the presence of luminal bacteria.2 Studies in humans have shown that the fecal stream is critical to disease development and progression.3 A loss of tolerance to specific bacterial antigens and autoantigens has been demonstrated in IBD patients.4 There are many reports in the literature referring to the reactivity to different microbial antigens in IBD patients.5
So far, anti-Saccharomyces cerevisiae mannan antibodies (ASCA) and anti-neutrophil cytoplasmic antibodies (ANCA) are the most widely studied markers.6 ASCA, occurring mainly in Crohn’s disease (CD) patients, recognize carbohydrate epitopes of yeast cell wall mannan. ANCA are predominantly found in ulcerative colitis (UC) patients as atypical p-ANCA, characterized by a broad inhomogeneous rim-like staining of the nuclear periphery. p-ANCA in CD were found to be associated with UC-like Crohn’s disease (mainly left-sided colitis).7 Antibodies to exocrine pancreas (PAB) are highly specific for Crohn’s disease, however, due to their low prevalence, the sensitivity is only moderate.8 The exact antigen for PAB has not yet been elucidated. Flagellin, the primary structural component of bacterial flagella, is recognized by Toll-like receptor 5 and activates the innate as well as adaptive immunity. Flagellins represent dominant antigens in CD.9 Phylogenic analyses have predicted that the origin of the flagellins is the Clostridium phylogenetic cluster XIVa.10 Duck et al. have isolated and characterized a number of flagellated bacteria from the Clostridium cluster XIVa.11 One particular bacterial strain, A4, expresses a flagellin related to the Fla-X flagellin to which individuals with CD are seropositive. Sequence comparisons of the 16S rDNA has placed A4 to the family of Lachnospiraceae (domain Bacteria, phylum Firmicutes, class Clostridia, order Clostridiales). The flagellins A4-Fla2 and Fla-X are quite similar in the amino acid sequence, Fla2 is one of the six flagellins expressed by A4 bacteria.
An association between clinical phenotypes and sero-reactivity to microbes and self-antigens respectively has already been reported by several groups. Targan et al. demonstrated that anti-CBir1 expression was associated independently with small bowel, internal-penetrating, and fibrostenosing disease features.12 According to these findings, Mow et al. demonstrated an association between antibody responses to microbial antigens and complications of small bowel Crohn’s disease.13
Besides serological responses, genetic alterations are also linked with disease characteristics. Ahmad et al.14 demonstrated an association between mutations in the NOD2 genes and ileal disease. NOD2 mutations are found in 20–40% of European and American CD patients.15 Several groups have reported an association of NOD2 mutations with fibrostenotic disease, small bowel disease, and a younger age of disease onset.16 In this study we aim to evaluate the phenotypic associations of two new anti-flagellin antibodies in comparison to ASCA, p-ANCA, PAB, and NOD2 mutations. We hypothesize that these new anti-flagellin antibodies are associated with complicated CD. Thus, these biomarkers may be useful in clinical practice for the identification of patients most likely to develop CD complications.
Materials and Methods
Patients
The cohort of 305 patients was drawn from patients assessed at the Department of Gastroenterology of the University of Bern from August 2005 to September 2007. The study was performed with the approval of the local ethics committees. The diagnosis of CD and UC was based on standard endoscopic, histological, and radiographic features.17 Patients with indeterminate colitis as well as other reasons for enterocolitis than IBD were excluded (infectious enterocolitis, medication-induced enterocolitis, ischemic enterocolitis). The controls were healthy persons willing to provide blood samples. All healthy controls were free of symptoms and had a normal clinical examination and abdominal ultrasonography, none of them had a patient with IBD in their families. Except for birth control pills taken by some women, the healthy controls were not taking any medication on a regular basis. For understandable reasons, the healthy control group had no endoscopic workup.
Phenotype Designations
The CD patients were allocated to different phenotypes based on the published Vienna criteria.18 The Vienna classification discriminates between nonstricturing-nonpenetrating = inflammatory and stricturing and fistulizing phenotype.
Phenotype designation was performed at the time of consent for serological testing. Most patients (n=217, 86%) were enrolled during the first consultation in the IBD clinic, some were enrolled at the time of surgery. A small proportion of patients (n=35, 14%) were updated in phenotype because of development of either stenosis or fistulizing-penetrating disease during the 25-month enrollment period. Surgery occurred mostly before enrollment or at the time of enrollment. If CD-related surgery was performed after enrollment, updates were made in the database. Significant surgery included small bowel or colonic segment resections, ileocolonic resections, colectomies, proctocolectomies, and stricturoplasties. The disease location was based on endoscopic, histopathologic, and radiographic evidence of chronic inflammation. Patients characterized as having small bowel disease included those with only small bowel disease and those with both small bowel and colonic disease. Phenotype and disease location were assigned after discussion of the clinical data by IBD physicians (AMS, FS). Both IBD physicians were blinded to the results of serological information.
Disease duration was defined as the time in years from the initial diagnosis of IBD until inclusion in the study (with serum sampling).
Genotyping
DNA was extracted from peripheral blood samples, using the QIAamp DNA Blood Minikit (QIAGEN, Hombrechtikon, Switzerland) according to the manufacturer’s protocol. The allelic variants G908R, L1007fsinsC, and R702W were assayed by polymerase chain reaction (PCR) amplification followed by restriction fragment length polymorphism (RFLP) analysis as described elsewhere.19 CD patients with heterozygous as well as compound heterozygous and homozygous mutant alleles were counted as positive NOD2 mutation. The scientist performing the NOD2 analyses (EV) was blinded to the clinical and serological data.
IBD Antibodies
The following antibodies were measured: Antibodies to the flagellins A4-Fla2 and Fla-X, ASCA, PAB, and p-ANCA. The laboratory scientists (TS, SM, BF, BS) were blinded to the patient diagnosis and the study hypothesis. All ELISA were read on a microplate reader (BioTek Instruments, Winooski, VT) at an OD of 450nm.
Flagellin ELISA
We evaluated two flagellins. Both flagellin A4-Fla2 as well as Fla-X were kindly provided by CO Elson, MD, University of Alabama in Birmingham. Both ELISA for A4-Fla2 and Fla-X were first standardized and validated in a cohort of 78 CD patients, 32 with UC and 30 healthy controls. ELISA plates were coated overnight either with 1μg/mL A4-Fla2 or Fla-X and then blocked with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 2 hours. Plates were washed and serum in duplicates was added at a dilution of 1:1000 in 1% BSA-PBS and incubated for 60 minutes. Antibodies against flagellin in patient sera were detected using a peroxidase-marked anti-human IgA and IgG goat antibody respectively (dilution 1:5000). After another wash, the plates were incubated with tetramethylbenzidien (TMB) substrate for 15 minutes (Sigma, Buchs, Switzerland). The reaction was stopped with 0.5M sulphuric acid. A patient was considered positive when either anti-flagellin IgG, IgA, or both were present. The cutoff was determined by measuring all 43 sera from the healthy controls. The mean of the OD450 of these healthy control sera plus two times the standard deviation was determined as cutoff between negative and positive antibody titers against flagellin.
ASCA ELISA
Phosphopeptidomannans from baker’s yeast (Hefe Vital Gold, Deutsche Hefewerke, Nürnberg, Germany) were extracted as previously described.20 Briefly, ninety-six well ELISA plates (MaxiSorb, Nunc, Wiesbaden, Germany) were coated with 100μL of 0.25μg/mL phosphopeptidomannans in carbonate-bicarbonate buffer, pH 9.6 and incubated overnight at 4°C. Serum diluted 1/1000 was applied to the coated plates in duplicates. The plates were incubated for 1h at 37°C. Secondary antibody was added and plates were incubated for 1h at room temperature. The secondary antibodies (Sigma, Buchs, Switzerland) were as follows: goat anti-human IgA (alpha-chain specific) peroxidase conjugate, and goat anti-human IgG (gamma-chain specific) peroxidase conjugate, both at 1/5000 (Sigma). The plates were developed using TMB substrate and the reaction was stopped with sulfuric acid. The absorbance was read at 450nm. The mean absorbance value for the healthy control sera plus two standard deviations was used to discriminate between positive and negative subjects. A patient was considered ASCA+, when being positive for ASCA IgG, IgA, or both. To define arbitrary units for ASCA titers, one serum highly positive for ASCA was used to obtain a standard curve at dilutions ranging from 1/100 to 1/12800. The curve was fitted using a four parameter logistic function to the logarithmically scaled dilutions.
PAB-Immunofluorescence
Frozen 5μm sections of human pancreas (blood group 0) were used. Patients sera were incubated for 30 min in a moist chamber at room temperature with 50μL serum samples diluted 1:100 in PBS. After washing in PBS, slides were incubated with polyvalent fluorescein-conjugated rabbit anti-human immunoglobulin (Dako, Baar, Switzerland) (diluted 1:100 in PBS) for 30 min. After washing, the sections were embedded in Immuno-Mount (Shandon, Pittsburgh, PA) and read using a Nikon fluorescence microscope. All slides were coded for anonymity and read independently by two investigators (FS, BS).
p-ANCA testing was done on cytospins of neutrophils as described elsewhere.21
Statistical Analysis
Clinical data were retrieved from our IBD database (Access 2000, Microsoft Switzerland Ltd Liab. Co, 8304 Wallisellen Switzerland). All statistical examinations were performed with a statistical package program (stata Vs 9, College Station, Texas, USA). A power analysis revealed that at least 163 CD patients had to be included (alpha 5%, beta 10%, d=0.05). Chi-squared tests were used for analysis of unpaired categorical data. Multivariate logistic regression modeling was performed to determine the primary associations among qualitative serological responses with disease phenotypes. Odds ratios (OR) and 95% confidence intervals were calculated to compare the odds of positive serum reactivity toward antigens in the group with a certain disease characteristic to these odds in the group of patients without such a characteristic. A P<0.05 was considered statistically significant. A Bonferoni adjustment was performed in case of multiple testing. For evaluation of the association between disease phenotype characteristics and the combination of the level of the immune response toward A4-Fla2, Fla-X, and oligomannan, the sums of quartile scores for A4-Fla2, Fla-X, and ASCA were calculated.13 For each antigen, patients whose antibody levels were in the 1st, 2nd, 3rd, and 4th quartile of the distribution were assigned a quartile score of 1, 2, 3, and 4 respectively. By adding individual quartile scores for each microbial antigen, a quartile sum score (range, 3–12) was obtained to represent the cumulative quantitative immune response against all 3 microbial antigens for each patient. The Cochran-Armitage test for trend was used to evaluate whether there exists a linear trend in the proportion of patients with a disease phenotype characteristic as the level of antibody responses increased by quartiles. A P-value (P trend) <0.05 indicates that the linear trend is statistically significant.
Results
Clinical Characteristics of the Study Population
The clinical characteristics of the study population are shown in Table 1. The CD patients are comparable with the UC patients regarding mean age, median age at onset of disease, as well as the mean disease duration.
Table 1.
Clinical characteristics of the study population
| Crohn’s disease | Ulcerative colitis | HC | |
|---|---|---|---|
| Number of patients | 252 | 53 | 43 |
|
| |||
| Female | 117 (46%) | 21 (40%) | 27 (63%) |
|
| |||
| Age (years): mean±SD, range | 42±17 (18–82) | 40±12 (22–73) | 37±8 (23–52) |
|
| |||
| Age at onset (years) | 28±11 (16–69) | 30±9 (14–70) | |
|
| |||
| Disease duration (years) | 11±13 (1–44) | 10±11 (2–34) | |
|
| |||
| Disease phenotype | – | ||
| - Inflammatory (B1) | 94 (37%) | - | |
| - Stricturing (B2) | 91 (36%) | ||
| - Fistulizing (B3) | 67 (27%) | ||
|
| |||
| Disease location | Small bowel 192 (76%) | Proctitis 11% | - |
| Proctosigmoiditis 23% | |||
| Left sided 13% | |||
| Extensive 9% | |||
| Pancolitis 44% | |||
|
| |||
| Small bowel surgery | 67 (27%) | - | - |
|
| |||
| Small bowel and colonic surgery | 91 (36%) | - | - |
Abbreviations: HC, healthy controls
Serological and Genetic Characteristics of the Study Population
The serological and genetic characteristics are presented in Table 2. The frequency of anti-flagellin A4-Fla2 and Fla-X serum reactivity was 59% and 57% in CD patients and 6% in UC patients respectively. The agreement between antibodies (IgG and/or IgA) to flagellins A4-Fla2 and Fla-X was overall 85%. In CD patients the agreement was 82%, in UC patients 73%, compared to 96% in HC. In all patient groups, the frequency of IgA antibodies to flagellins was below 20 percent. Figure 1 shows the comparative scatter plot of the serological responses toward A4-Fla2, Fla-X and ASCA.
Table 2.
Serologic and genetic characteristics of the patient cohort
| Crohn’s disease (n=252) | Ulcerative colitis (n=53) | HC (n=43) | |
|---|---|---|---|
| Serological Profile (%) | |||
| - A4-Fla2-positive | 59% | 6% | 0% |
| - Fla-X-positive | 57% | 6% | 2% |
| - ASCA-positive | 62% | 4% | 5% |
| - p-ANCA-positive | 12% | 51% | 0% |
| - PAB-positive | 22% | 0% | 0% |
|
| |||
| NOD2 genotype for SNP 8, 12, and 13 (%) | |||
| - No mutations | 64% | 85% | 98% |
| - Heterozygous | 29% | 15% | 2% |
| - Compound heterozygous/homozygous | 7% | 0% | 0% |
Abbreviations: HC, healthy controls
Figure 1.
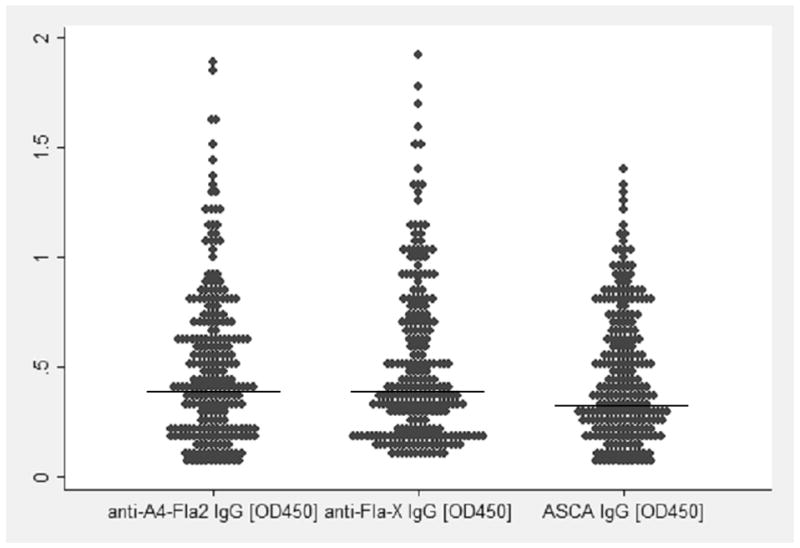
The comparative scatter plot illustrates the spread of the values (extinctions at OD450) for anti-A4-Fla2 IgG, anti-Fla-X IgG, and ASCA IgG. The horizontal line discriminates the positive values (above the line) from the negatives (below the line).
In the CD group, 149 patients were positive for A4-Fla2, of these, 140 (94%) had IgG, 21 (14%) had IgA, and 9 (6%) had IgA only without IgG. The results for antibodies to Fla-X in CD patients are as follows: 144 patients tested positive for Fla-X, of these, 134 (93%) had IgG, 18 (13%) had IgA, and only 10 (7%) had IgA without IgG.
ASCA-positivity was found in 62% of CD patients, in 4% of UC patients, and in 5% of healthy controls. Compound heterozygous and homozygous mutant NOD2 alleles were found in 7% of CD patients, but not in UC patients or healthy controls.
Antibodies to A4-Fla2, Fla-X, and ASCA are associated with complicated CD phenotypes, small bowel disease, small bowel surgery, and negatively associated with inflammatory CD
We evaluated if the antibody responses to different microbial antigens and autoantigens are associated with distinct disease phenotypes. Table 3 shows the proportion of patients with each phenotype segregated by response to flagellin A4-Fla2, Fla-X, ASCA, p-ANCA, and PAB. Anti-A4-Fla2 and anti-Fla-X were significantly associated with the occurrence of stricturing and fistulizing phenotype, as well as small bowel disease and small bowel surgery. A negative association of anti-A4-Fla2 and anti-Fla-X was found with inflammatory CD phenotype.
Table 3.
Antibody responses (in percent) to different microbial antigens and autoantigens in association with distinct disease phenotypes in CD patients
| Clinical phenotype | Stricturing | Fistulizing | Inflammatory | Small bowel disease | Small bowel surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | ||
| A4-Fla2 | % Positive | 72.5 | 52.8 | 72.4 | 55.5 | 40.5 | 71.7 | 65.7 | 41.5 | 71.7 | 55.9 |
| P value | 0.006 | 0.029 | <0.001 | 0.002 | 0.03 | ||||||
| Fla-X | % Positive | 67.5 | 52.1 | 72.4 | 52.4 | 38.1 | 69.6 | 65.1 | 37.5 | 71.7 | 51.9 |
| P value | 0.023 | 0.01 | <0.001 | <0.001 | 0.01 | ||||||
| ASCA | % Positive | 85 | 49.3 | 75.9 | 57.3 | 33.3 | 79.7 | 75.9 | 21.4 | 83 | 53.7 |
| P value | <0.001 | 0.015 | <0.001 | <0.001 | <0.001 | ||||||
| p-ANCA | % Positive | 2.5 | 16.9 | 13.8 | 11 | 19 | 7.2 | 7.2 | 23.2 | 5 | 13.6 |
| P value | 0.001 | 0.566 | 0.008 | 0.001 | 0.072 | ||||||
| PAB | % Positive | 27.5 | 20.4 | 27.6 | 20.7 | 15.7 | 27.8 | 25.9 | 13.5 | 31 | 19.7 |
| P value | 0.285 | 0.264 | 0.039 | 0.063 | 0.08 | ||||||
| NOD2 | % Positive | 38.9 | 34.1 | 36.8 | 35.5 | 32.1 | 38 | 40.9 | 25 | 43.9 | 32.5 |
| P value | 0.558 | 0.883 | 0.471 | 0.025 | 0.104 | ||||||
Abbreviations: Yes, phenotype present; No, phenotype absent
ASCA showed the most significant associations with complicated CD phenotypes (strictures and fistulae), small bowel involvement, as well as small bowel surgery. We found a significantly negative correlation between ASCA and inflammatory CD phenotype. p-ANCA were positively associated with inflammatory (“UC-like”) disease and negatively associated with stricturing phenotype, small bowel disease, and small bowel surgery. We found PAB to be significantly negatively associated with inflammatory CD, and furthermore, there was a trend toward small bowel disease and small bowel surgery (P<0.1).
NOD2 mutations are associated with small bowel disease in CD patients
As shown in Table 3, there was a clear correlation between NOD2 mutations and small bowel disease (P=0.025) and a trend towards a need for small bowel surgery (P=0.1). However, NOD2 mutations were not associated with distinct disease phenotypes.
Logistic regression modeling evaluating the association of antibody responses and NOD2 mutations with disease phenotypes
We performed multivariate logistic regression analysis to determine which antibody responses were independently associated with disease characteristics. The odds ratios as well as the corresponding P-values are shown in Table 4.
Table 4.
Clinical features: Results of Multivariate Logistic Regression (odds ratios and P values).
| Variable | Stricturing | Fistulizing | Inflammatory | Small bowel disease | Small bowel surgery | |
|---|---|---|---|---|---|---|
| A4-Fla2 | Odds | 3.41 | 2.38 | 0.171 | 3.87 | 1.83 |
| P-value | 0.027 | 0.078 | 0.001* | 0.013 | 0.137 | |
| Fla-X | Odds | 2.95 | 2.04 | 0.214 | 4.26 | 2.27 |
| P-value | 0.041 | 0.115 | 0.005* | 0.01 | 0.094 | |
| ASCA | Odds | 7.49 | 3.02 | 0.2 | 7.52 | 2.99 |
| P-value | <0.001 | 0.007 | <0.001* | <0.001 | 0.009 | |
| p-ANCA | Odds | 0.15 | 1.4 | 2.6 | 0.18 | 0.37 |
| P-value | 0.016* | 0.484 | 0.018 | 0.016* | 0.137 | |
| PAB | Odds | 1.04 | 1.34 | 0.67 | 1.52 | 1.48 |
| P-value | 0.91 | 0.413 | 0.329 | 0.388 | 0.276 | |
| NOD2 | Odds | 1.08 | 1.2 | 0.99 | 2.45 | 1.52 |
| P-value | 0.90 | 0.88 | 0.991 | 0.04 | 0.294 | |
negative association.
Antibodies to flagellin A4-Fla2 and Fla-X were significantly associated with stricturing disease and small bowel disease. A trend was found for fistulizing phenotype and small bowel surgery. We detected a negative correlation of anti-flagellin antibodies and inflammatory CD phenotype.
ASCA had the strongest association with stricturing and fistulizing CD phenotype as well as small bowel disease and small bowel surgery, a significantly negative association was found with inflammatory CD phenotype. We found NOD2 mutations independently correlated with small bowel disease (P=0.04), however, there was no significant association with complicated CD phenotypes.
Complicated CD phenotypes are more frequently found in CD patients with multiple antibody responses to microbial antigens
Immune reactivity toward more than one of the described microbial antigens (A4-Fla2, FlaX, and oligomannan) was found in several patients. We illustrate the distribution of antimicrobial antibodies in CD patients in Figure 2 by a Venn diagram. Of note, only 21.1% of patients were negative for all anti-microbial antibodies.
Figure 2.
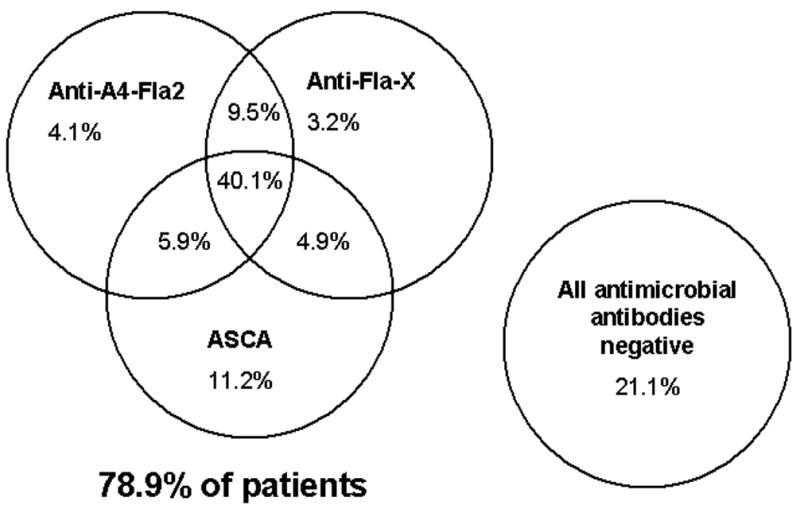
The Venn diagram shows the relationship between the antimicrobial biomarkers (anti A4-Fla2, anti-Fla-X, ASCA) in the CD cohort (n=252) by presence vs absence. The percentage of patients positive for each marker, any combination of 2 markers, and all markers is shown.
We questioned whether patients with antibody responses against an increasing number of microbial antigens would have an increased likelihood of suffering from a complicated CD phenotype. Table 5 shows the relationship between serum reactivity toward 1, 2, or all 3 of the microbial antigens (A4-Fla2, Fla-X, oligomannan) and CD phenotype. We found that CD patients with increasing number of antibodies suffered significantly more often from a complicated CD phenotype and small bowel disease as well as small bowel surgery.
Table 5.
Disease characteristics in patients with antibody reactivity against microbial antigens.
| Number of antibodies toward microbes* | |||||||
|---|---|---|---|---|---|---|---|
| Phenotype | 0 | 1 | 2 | 3 | P | OR | 95%-CI |
| Stricturing (%) | 6.3 | 20 | 16.2 | 57.5 | <0.001 | 3.3 | 1.6–6.9 |
| Fistulizing (%) | 8.8 | 14 | 31.6 | 45.6 | 0.002 | 1.7 | 1.3–2.6 |
| Inflammatory (%) | 44 | 20.2 | 16.8 | 19 | 0.001 | 0.09 | 0.02–0.04 |
| Small bowel disease (%) | 12.6 | 19.8 | 18 | 49.6 | <0.001 | 8.4 | 2.5–19.3 |
| Small bowel surgery (%) | 6.7 | 17 | 22 | 54.3 | 0.002 | 1.5 | 1.1–2.1 |
Numbers represent the percentage of antibody frequency in a specific disease phenotype. The microbial antigens include A4-Fla2, Fla-X, and ASCA.
Abbreviations: OR: odds ratio; CI: confidence interval.
In summary, this analysis indicates that patients who have antibodies against a higher number of microbial antigens (A4-Fla2, Fla-X, and oligomannan) are at increased risk for stricturing and fistulizing disease, small bowel involvement, as well as consecutive small bowel surgery compared to patients with no serological response against these microbial antigens.
Higher levels of antibody response against individual and multiple microbial antigens are associated with a higher risk for complicated CD phenotype and small bowel disease
The association between qualitative antibody responses against microbial antigens and disease phenotypes has already been shown, however, such analyes do not represent the concentration of antibodies (quantitative analysis). Landers et al. demonstrated that the level of antibody responses toward microbial antigens could be analyzed by breaking it down into quartiles.4
As sensitivity of PAB and pANCA for CD detection was low and as no significant association with complicated CD phenotypes was detected, we focused our analyses on anti-microbial antigens. We sought to investigate the association between the levels of antibody responses divided into quartiles, against A4-Fla2, Fla-X, and oligomannan, and the frequency of the various CD phenotypes. The population was subdivided into 4 quartiles according to OD450 values obtained for anti-A4-Fla2, Fla-X, and ASCA. Quartile sums were calculated by the addition of each individual’s quartile values for each microbial antigen (range 3–12). The patients with the lowest levels of reactivity against all 3 antigens had a quartile score of 3, whereas patients with the highest levels of antibody reactivity against all 3 antigens had a quartile sum score of 12. Figure 3 shows the quartile analysis of the CD cohort.
Figure 3.
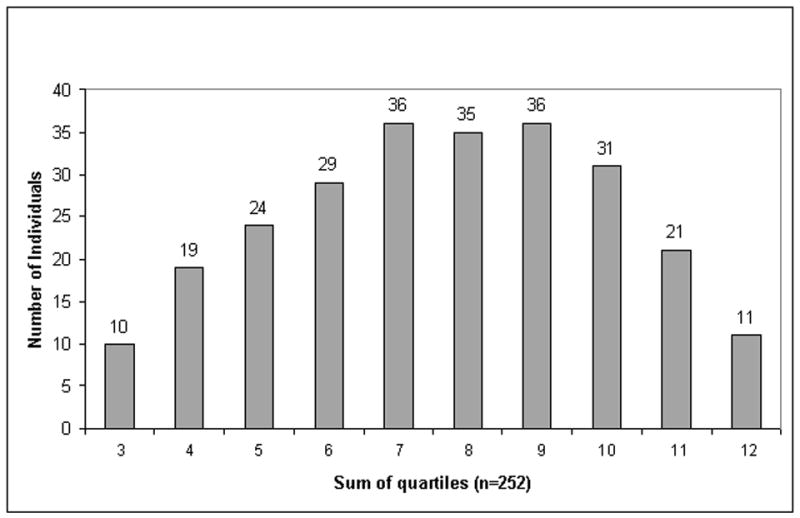
The distribution of quartile sums is shown. For each of the different markers (anti-A4-Fla2, anti-Fla-X, and ASCA) the population was subdivided into four equal quartiles (each n=63) based on the antibody response (ELISA units). Quartile sums were calculated by adding individual quartile scores for each microbial antigen, ranging from 3 to 12.
Table 6 shows the results of quartile analysis for A4-Fla2, Fla-X, and ASCA for each disease characteristic. There was an increasing percentage of patients with stricturing disease, fistulizing disease, small bowel disease, and small bowel surgery and a decreasing likelihood of inflammatory disease phenotype as the levels of the antibody responses against microbial antigens increased. We further compared the frequency of disease characteristics in the patients with quartile scores of 10–12 (representing CD patients with high antimicrobial reactivity) with the frequency in patients with quartile scores from 3–5. We found the following associations for patients with quartile sum scores of 10–12: stricturing phenotype (OR, 3.2; 95%-CI, 1.8–5.4; P <0.001), fistulizing phenotype (OR, 2.7; 95%-CI, 1.6–4.2; P=0.013), inflammatory phenotype (OR, 0.2; 95%-CI, 0.1–0.5; P<0.001), small bowel disease (OR, 4.1; 95%-CI 1.9–8.2; P <0.001), small bowel surgery (OR, 1.6; 95%-CI, 0.7–2.8; P=0.358).
Table 6.
Association of individual immune response quartiles to ASCA, A4-Fla2, and Fla-X with disease phenotype.
| ASCA | Percent of antibodies in quartile | ||||||
|---|---|---|---|---|---|---|---|
| Phenotype | 1 | 2 | 3 | 4 | P trend | OR | 95%-CI |
| Stricturing (%) | 8.9 | 22.8 | 32.9 | 35.4 | <0.001 | 3.1 | 1.9–4.6 |
| Fistulizing (%) | 8.6 | 31 | 32.8 | 27.6 | 0.001 | 2.5 | 1.6–3.9 |
| Inflammatory (%) | 38.1 | 48.8 | 13.1 | 0 | 0.003 | 0.27 | 0.1–0.5 |
| Small bowel disease (%) | 16.8 | 28.7 | 31.7 | 22.8 | <0.001 | 5.1 | 1.9–15.6 |
| Small bowel surgery (%) | 11.7 | 25 | 35 | 28.3 | 0.017 | 2.2 | 1.3–3.2 |
| A4-Fla2 | Percent of antibodies in quartile | ||||||
| Phenotype | 1 | 2 | 3 | 4 | P trend | OR | 95%-CI |
| Stricturing (%) | 16.3 | 22.5 | 32.5 | 28.7 | 0.001 | 2.1 | 1.3–3.8 |
| Fistulizing (%) | 15.5 | 24.1 | 34.5 | 25.9 | 0.01 | 1.9 | 1.3–3.1 |
| Inflammatory (%) | 35.7 | 33.4 | 21.4 | 9.5 | <0.001 | 0.26 | 0.1–0.5 |
| Small bowel disease (%) | 18.6 | 25.7 | 34.1 | 21.6 | 0.035 | 2 | 1.2–4.3 |
| Small bowel surgery (%) | 16.7 | 26.7 | 38.3 | 18.3 | 0.364 | 1.2 | 0.8–1.9 |
| Fla-X | Percent of antibodies in quartile | ||||||
| Phenotype | 1 | 2 | 3 | 4 | P trend | OR | 95%-CI |
| Stricturing (%) | 13.8 | 20 | 32.5 | 33.7 | 0.002 | 1.9 | 1.2–3.5 |
| Fistulizing (%) | 17.2 | 24.2 | 29.3 | 29.3 | 0.013 | 1.7 | 1.1–2.8 |
| Inflammatory (%) | 38.1 | 29.8 | 21.4 | 10.7 | 0.001 | 0.32 | 0.2–0.6 |
| Small bowel disease (%) | 17.4 | 28.7 | 34.7 | 19.2 | 0.04 | 1.8 | 1.2–3.1 |
| Small bowel surgery (%) | 18.3 | 28.3 | 36.7 | 16.7 | 0.643 | 1.2 | 0.5–2.3 |
Abbreviations: OR: odds ratio; CI: confidence interval.
We wondered if there exists an association between the disease duration and positivity toward antimicrobial antibodies as well as their titers. We therefore grouped our CD cohort according the disease duration into the following categories: 0–5 years (n=54), 6–10 years (n=60), 11–15 years (n=73) and >15 years (n=65). We found neither a significant association between disease duration and positivity for A4-Fla2, Fla-X, and ASCA nor for their titers (Kruskal-Wallis test).
NOD2 mutations are associated with the frequency and titers of ASCA
We hypothesized that NOD2 mutations might be associated with a higher frequency and/or titers of anti-microbial antibodies. We found ASCA frequency significantly associated with mutant NOD2 alleles ( ASCA frequency 71.4% in CD patients with NOD2 mutations compared to 57.2% ASCA frequency in CD patients with wild type, P=0.038). However, no significant association with NOD2 mutations could be detected for anti-flagellin-antibodies A4-Fla2 and Fla-X, p-ANCA, and PAB.
When the quartiles of each microbial antigen (ASCA, A4-Fla2, Fla-X) were correlated to the NOD2 status, we found significantly higher ASCA titers in patients with NOD2 mutations compared to CD patients without NOD2 mutations (mean ASCA quartile in CD patients with NOD2 mutations 2.54±0.78, compared to 1.97±0.99 in wild type CD patients, P=0.045). A trend toward higher anti-A4-Fla2 and anti-FlaX titers was observed for CD patients with NOD2 mutations (P=0.092 and P=0.127).
Discussion
In this study, we evaluated two new anti-flagellin-antibodies in comparison with other antibodies such as ASCA, p-ANCA, PAB, and NOD2 mutations regarding their associations with complicated CD phenotypes.
To the best of our knowledge we evaluated these anti-flagellin antibodies for the first time regarding their correlation with distinct disease phenotypes. Our anti-A4-Fla2 and anti-FlaX antibody results are in good accordance with the anti-CBir1 flagellin data regarding its frequency as well as its association with complicated CD phenotypes. Anti-CBir1 was the first flagellin shown to be a dominant antigen capable of inducing colitis in mice. In CD patients, anti-CBir1 antibodies were associated with complicated phenotypes.9 These initial findings were later corroborated by Papadakis et al. who demonstrated that anti-CBir1 reactivity was significantly associated with fibrostenosis, internal penetrating disease, small bowel involvement, as well as small bowel surgery, but negatively associated with UC-like (=inflammatory) CD.22 In multivariate logistic regression they found an independent association of anti-CBir1 reactivity with fibrostenosis and UC-like disease irrespective of the reactivity to other microbial antigens (I2, oligomannan, OmpC). In the multivariate regression model we observed a trend but no significance for anti-flagellin antibodies and fistulizing CD phenotype. This finding is probably related to the fact that the percentage and absolute number of patients with fistulizing disease (27%) in our cohort was lower compared with other groups.13
We found ASCA to have the strongest association with stricturing and fistulizing disease as well as with small bowel involvement and small bowel surgery; a significant negative association was found with purely inflammatory CD phenotype. The ASCA frequencies in our CD cohort are comparable with the findings of other groups.23 The significant associations of ASCA with complicated CD phenotyes are in accordance with groups who found that ASCA are linked to stricturing and fistulizing CD as well as with small bowel disease.13
Our findings regarding p-ANCA being significantly associated with inflammatory phenotype, and negatively associated with stricturing phenotype, small bowel disease, and small bowel surgery, are in agreement with other groups demonstrating that p-ANCA are associated with an uncomplicated CD phenotype.13
In accordance with other groups, we found antibodies against exocrine pancreas in low frequencies in CD patients.24 Based on our findings we agree with others that, despite their high specificity, the low sensitivity of PAB limits their value for the discrimination of CD phenotypes.8
As expected, in our cohort of CD patients, NOD2 mutations associated with small bowel disease. Defects in NOD2, located on chromosome 16, are thought to render the host hyporesponsive to bacterial products, what could lead to a diminished ability to clear bacteria in close approximation to the gut mucosa leading consecutively to an enhanced adaptive immune response and inflammation.25,26 Our findings are in accordance with the results in a large patient cohort demonstrating that NOD2 mutations (R702W, G908R, 1007fs) enhance the risk for small bowel disease.27 Despite the fact, that CD patients with NOD2 mutations were more frequently ASCA-positive, there was no association between complicated CD phenotypes and the presence of NOD2 mutations. This may indicate that in CD as a multifactorial disorder, the phenotype behaviour is more closely linked to immune responses against microbial antigens in general than to an isolated genetic trait. Also, our observation is in accordance with the data published by Louis et al. who demonstrated that NOD2 status did not result in rapid progression to ileal complications. 28 We demonstrated that CD patients with high-level antibody responses against multiple microbial antigens (flagellins A4-Fla2 and Fla-X, oligomannan) have the greatest risk of suffering from stricturing and fistulizing disease phenotype and from small bowel disease. Approximately 80% of our CD patients expressed a response toward at least 1 microbial antigen (A4-Fla2, Fla-X, oligomannan). By this, our findings corroborate the results of Targan et al. and Mow et al.12,13
The finding that high ASCA titers are associated with a greater frequency of mutant NOD2/CARD15 alleles and with a higher probability of complicated CD phenotypes was published recently by Dassopoulos et al.29
One possible limitation of our study lies in the uncertainty of phenotype assessment over time. It has already been postulated that longer disease follow-up results in changes in phenotypes and possibly complications of CD.30 Mow et al. found no correlation between the duration of disease and serological responses which indicates that the disease duration was not a major factor in the development of disease complications and that immune reactions have a more significant effect on disease phenotype.13 However, these findings contrast the results of Ferrante et al. who found that patients being positive for antimicrobial markers (gASCA, ACCA, AMCA, Omp) had significantly longer disease duration than the serologically negative group and also significantly higher antibody responses against gASCA, AMCA and Omp with increasing disease duration.31 These different findings indicate that prospective longitudinal studies over a period of several years are necessary to conclude on the impact of disease duration on serological response.
This study validates the already existing evidence that seroreactivity to microbial antigens is significantly associated with complicated CD phenotypes. Furthermore, our data support the appraisal that flagellins represent dominant antigens in CD. Despite the growing evidence that these biomarkers may be useful for the clinician in evaluating the individual patient’s risk of developing a complicated disease phenotype, these markers are not routinely used in clinical practice. This is related to the current absence of hard evidence that antibody-driven risk stratification alters the course of CD. Randomized prospective studies are needed to prove the advantage of antibody determinations to guide clinical decisions.
In summary, this study shows that reactivity to two newly evaluated flagellins, A4-Fla2 and Fla-X, and oligomannan (ASCA) is significantly associated with complicated disease phenotype, whereas p-ANCA are associated with inflammatory phenotype. Knowledge of such serological markers may support an individual risk assessment, allowing an individually tailored therapy. By this, CD patients may ultimately profit from biomarker-based clinical decisions. Prospective studies are needed to investigate if biomarker-based clinical decisions can change the clinical outcome of CD patients.
Acknowledgments
SNSF grant 3247B0-118112/1 to Frank Seibold, NIH grant DK64400 to Charles O. Elson, SNSF Grant 3347C0-108792/1 to Pierre Michetti (Swiss IBD Cohort Study)
This work was further supported by ibdnet.ch.
The authors thank Professor CO Elson, MD, Division of Gastroenterology and Hepatology, Department of Medicine, University of Alabama at Birmingham, for critical discussion and the donation of the flagellins. We are grateful to Adrian Spoerri, MSc, Institute for Social and Preventive Medicine, University of Bern, for statistical support. The authors also thank Linda Bolzern for proofreading the text.
Footnotes
Disclaimers: none
Conflict of interest: none
Parts of the results were presented in abstract form (poster) at DDW 2008 in San Diego, USA, 18 May 2008.
Disclosures
none
References
- 1.Satsangi J, Morecroft J, Shah NB, et al. Genetics of inflammatory bowel disease: Scientific and clinical implications. Best Pract Res Clin Gastroenterol. 2003;17:3–18. doi: 10.1053/bega.2002.0349. [DOI] [PubMed] [Google Scholar]
- 2.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 3.D’Haens GR, Geboes K, Peeters M. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262–267. doi: 10.1016/s0016-5085(98)70476-7. [DOI] [PubMed] [Google Scholar]
- 4.Landers CJ, Cohavy O, Misra R, et al. Selected loss of tolerance evidenced by Crohn’s disease-associated immune responses to auto- and microbial antigens. Gastroenterology. 2002;123:689–699. doi: 10.1053/gast.2002.35379. [DOI] [PubMed] [Google Scholar]
- 5.Adams RJ, Heazlewood SP, Gilshenan KS, et al. IgG antibodies against common gut bacteria are more diagnostic for Crohn’s disease than IgG against mannan or flagellin. Am J Gastroenterol. 2008;103:386–396. doi: 10.1111/j.1572-0241.2007.01577.x. [DOI] [PubMed] [Google Scholar]
- 6.Vernier G, Sendid B, Poulain D, et al. Relevance of serologic studies in inflammatory bowel disease. Curr Gastroenterol Rep. 2004;6:482–487. doi: 10.1007/s11894-004-0070-x. [DOI] [PubMed] [Google Scholar]
- 7.Vasiliauskas EA, Plevy SE, Landers CJ, et al. Perinuclear antineutrophil cytoplasmic antibodies in patients with Crohn’s disease define a clinical subgroup. Gastroenterology. 1996;110:1810–1819. doi: 10.1053/gast.1996.v110.pm8964407. [DOI] [PubMed] [Google Scholar]
- 8.Klebl FH, Bataille F, Huy C, et al. Association of antibodies to exocrine pancreas with subtypes in Crohn’s disease. Eur J Gastroenterol Hepatol. 2005;17:73–77. doi: 10.1097/00042737-200501000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn’s disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins MD, Lawson PA, Willems A, et al. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- 11.Duck LW, Walter MR, Novak J, et al. Isolation of flagellated bacteria implicated in Crohn’s disease. Inflamm Bowel Dis. 2007;13:1191–1201. doi: 10.1002/ibd.20237. [DOI] [PubMed] [Google Scholar]
- 12.Targan SR, Landers CJ, Yang H, et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128:2020–2028. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 13.Mow WS, Vasiliauskas EA, Ying-Chao L, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology. 2004;126:414–424. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad T, Armuzzi A, Bunce M, et al. The molecular classification of the clinical manifestations of Crohn’s disease. Gastroenterology. 2002;122:854–866. doi: 10.1053/gast.2002.32413. [DOI] [PubMed] [Google Scholar]
- 15.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:537–539. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 16.Lesage S, Zouali H, Cézard JP, et al. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am J Hum Genet. 2002;70:845–857. doi: 10.1086/339432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–6. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- 18.Gasche C, Schoelmerich J, Brynskov J, et al. A simple classification of Crohn’s disease: report of the working party for the world congress of gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Esters N, Pierik M, van Steen K, et al. Transmission of CARD15 (NOD2) variants within families of patients with inflammatory bowel disease. Am J Gastroenterol. 2004;99:299–305. doi: 10.1111/j.1572-0241.2004.04040.x. [DOI] [PubMed] [Google Scholar]
- 20.Schaffer T, Müller S, Flogerzi B, et al. Anti-Saccharomyces cerevisiae mannan antibodies (ASCA) of Crohn’s patients crossreact with mannan from other yeast strains, and murine ASCA IgM can be experimentally induced with Candida albicans. Inflamm Bowel Dis. 2007;13:1339–1346. doi: 10.1002/ibd.20228. [DOI] [PubMed] [Google Scholar]
- 21.Seibold F, Weber P, Klein R, et al. Clinical significance of antibodies against neutrophils in patients with inflammatory bowel disease and primary sclerosing cholangitis. Gut. 1992;33:657–662. doi: 10.1136/gut.33.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papadakis KA, Yang H, Ippoliti A, et al. Anti-flagellin (CBir1) phenotypic and genetic Crohn’s disease associations. Inflamm Bowel Dis. 2007;13:524–530. doi: 10.1002/ibd.20106. [DOI] [PubMed] [Google Scholar]
- 23.Peeters M, Joossens S, Vermeire S, et al. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Am J Gastroenterol. 2001;96:730–734. doi: 10.1111/j.1572-0241.2001.03613.x. [DOI] [PubMed] [Google Scholar]
- 24.Stocker W, Otte M, Ulrich S, et al. Autoimmunity to pancreatic juice in Crohn’s disease. Scand J Gastroenterol. 1987;139:41–52. doi: 10.3109/00365528709089774. [DOI] [PubMed] [Google Scholar]
- 25.Devlin SM, Yang H, Ippoliti A, et al. NOD2 variants and antibody response to microbial antigens in Crohn’s disease patients and their unaffected relatives. Gastroenterology. 2007;132:576–586. doi: 10.1053/j.gastro.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Oostenbrug LE, Nolte IM, Oosterom E, et al. CARD15 in inflammatory bowel disease and Crohn’s disease phenotypes: an association study and pooled analysis. Dig Liver Dis. 2006;38:834–845. doi: 10.1016/j.dld.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 28.Louis E, Michel V, Hugot JP, et al. Early development of stricturing or penetrating pattern in Crohn’s disease is influenced by disease location, number of flares, and smoking but not NOD2 genotype. Gut. 2003;52:552–557. doi: 10.1136/gut.52.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dassopoulos T, Frangakis C, Cruz-Correa M, et al. Antibodies to saccharomyces cerevisiase in Crohn’s disease: higher titers are associated with a greater frequency of mutant NOD2/CARD15 alleles and with a higher probability of complicated disease. Inflamm Bowel Dis. 2007;13:143–151. doi: 10.1002/ibd.20031. [DOI] [PubMed] [Google Scholar]
- 30.Louis E, Collard A, Oger AF, et al. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrante M, Henckaerts L, Joossens M, et al. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut. 2007;56:1394–1403. doi: 10.1136/gut.2006.108043. [DOI] [PMC free article] [PubMed] [Google Scholar]


