Abstract
Background/Aims
The increasing incidence of Candida glabrata and Candida krusei infections is a significant problem because they are generally more resistant to fluconazole. We compared the risk factors associated with C. glabrata and C. krusei fungemia with Candida albicans fungemia and examined the clinical manifestations and prognostic factors associated with candidemia.
Methods
We retrospectively reviewed demographic data, risk factors, clinical manifestations, and outcomes associated with C. glabrata and C. krusei fungemia at a tertiary-care teaching hospital during a 10-years period from 1997 to 2006.
Results
During the study period, there were 497 fungemia episodes. C. glabrata fungemia accounted for 23 episodes and C. krusei fungemia accounted for 8. Complete medical records were available for 27 of these episodes and form the basis of this study. Compared to 54 episodes of C. albicans fungemia, renal insufficiency and prior fluconazole prophylaxis were associated with development of C. glabrata or C. krusei fungemia. The overall mortality was 67%. The fungemia-related mortality of C. glabrata and C. krusei was higher than that of C. albicans (52 vs. 26%, p=0.021). Empirical antifungal therapy did not decrease the crude mortality. Multiple logistic regression analysis showed that high APACHE II scores, catheter maintenance, and shock were independently associated with an increased risk of death.
Conclusions
Renal insufficiency and prior fluconazole prophylaxis were associated with the development of C. glabrata or C. krusei fungemia. Fungemia-related mortality of C. glabrata or C. krusei was higher than that of C. albicans. Outcomes appeared to be related to catheter removal, APACHE II scores, and shock.
Keywords: Candidemia, Risk factors, Mortality
INTRODUCTION
Recently, we have seen an increase in the frequency of non-albicans species of Candida, such as C. glabrata, C. krusei, C. tropicalis, and C. parapsilosis, as the cause of fungemia [1,2]. C. krusei is intrinsically resistant to fluconazole because of a decreased susceptibility of 14α-demethylase [3], and C. glabrata is relatively resistant to fluconazole due to an energy-dependent efflux mechanism [4]. The increasing proportion of fungemia due to C. glabrata and C. krusei has important implications for therapy.
We evaluated the risk factors associated with C. glabrata and C. krusei fungemia in comparison with Candida albicans fungemia. We also examined the clinical manifestations and prognostic factors associated with candidemia.
METHODS
Study design
All episodes of fungemia that occurred between January 1997 and December 2006 at Severance Hospital, Yonsei University College of Medicine, Seoul, Korea, a 1500-bed tertiary-care teaching hospital, were identified. We retrospectively reviewed demographic data, risk factors, clinical manifestations, and outcomes associated with C. glabrata and C. krusei fungemia. In addition, we selected 54 patients who had C. albicans fungemia as the control group. Cases and controls were matched 1 to 2 by age, sex, and the time of fungemia.
Definitions
An episode of fungemia was defined as the isolation of any pathogenic species of Candida from at least one blood culture specimen from a patient with signs and symptoms of infection. A second episode of fungemia occurring in the same patient within 4 weeks of the first episode was counted as the same episode. Recovery from candidemia was defined as the resolution of all clinical manifestations and no further positive blood cultures within 1 weeks after therapy. Failure to respond was defined as the persistence of clinical signs and symptoms or persistent candidemia caused by the same Candida species after the onset of therapy.
Death was attributed to Candida infection if the patient did not respond to therapy and there was no other obvious cause of death, such as a major hemorrhage or other infection. We defined early mortality as death within 3 to 7 days after diagnosis and late mortality as death between days 8 and 30. Patients who received no antifungal therapy were excluded from the analysis.
Statistical analysis
The χ2 test and Fisher's exact test were used to determine categorical predictors of infection and outcome. Continuous variables were compared using the t-test. Multiple logistic regression analysis was performed to identify independent predictors of death; variables included the APACHE II scores, catheter maintenance, shock, non-albicans species, and early treatment. P values of less than 0.05 were considered statistically significant. All statistical analysis was performed with (SPSS Inc., Chicago, IL, USA).
RESULTS
Incidence, demographic and clinical characteristics
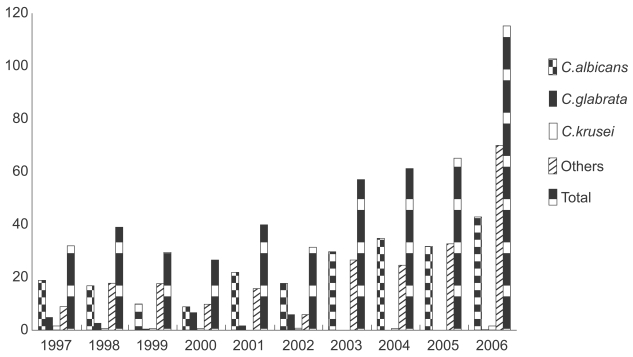
During the study period, there were 497 fungemia episodes. C. glabrata fungemia accounted for 23 episodes (4.6%) and C. krusei fungemia accounted for eight (1.6%). The proportion of fungemias due to C. glabrata or C. krusei ranged from 0-29% per year (Fig. 1).
Figure 1.
Annual fungemia episodes and causative organisms over a 10 year period at Severance Hospital.
Complete medical records were available for 27 of the 31 episodes with non-albicans infections and 54 of the 234 episodes with C. albicans infections; therefore, 27 and 54 cases of each bloodstream infection were compared.
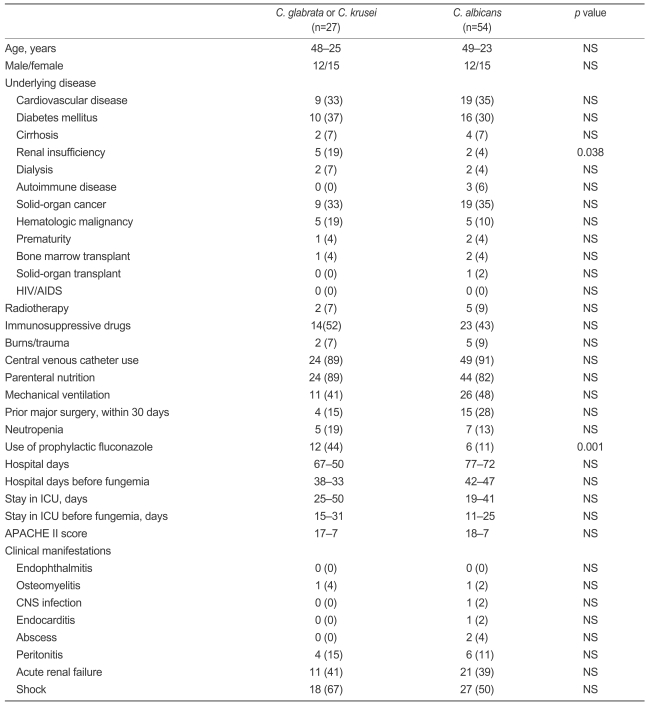
Candida glabrata fungemia occurred in 12 men (44% of subjects) and 15 women (56% of subjects). Their mean age was 48±25 years. The majority of patients had multiple underlying illnesses and other risk factors that have been associated with fungemia. The most common underlying illnesses were diabetes mellitus (37%), cardiovascular diseases (33%), and solid-organ cancer (33%). No patient developed endophthalmitis, central nervous system infection, endocarditis, or abscesses during the follow-up period. One patient had osteomyelitis and four had peritonitis as a primary infection. Eleven patients developed acute renal failure (ARF) and 18 presented with septic shock (Table 1).
Table 1.
Demographics, clinical manifestations, and risk factors of C. glabrata and C. krusei fungemia
Values are number (percentage) or mean±SD.
NS, not significant; HIV, human immunodeficiency virus; AIDS, acquired immune deficiency syndrome; ICU, intensive care unit ; CNS, central nervous system.
Risk factors for C. glabrata or C. krusei
Compared to 54 episodes of C. albicans fungemia, renal insufficiency and prior fluconazole prophylaxis were associated with the development of C. glabrata or C. krusei fungemia (Table 1).
Treatment and outcomes of fungemia
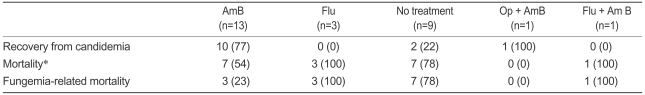
Of the 27 episodes, 18 (67%) were treated with an antifungal agent. Nine episodes (33%) were not treated because of death before diagnosis (5 patients), discharge to another hospital (1 patient), or no reason was documented (3 patients). Of the nine episodes for which an antifungal agent was not used, two patients showed recovery from the Candida infection. They were not treated with any antifungal agent, but the central venous catheter was removed. Of the 18 episodes for which an antifungal agent was used, three were treated with fluconazole alone, 13 episodes were treated with amphotericin B formulation alone, and one case with osteomyelitis was treated with amphotericin B formulation and surgery. One patient was treated with fluconazole initially, and this was switched to amphotericin B after the species was documented to be C. glabrata. For 10 of the 13 episodes (77%) treated with amphotericin B, additional blood cultures grew no yeast by the end of therapy. The mortality rate for episodes treated with amphotericin B was 54% (7 of 13 episodes), and fungemia-related mortality constituted 23% (3 of 13 episodes). All patients who did not achieve microbiological success died of fungemia (Table 2).
Table 2.
Treatment outcomes of 27 episodes of C. glabrata and C. krusei
Values are number (percentage).
AmB, amphotericin B; Flu, fluconazole; Op, operation.
*Overall mortality at day 30.
Overall, the early and late mortality of C. glabrata and C. krusei fungemia were not significantly different from that due to C. albicans. However, the fungemia-related mortality for C. glabrata and C. krusei was higher (52%, 14/27) than that due to C. albicans (26%, 14/54, p=0.021, Table 3).
Table 3.
Differences in outcome between C. glabrata, C. krusei, and C. albicans
Values are number (percentage).
NS, not significant.
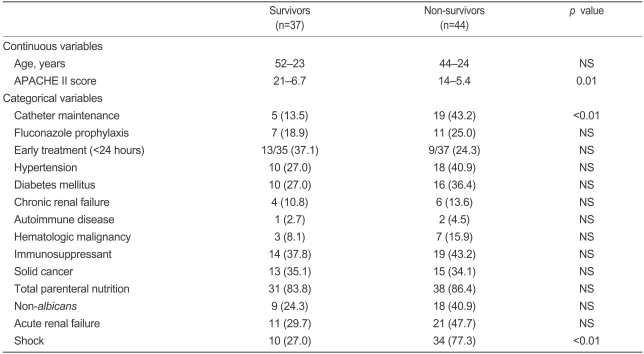
In the univariate analysis, patients whose central venous catheters were not removed (p<0.01) or who had high APACHE II scores (p=0.01) had a higher crude mortality. Receiving appropriate antifungal therapy within 24 hours was not associated with a risk of mortality (p=0.21). Septic shock was associated with an increased risk of mortality (p<0.01, Table 4).
Table 4.
Univariate analysis of the factors associated with death in patients with candidemia
Values are number (percentage) or mean±SD.
NS, not significant.
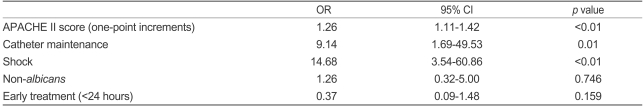
Multiple logistic regression analysis showed that increasing APACHE II scores (one-point increments) (odds ratio [OR] 1.262, 95% CI 1.116-1.427, p<0.01), catheter maintenance (OR 8.982, 95% CI 1.715-47.032, p<0.01), and shock (OR 14.465, 95% CI 3.573-58.557, p<0.01) were independently associated with death (Table 5).
Table 5.
Multivariate analysis of the factors associated with death in patients with candidemia
OR, odds ratio; CI, confidence interval.
DISCUSSION
Candida species are now the fourth most common cause of nosocomial bloodstream infections and are associated with a crude mortality rate of 39%, which is the highest mortality rate associated with any form of nosocomial bloodstream infection [1]. During the past decade, an increase in the incidence of bloodstream infections due to Candida species other than C. albicans has been reported [2,5-7]. However, no increase in C. glabrata or C. krusei fungemia was evident in our study.
Candida krusei and C. glabrata infections can cause serious complications as they have higher crude mortality than other Candida species, such as C. albicans or C. parapsilosis [1]. In our study, non-albicans fungemia did not cause complications such as endophthalmitis or endocarditis. However, the true incidence of these complications is likely to have been higher, because echocardiography was performed in fewer than half of the episodes and ophthalmological examinations were conducted in five cases only. In our analysis, the crude mortality for non-C. albicans (C. glabrata or C. krusei) fungemia was not different from that due to C. albicans fungemia. This is consistent with the findings of Klevay et al. [8], Blot et al. [9], and Bassetti et al. [10], who found that mortality did not differ by species. However, fungemia-related mortality was significantly higher among patients with C. glabrata or C. krusei fungemia.
Previous investigations have suggested that prior exposure to antifungal agents [2,11,12], exposure to anti-microbial agents, especially vancomycin or piperacillin-tazobactam [13], age [14,15], and underlying hematologic malignancy [11,16] were predisposing factors for non-albicans fungemia.
Although there is a historical association between fluconazole use and the increased incidence of relatively resistant non-albicans Candida species, the degree to which fluconazole plays a role in the risk to individual patients remains unclear [17]. Fluconazole prophylaxis was a predisposing factor in our analysis. However, not all studies have linked fluconazole use to increased colonization and infection by C. glabrata or C. krusei. Lin et al. [13] reported that exposure to fluconazole was not found to be a significant risk factor for developing non-C. albicans candidemia. Similarly, Safdar et al. [18] reported that the predominance of C. glabrata and C. krusei breakthrough infections was similar to that seen with high-dose fluconazole (400 mg) prophylaxis, and no adverse effect of low-dose fluconazole in terms of an increased incidence of non-susceptible Candida species was seen.
In several studies, predictors of death from candidemia were APACHE II scores at the time of fungemia [18-22], length of hospitalization [23], delayed or inappropriate treatment [21,24,25], neutropenia [19,20,26,27], and central venous catheter maintenance [20,27]. We observed that increasing APACHE II scores (OR 1.262, 95% CI 1.116-1.427, p<0.01), catheter maintenance (OR 8.982, 95% CI 1.715-47.032, p<0.01), and shock (OR 14.465, 95% CI 3.573-58.557, p<0.01) were independently associated with the risk of death. This was similar to previous studies, while empirical antifungal therapy, which was started within 24 hours from the time when the blood samples were obtained, but before identification of the organism, did not improve outcome. Bias was likely in the selection of antifungal agents used in various patients. Immunocompromised patients were perhaps more likely to have been given amphotericin B, and very sick patients received no therapy because of early death due to fungemia.
There are several limitations to this study. First, data were collected from a single center, resulting in a limited sample size that could be influenced by local outbreaks, specific infection-control practices, or regional susceptibility patterns. Second, the retrospective nature of this analysis may be susceptible to reviewer bias. Third, the small sample size when various subsets were assessed with regard to mortality precludes meaningful statistical analysis of many of these factors.
In conclusion, renal insufficiency and prior fluconazole prophylaxis were associated with the development of C. glabrata or C. krusei fungemia. Fungemia-related mortality due to C. glabrata or C. krusei was higher than due to C. albicans.
The outcomes appeared to be related to catheter removal, APACHE II scores, and shock. Future prospective studies including data from multiple centers that analyze antimicrobial use and subsequent colonization and infection by C. glabrata and C. krusei will lead to a better understanding of the epidemiology of these increasingly important pathogens.
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Abi-Said D, Anaissie E, Uzun O, Raad I, Pinzcowski H, Vartivarian S. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin Infect Dis. 1997;24:1122–1128. doi: 10.1086/513663. [DOI] [PubMed] [Google Scholar]
- 3.Orozco AS, Higginbotham LM, Hitchcock CA, et al. Mechanism of fluconazole resistance in Candida krusei. Antimicrob Agents Chemother. 1998;42:2645–2649. doi: 10.1128/aac.42.10.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkinson T, Falconer DJ, Hitchcock CA. Fluconazole resistance due to energy-dependent drug efflux in Candida glabrata. Antimicrob Agents Chemother. 1995;39:1696–1699. doi: 10.1128/aac.39.8.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen MH, Peacock JE, Jr, Morris AJ, et al. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med. 1996;100:617–623. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 6.Voss A, Kluytmans JA, Koeleman JG, et al. Occurrence of yeast bloodstream infections between 1987 and 1995 in five Dutch university hospitals. Eur J Clin Microbiol Infect Dis. 1996;15:909–912. doi: 10.1007/BF01690507. [DOI] [PubMed] [Google Scholar]
- 7.Pfaller MA, Jones RN, Doern GV, Sader HS, Hollis RJ, Messer SA. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and antifungal susceptibilities of isolates collected in 1997 in the United States, Canada, and South America for the SENTRY Program. J Clin Microbiol. 1998;36:1886–1889. doi: 10.1128/jcm.36.7.1886-1889.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klevay MJ, Ernst EJ, Hollanbaugh JL, Miller JG, Pfaller MA, Diekema DJ. Therapy and outcome of Candida glabrata versus Candida albicans bloodstream infection. Diagn Microbiol Infect Dis. 2008;60:273–277. doi: 10.1016/j.diagmicrobio.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Blot S, Vandewoude K, Hoste E, Poelaert J, Colardyn F. Outcome in critically ill patients with candidal fungaemia: Candida albicans vs. Candida glabrata. J Hosp Infect. 2001;47:308–313. doi: 10.1053/jhin.2000.0918. [DOI] [PubMed] [Google Scholar]
- 10.Bassetti M, Trecarichi EM, Righi E, et al. Incidence, risk factors, and predictors of outcome of candidemia: survey in 2 Italian university hospitals. Diagn Microbiol Infect Dis. 2007;58:325–331. doi: 10.1016/j.diagmicrobio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Abbas J, Bodey GP, Hanna HA, et al. Candida krusei fungemia: an escalating serious infection in immunocompromised patients. Arch Intern Med. 2000;160:2659–2664. doi: 10.1001/archinte.160.17.2659. [DOI] [PubMed] [Google Scholar]
- 12.Marr KA, Seidel K, White TC, Bowden RA. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J Infect Dis. 2000;181:309–316. doi: 10.1086/315193. [DOI] [PubMed] [Google Scholar]
- 13.Lin MY, Carmeli Y, Zumsteg J, et al. Prior antimicrobial therapy and risk for hospital-acquired Candida glabrata and Candida krusei fungemia: a case-case-control study. Antimicrob Agents Chemother. 2005;49:4555–4560. doi: 10.1128/AAC.49.11.4555-4560.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diekema DJ, Messer SA, Brueggemann AB, et al. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J Clin Microbiol. 2002;40:1298–1302. doi: 10.1128/JCM.40.4.1298-1302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malani A, Hmoud J, Chiu L, Carver PL, Bielaczyc A, Kauffman CA. Candida glabrata fungemia: experience in a tertiary care center. Clin Infect Dis. 2005;41:975–981. doi: 10.1086/432939. [DOI] [PubMed] [Google Scholar]
- 16.Bodey GP, Mardani M, Hanna HA, et al. The epidemiology of Candida glabrata and Candida albicans fungemia in immunocompromised patients with cancer. Am J Med. 2002;112:380–385. doi: 10.1016/s0002-9343(01)01130-5. [DOI] [PubMed] [Google Scholar]
- 17.White MH. The contribution of fluconazole to the changing epidemiology of invasive candidal infections. Clin Infect Dis. 1997;24:1129–1130. doi: 10.1086/513661. [DOI] [PubMed] [Google Scholar]
- 18.Safdar A, van Rhee F, Henslee-Downey JP, Singhal S, Mehta J. Candida glabrata and Candida krusei fungemia after high-risk allogeneic marrow transplantation: no adverse effect of low-dose fluconazole prophylaxis on incidence and outcome. Bone Marrow Transplant. 2001;28:873–878. doi: 10.1038/sj.bmt.1703252. [DOI] [PubMed] [Google Scholar]
- 19.Pappas PG, Rex JH, Lee J, et al. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis. 2003;37:634–643. doi: 10.1086/376906. [DOI] [PubMed] [Google Scholar]
- 20.Viudes A, Peman J, Canton E, Ubeda P, Lopez-Ribot JL, Gobernado M. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. Eur J Clin Microbiol Infect Dis. 2002;21:767–774. doi: 10.1007/s10096-002-0822-1. [DOI] [PubMed] [Google Scholar]
- 21.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michalopoulos AS, Geroulanos S, Mentzelopoulos SD. Determinants of candidemia and candidemia-related death in cardiothoracic ICU patients. Chest. 2003;124:2244–2255. doi: 10.1378/chest.124.6.2244. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Slavin M, Nguyen Q, et al. Active surveillance for candidemia, Australia. Emerg Infect Dis. 2006;12:1508–1516. doi: 10.3201/eid1210.060389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antoniadou A, Torres HA, Lewis RE, et al. Candidemia in a tertiary care cancer center: in vitro susceptibility and its association with outcome of initial antifungal therapy. Medicine. 2003;82:309–321. doi: 10.1097/01.md.0000091182.93122.8e. [DOI] [PubMed] [Google Scholar]
- 25.Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 26.Goodrich JM, Reed EC, Mori M, et al. Clinical features and analysis of risk factors for invasive candidal infection after marrow transplantation. J Infect Dis. 1991;164:731–740. doi: 10.1093/infdis/164.4.731. [DOI] [PubMed] [Google Scholar]
- 27.Anaissie EJ, Rex JH, Uzun O, Vartivarian S. Predictors of adverse outcome in cancer patients with candidemia. Am J Med. 1998;104:238–245. doi: 10.1016/s0002-9343(98)00030-8. [DOI] [PubMed] [Google Scholar]