Abstract
We have investigated the function of endogenous galectin-3 in T cells. Galectin-3-deficient (gal3−/−) CD4+ T cells secreted more IFN-γ and IL-4 than gal3+/+CD4+ T cells after T-cell receptor (TCR) engagement. Galectin-3 was recruited to the cytoplasmic side of the immunological synapse (IS) in activated T cells. In T cells stimulated on supported lipid bilayers, galectin-3 was primarily located at the peripheral supramolecular activation cluster (pSMAC). Gal3+/+ T cells formed central SMAC on lipid bilayers less effectively and adhered to antigen-presenting cells less firmly than gal3−/− T cells, suggesting that galectin-3 destabilizes the IS. Galectin-3 expression was associated with lower levels of early signaling events and phosphotyrosine signals at the pSMAC. Additional data suggest that galectin-3 potentiates down-regulation of TCR in T cells. By yeast two-hybrid screening, we identified as a galectin-3-binding partner, Alix, which is known to be involved in protein transport and regulation of cell surface expression of certain receptors. Co-immunoprecipitation confirmed galectin-3-Alix association and immunofluorescence analysis demonstrated the translocation of Alix to the IS in activated T cells. We conclude that galectin-3 is an inhibitory regulator of T-cell activation and functions intracellularly by promoting TCR down-regulation, possibly through modulating Alix's function at the IS.
Galectins are beta-galactoside-binding proteins with evolutionarily conserved carbohydrate-recognition domains (CRD). The family members are expressed by organisms from nematodes to mammals. Currently, 15 members have been identified in mammals (reviewed in ref. 1). Each member contains either one or two CRDs, but galectin-3 is unique in that it contains a single CRD in the C-terminal region connected to an N-terminal domain consisting of tandem repeats of short proline-rich motifs. Galectins play important roles in immune responses and tumor progression and other physiological and pathological processes (reviewed in refs. 2–5).
Galectin-3 is widely distributed and is expressed by various immune cells (reviewed in ref. 6). Like other galectins, it does not have a classical signal sequence and is found in the cytosol and nucleus, but is also detected extracellularly. Recombinant galectin-3 has been shown to either induce or suppress cell activation and promote or inhibit cell adhesion in vitro when delivered exogenously, depending on the experimental systems (reviewed in ref. 1). Endogenous galectin-3 has been shown to inhibit apoptosis (reviewed in refs. 7 and 8), promote mediator release and cytokine production by mast cells (9), promote phagocytosis by macrophages (10), and drive alternative macrophage activation (11). While it is clear recombinant galectin-3 exerts its functions by engaging cell surface glycoproteins or glycolipids, the mechanisms by which endogenous galectin-3 functions are largely unknown.
With regard to T cells, galectin-3 is expressed by CD4+ and CD8+ T cells after these cells are activated by anti-CD3 antibody or mitogens (12). Exogenously delivered galectin-3 has been shown to induce IL-2 production by Jurkat cells (13) and cause apoptosis in activated T cells (14, 15). Endogenous galectin-3, however, inhibits apoptosis in Jurkat cell transfectants overexpressing the protein (16). Other than this, the function of endogenous galectin-3 in the T-cell response is largely unknown.
Activation of T cells by TCR engagement is associated with the recruitment of many receptors and signaling molecules to the stable contact region between T cells and antigen-presenting cells (APCs) called the immunological synapse (IS), which is important in tolerance and immunity (17). T-cell receptor signaling in the IS involves continual formation of TCR microclusters that recruit signaling molecules (18, 19). These microclusters rapidly coalesce to form supramolecular activation clusters (SMAC) (20, 21). There is a central zone (cSMAC) containing TCR/CD3, which is surrounded by a peripheral zone (pSMAC) marked by lymphocyte function-associated antigen-1 (LFA-1), and a distal zone (dSMAC) (22). Current models suggest that cSMAC is engaged in TCR degradation and costimulation, pSMAC in adhesion and TCR microcluster transport, and dSMAC in TCR and LFA-1 microcluster formation (23, 24).
Here, we report that gal3−/− CD4+ T cells secreted higher levels of IFN-γ and IL-4 compared with gal3+/+ cells. Galectin-3 was recruited to the cytoplasmic side of the IS in CD4+ T cells after TCR engagement and was primarily located at the pSMAC. Our findings suggest that galectin-3 destabilizes IS formation. We also obtained evidence that galectin-3 suppresses the activation of the early events in TCR-mediated signal transduction and potentiates down-regulation of TCR in cells activated by engagement of the receptor. Finally, we found that galectin-3 is associated with a component of the endosomal sorting complex required for transport (ESCRT), Alix, known to regulate cell surface expression of certain receptors.
Results
Naïve Gal3−/− CD4+ T Cells Are Hyperresponsive with Regard to Cytokine Production.
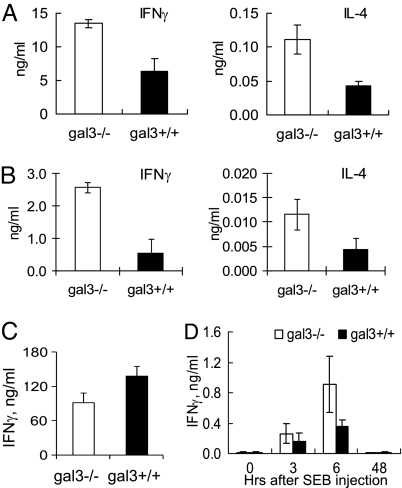
We studied the cytokine response of purified naïve CD4+ T cells activated by TCR engagement in vitro. We first compared CD4+ T cells from gal3+/+ and gal3−/− mice stimulated by anti-CD3/CD28 and found that gal3−/− cells secreted significantly higher amounts of IFN-γ and IL-4 than gal3+/+ cells (Fig. 1A). We then compared CD4+ T cells from gal3+/+ and gal3−/− mice expressing transgenic TCR that recognizes an OVA peptide (gal3+/+OTII and gal3−/−OTII mice) by stimulating them in vitro with the peptide in the presence of APCs. The hyprerresponsiveness of gal3−/− cells compared with gal3+/+ cells was also observed (Fig. 1B). This is limited to responses mediated through TCR, as gal3−/− CD4+ T cells did not secrete higher amounts of IFN-γ when stimulated with PMA/Ionomycin (Fig. 1C). To determine the function of galectin-3 in T cells in vivo, we compared the cytokine response between gal3+/+ and gal3−/− mice to treatment with the superantigen Staphylococcal Enterotoxin B (SEB). We found that after injection of the superantigen, gal3−/− mice produced significantly higher IFN-γ levels in the sera compared with gal3+/+ mice (Fig. 1D).
Fig. 1.
Naïve gal3−/− CD4+ T cells are hyperresponsive. (A) Purified naïve CD4+ T cells were stimulated with plate-bound anti-CD3/CD28 and the concentrations of secreted IFN-γ and IL-4 in the media were measured, P < 0.05. (B) CD4+ T cells purified from gal3−/− and gal3+/+ OTII mice were mixed with T cell-depleted splenocytes that were fixed and pulsed with a peptide antigen (OVA323–339), as the APCs. The concentrations of the cytokines in the media were measured, P < 0.05. (C) CD4+ T cells were stimulated with PMA and ionomycin and the amounts of the cytokines were measured, P > 0.05. (D) Gal3+/+ and gal−/− mice were treated with SEB and their serum IFN-γ levels were measured at different time points. ANOVA, P < 0.005.
Galectin-3 Is Recruited to the IS.
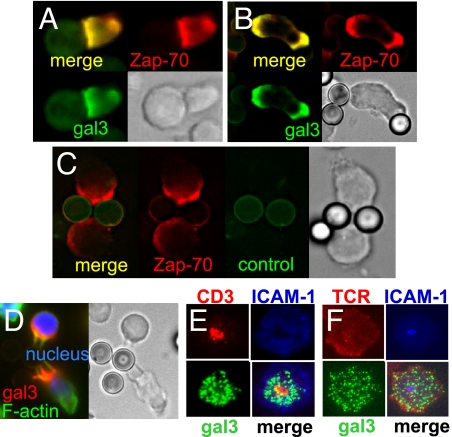
To elucidate the mechanism of suppression of the T-cell response by galectin-3, we analyzed the location of galectin-3 in activated T cells. We incubated galectin-3-transfected Jurkat cells with superantigen SEE-pulsed human B cells and performed immunofluorescence staining. The results showed that galectin-3 was recruited to the IS and co-localized with Zap-70, which is known to be localized at the IS (Fig. 2A). Approximately 71% of Zap-70-positive synapses (n = 21) showed galectin-3 accumulation at the IS. Similar translocation of galectin-3 was also found when these cells were stimulated with anti-CD3-coated beads (Fig. 2B). Seventy-four percent of Zap-70-positive cell/bead interfaces (n = 35) showed galectin-3 accumulation. The staining of galectin-3 is specific, as the use of a control antibody only resulted in faint uniform signal (Fig. 2C). We also examined the localization of galectin-3 in mouse CD4+ T cells activated with anti-CD3-coated beads. The results showed that galectin-3 was recruited to the contact region between T cells and the beads (Fig. 2D). Sixty-eight percent of galectin-3-positive cells in contact with beads (n = 44) showed galectin-3 accumulation in the interface. Galectin-3 is intracellular as the staining was revealed only in permeabilized cells.
Fig. 2.
Galectin-3 is recruited to the IS. (A) Galectin-3-transfected (Gal3+) Jurkat T cells were stimulated for 5 min with SEE-pulsed RPMI 8866 B cells. The cells were processed for immunofluorescence staining of galectin-3. (B and C) Gal3+ Jurkat cells were stimulated with anti-CD3-coated latex beads and stained with anti-galectin-3 (B) or control antibody (C). (D) Activated mouse CD4+ T cells from wild-type mice were stimulated with anti-CD3-coated beads and the cells were stained for galectin-3. (E) Gal3+ Jurkat cells were stimulated to form the IS on supported lipid bilayers preloaded with fluorescence-labeled anti-CD3 and ICAM-1. The cells were stained for galectin-3. (F) Gal3+/+OTII CD4+ T cells were mixed with fluorescence-labeled anti-TCRβ and injected into flow cells coated with lipid bilayers preloaded with pMHC and fluorescence-labeled ICAM-1 to form the IS. The cells were stained for galectin-3.
We used lipid bilayers loaded with GPI-ICAM-1-Cy5 (that binds LFA) and anti-TCR-AF-564 (that binds CD3/TCR) to induce formation of mature IS in galectin-3-transfected Jurkat T cells. We then fixed and stained the cells and visualized the location of galectin-3 by using total internal reflection fluorescence microscopy (TIRFM). The staining pattern indicated that galectin-3 was primarily located in the pSMAC, although it was also present in the cSMAC (Fig. 2E). The recruitment of galectin-3 to the IS in the lipid bilayer system was also confirmed by applying activated mouse CD4+ T cells onto lipid bilayers preloaded with ICAM-1 and MHC-peptide complex (pMHC) (Fig. 2F).
Galectin-3 Inhibits cSMAC Formation and Destabilizes T-Cell Binding to APCs.
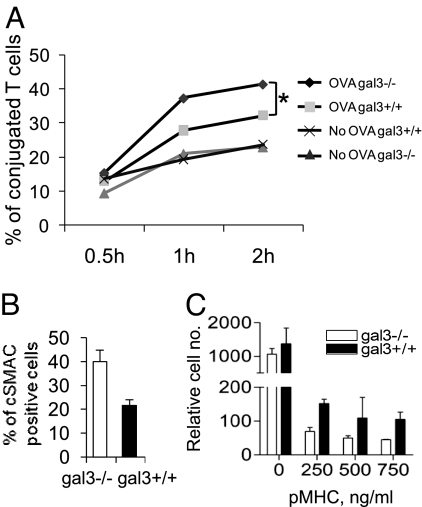
T-cell activation stimulated by cognate peptide-loaded APCs involves three phases: 1) contact acquisition; 2) formation of an interaction plane (i.e., the IS); and 3) detachment of the T cell. We next studied the effects of galectin-3 on the three phases. We first determined the effects of galectin-3 on binding of CD4+ T cells to APCs. Fluorescence (DiO) labeled gal3−/− and gal3+/+ OTII T cells were mixed with DiI-labeled APCs (bone marrow-derived dendritic cells; BMDCs) in the presence of the peptide antigen. The amounts of cell conjugates were then measured by flow cytometry at different time points. The results showed that gal3+/+ OTII T cells formed fewer conjugates than gal3−/− OTII T cells (Fig. 3A). This indicates that galectin-3 negatively regulates the T-cell function by inhibiting binding of T cells to APCs. Since the mature IS can be identified by formation of cSMAC and pSMAC, we next analyzed the effects of galectin-3 expression on the maturation of the IS by measuring the number of cSMAC. We found there was a lower number of gal3+/+ CD4+ T cells that formed the cSMAC compared with gal3−/− CD4+ cells (Fig. 3B).
Fig. 3.
Galectin-3 inhibits cSMAC formation and destabilizes T-cell binding to APCs. (A) DiO-labeled gal3−/− and gal3+/+ OTII CD4+ T cells were mixed with DiI-labeled BMDC in the presence of 1 μg/mL OVA 323–339 peptide. The double fluorescence-labeled cell conjugates were detected by flow cytometry at the indicated time points. The percentages of cell conjugates were calculated by dividing the double-fluorescence labeled cell counts over the total stained T-cell counts. ANOVA, P < 0.05. (B) Gal3−/− and gal3+/+ CD4+ T cells were stimulated as mentioned in Fig. 2F and the ratios of the cSMAC-positive cell numbers over the total cell numbers were calculated. P < 0.01. (C) Gal3−/− and gal3+/+OTII CD4+ T cells were placed on the upper chamber of a transwell device on top of a membrane coated with ICAM-1 and pMHC. Cells migrated to the lower chambers were enumerated by flow cytometry. ANOVA, P < 0.0001.
Following maturation, the IS is destabilized and T cells migrate away from APCs. We used an assay to study the effect of endogenous galectin-3 on synapse stability by analyzing the number of T cells transmigrating through a membrane coated with ICAM-1 and pMHC. We compared T-cell blasts from gal3−/−OTII and gal3+/+OTII mice. The two genotypes displayed identical transmigration in the absence of pMHC (Fig. 3C). In the presence of agonist pMHC on the membrane, more gal3+/+ T cells transmigrated than gal3−/− cells (Fig. 3C). The results strongly support that galectin-3 inhibits cSMAC formation and destabilizes the IS.
Gal3−/− CD4+ T Cells Exhibit Higher TCR-Mediated Signaling.
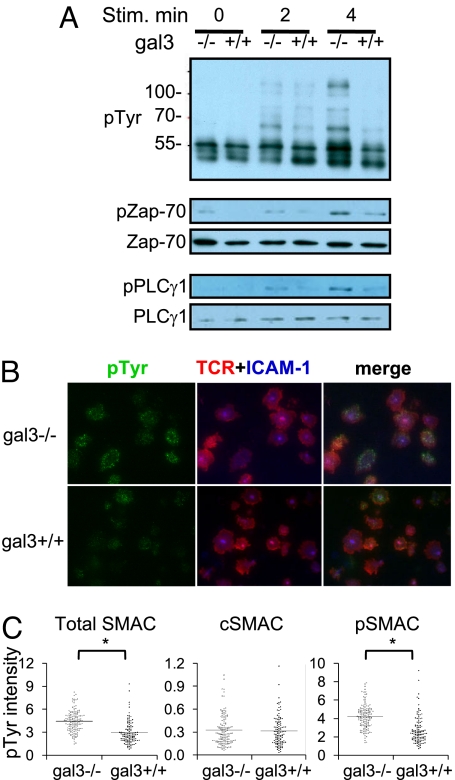
To determine whether the inhibitory function of galectin-3 on CD4+ T cells involves suppression of TCR-mediated signal transduction, we stimulated the cells with anti-CD3/CD28 and analyzed the activation of signal transduction molecules. We found that activated gal3−/− T cells produced higher levels of total tyrosine phosphorylation and enhanced activation of the early signaling molecules Zap-70 and PLCγ1 (Fig. 4A). These results indicate that galectin-3 inhibits TCR-mediated activation of CD4+ T cells, by affecting the early events in signal transduction. The differences in cytokine production observed in Fig. 1 are likely caused by these signaling alterations rather than intrinsic defects in cytokine production, as stimulation with PMA and ionomycin resulted in identical cytokine responses in gal3−/− and gal3+/+ CD4+ T cells (Fig. 1C).
Fig. 4.
Gal3−/− CD4+ T cells exhibit higher TCR-mediated signaling. (A) Purified CD4+ T cells were stimulated with plate-bound anti-CD3/CD28 for the indicated time periods and subjected to immunoblotting analysis. (B) Gal3−/− and gal3+/+ CD4+ T cells were stimulated as mentioned in Fig. 2F. The cells were fixed and stained for phosphotyrosine (pTyr) signals and the images were taken by TIRFM. (C) The relative intensities of phosphotyrosine staining of total SMAC, cSMAC, and pSMAC regions are shown. *Mann Whitney U, P < 0.05.
The levels of tyrosine phosphorylation in activated gal3−/− and gal3+/+ CD4+ T cells in the lipid bilayer system were also examined. T-cell blasts were stimulated with ICAM-1 and pMHC loaded on lipid bilayers for 30 min and fixed. The phosphotyrosine signals were detected with TIRFM and the results showed that gal3−/− CD4+ T cells exhibited higher phosphotyrosine signals at the IS compared with gal3+/+ CD4+ T cells (Fig. 4B). Comparison of the phosphotyrosine signal levels within the SMACs among those CD4+ T cells revealed that the higher signals were generated from the d and pSMACs rather than the cSMAC (Fig. 4C). The results indicate that galectin-3 inhibits TCR-mediated signaling primarily at the pSMAC.
Galectin-3 Potentiates Down-Regulation of TCR/CD3 in T Cells Following TCR Engagement.
When T cells are stimulated by TCR/CD3 engagement, surface TCR is internalized by endocytosis resulting in a down-regulation of TCRs, and it has been proposed that this down-regulation is a mechanism by which over-activation of T cells is prevented (25). Since galectin-3 is recruited to the IS and attenuates T-cell activation, we next determined the effect of galectin-3 on TCR down-regulation.
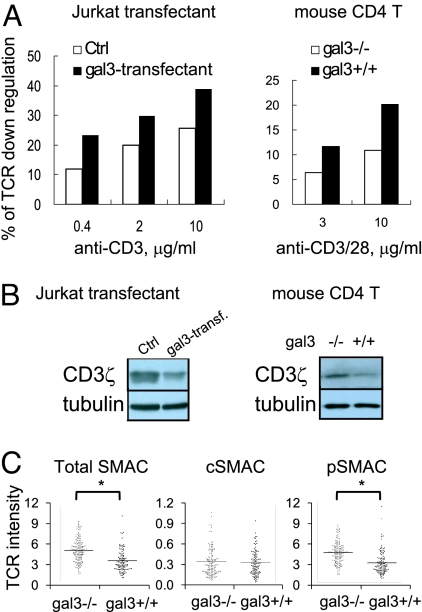
This was performed by flow cytometric analysis of TCR after activation with anti-CD3. Both galectin-3-transfected Jurkat T cells and gal3+/+ mouse CD4+ T cells showed higher TCR down-regulation than galectin-3-negative Jurkat cells and gal3−/− CD4+ T cells, respectively (Fig. 5A). Immunoblotting analysis also showed lower levels of a component of the TCR complex, CD3ζ, in galectin-3-transfected Jurkat T cells compared with control cells, and in gal3+/+ CD4+ T cells compared with gal3−/− cells (Fig. 5B). These results indicate that galectin-3 potentiates TCR down-regulation when T cells are activated through TCR engagement.
Fig. 5.
Galectin-3 potentiates down-regulation of TCR/CD3 in T cells following TCR engagement. (A) Gal3+ Jurkat cells or control transfectants (Ctrl) (Left) or gal3−/− and gal3+/+ mouse CD4+ T cells (right) were stimulated by plate-bound anti-CD3 (for Jurkat cells) or anti-CD3/CD28 (for mouse CD4+ T cells) for 2 h and then subjected to TCRβ staining followed by flow cytometric analysis. The mean fluorescence intensity (MFI) of stimulated T cells was compared with that of unstimulated cells and expressed as % of TCR down-regulation. (B) Jurkat-transfectants (Left) or mouse CD4+ T cells (Right) were stimulated with anti-CD3 (or anti-CD3/CD28) for 8 h and then subjected to immunoblotting analysis for the CD3ζ levels. (C) Gal3−/− and gal3+/+ CD4+ T cells were stimulated as mentioned in Fig. 2F. The relative fluorescence intensities of TCR on the total SMAC, cSMAC, and pSMAC are shown. *Mann Whitney U, P < 0.05.
The site of TCR down-regulation, the IS, was also examined in T cells stimulated with lipid bilayers loaded with agonist proteins. The TCR levels on the proximal surfaces (as visualized by TIRFM) in the synapse were compared. The results showed that the TCR levels in the pSMAC were lower in gal3+/+ CD4+ T cells compared with gal3−/− cells (Fig. 5C). Other than that gal3+/+ CD4+ T cells formed less cSMAC than gal3−/− counterparts, there was no difference in the intensity of the individual cSMAC formed in these two cell populations.
Carbohydrate-Binding Activity Is Unnecessary for Translocation of Galectin-3 to the IS and Galectin-3's Suppressive Effect on T Cells Is Not Dependent on its Binding to Cell Surface Glycans.
To determine whether recruitment of galectin-3 to the IS is mediated through its carbohydrate-binding activity, we studied Jurkat T cells transfected with a galectin-3 mutant lacking carbohydrate-binding activity. The mutant protein was found to be localized at the IS in Jurkat cells co-cultured with SEE-pulsed B cells as APCs (Fig. S1A) or placed on lipid bilayers (Fig. S1B). We also studied the translocation of galectin-3 in galectin-3-transfected Jurkat T cells in the presence of a galectin-3 inhibitor, lactose, which is known to inhibit galectin-3's carbohydrate-binding activity. If galectin-3's localization to the IS involves the protein's interaction with cell surface glycans, the amount of the protein in the IS should be diminished in the presence of lactose. We found the intensity of the galectin-3 signals in the IS was not affected (Fig. S1C).
We also determined whether the suppression of the T-cell response by galectin-3 occurs through binding of galectin-3 secreted by the cells to cell surface glycans. We activated gal3+/+ and gal3−/− CD4+ T cells with anti-CD3/CD28 in the presence of lactose. Gal3−/− cells secreted higher amounts of IFN-γ than gal3+/+ cells regardless of the presence of lactose (Fig. S1D). As lactose would inhibit the binding of extracellular galectin-3 to cell surface glycans, these results support an intracellular location of the functional site where galectin-3 attenuates T-cell activation.
Galectin-3 Binds to the ESCRT Protein Alix at the IS.
To identify potential interactors of galectin-3 that contribute to its functions described above, we used a yeast two-hybrid system using the full-length galectin-3 cDNA as a bait to screen a cDNA library derived from Jurkat T lymphoma. We identified a protein called PDCD6-interacting protein (ALG-2 interacting protein X, Alix). Alix is a component of ESCRT pathway which is required for biogenesis of multivesicular bodies (MVBs), cytokinesis, and retroviral budding (reviewed in refs. 26 and 27). Similar to galectin-3, which has a proline-rich domain in the N-terminal region, it contains a proline-rich domain in its C-terminal portion.
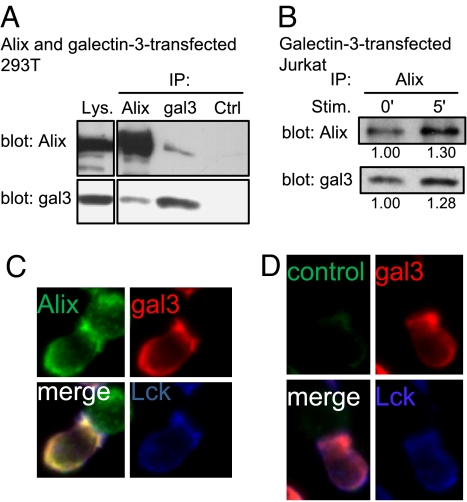
To confirm binding of galectin-3 to Alix inside the cell, we co–transfected 293T human embryonic kidney cells with plasmid DNAs coding for Alix and galectin-3. The cells were treated with a membrane permeable chemical crosslinker, dithiobis[succinimidylpropionate] (DSP), before lysis, and the cell lysates were subjected to immunoprecipitation using anti-Alix or anti-galectin-3 antibody. The results showed that Alix was co-immunoprecipitated with galectin-3 (Fig. 6A). Binding of Alix to galectin-3 was also demonstrated in galectin-3-transfected Jurkat T cells and there was an increased level of association between these two proteins, when these cells were activated by TCR engagement (Fig. 6B). Finally, we determined whether these two proteins are colocalized by immunofluorescence analysis. We stimulated galectin-3-transfected Jurkat cells with superantigen-pulsed RPMI-8866 B cells and processed them for immunofluorescence staining of Alix and galectin-3. As shown in Fig. 6 C and D, these two proteins are colocalized at the IS.
Fig. 6.
Galectin-3 is associated with Alix at the IS. (A) HEK-293T cells were co-transfected with plasmid DNAs containing Alix and galectin-3. The cells were treated with DSP and lysed, and the lysates were subjected to immunoprecipitation using anti-Alix, anti-galectin-3 (gal3) or control sera (Ctrl). The lysates (Lys.) and precipitates were immunoblotted with anti-Alix and anti-galectin-3 antibodies. (B) Gal3+ Jurkat cells were stimulated with anti-CD3-coated beads for 0 and 5 min and subjected to DSP cross-linkage. The lysates were immunoprecipitated with anti-Alix and immunoblotted with anti-Alix and anti-galectin-3 antibodies. The amounts of Alix and galectin-3 induced by the stimulus, relative to their baseline levels, are shown under each blot. (C and D) Galectin-3-transfected Jurkat T cells were stimulated for 5 min with SEE-pulsed RPMI 8866 B cells. The cells were fixed and stained with antibodies for galectin-3 (red) and Lck (blue), together with either anti-Alix antibody (green) (C) or a control antibody (D).
Discussion
Here, we report that endogenous galectin-3 exhibits an inhibitory function in the TCR-mediated cytokine response in CD4+ T cells. Expression of galectin-3 in these cells is associated with lower levels of cytokine production, when the cells are activated through TCR engagement, which is accompanied by attenuated signal transduction, including lower levels of tyrosine phosphorylation. Remarkably, we discovered that galectin-3 is translocated to the IS in cells activated by TCR engagement and it is mainly localized at the pSMAC. Galectin-3's suppression of phosphotyrosine signals also occurs at the pSMAC, but not the cSMAC. Galectin-3 expression suppresses the formation of cSMAC, suggesting that galectin-3 inhibits TCR aggregation and signaling mediated through these microclusters. Moreover, in the process of CD4+ T cell-APC interactions, galectin-3 expression in T cells is negatively correlated with the strength of the interaction, but positively correlated with the rate of dissociation of the conjugated cells, suggesting that galectin-3 destabilizes the IS. The inhibitory function of galectin-3 is associated with increased TCR down-regulation. Finally, we have identified Alix, a known regulator of receptor down-regulation, as a galectin-3-interacting protein, and shown that it is also recruited to the IS in activated T cells. Although galectin-3 is known to have extracellular functions that occur through engagement of cell surface glycans, our results suggest that this protein is localized at the cytosolic side of the IS and exerts its inhibitory effect in T cells by functioning intracellularly.
In the setting of T cell-APC interactions, our data suggest that the inhibitory function of galectin-3 on T-cell activation may be related to the fact that galectin-3 expression in T cells results in a weaker adhesion of these cells to APCs. This is evidenced by a higher degree of migration of T cells away from APCs (Fig. 3B) and a lower degree of adhesion of T cells to APCs (Fig. 3C). We believe that the lower association between CD4+ T cells and APCs is a result of lower TCR levels. Thus, we propose that by functioning intracellularly at the IS, galectin-3 negatively regulates the adhesion between T cells and APCs, and one possible mechanism is through down-regulation of TCR.
Both dSMAC and pSMAC have been shown to be the location of TCR-mediated signaling through recruitment of signaling molecules into TCR microclusters, followed by translocation of TCR microclusters toward the center of the IS to form the cSMAC. The cSMAC is thought to be the center of TCR internalization and down-regulation (25), but the amount of TCR in this compartment is not altered by galectin-3. Our results suggest that galectin-3 is localized primarily in the pSMAC, which is also the region in which TCR is down-regulated by galectin-3. There are a number of possible mechanisms, since TCR down-regulation involves the internalization of the receptor complexes by endocytosis, their degradation in lysosomes, and recycling back to the cell surface (28, 29). It is possible that TCR internalization takes place in the pSMAC and galectin-3 regulates this process, but the dense actin network may prevent internalization until TCR reaches the actin depleted cSMAC (23, 24). Galectin-3 may also mediate modifications of TCR that favor degradation rather than recycling, such as ubiquitination. It is also possible that pSMAC is the site of entry for recycled TCR and that galectin-3 may function by inhibiting endocytosed TCR from recycling back to the pSMAC.
Galectin-3 contains features that make it suitable for involvement in the TCR-mediated early signaling cascade intracellularly. First, a number of negative signaling proteins involved in TCR-mediated signal cascade contain proline-rich sequences (30) and galectin-3 contains proline-rich tandem repeats in the N-terminal region. Second, it is known that a number of inhibitory proteins involved in TCR signaling are recruited into the IS by interacting with other components containing a Src homology 3 (SH3) domain(s) [for example, Nef (31) and CD2AP (25)] and that proteins bound by the SH3 domains contain PXXP motifs (32). Interestingly, galectin-3 contains several PXXP motifs. Thus, galectin-3 may be recognized by other SH3 domain-containing signaling molecules recruited into the synapse. Thirdly, many molecules involved in the signal transduction cascade are phosphorylated. Galectin-3 can be phosphorylated at serine 6 and serine 12 (33) and phosphorylation at these residues has been associated with its intracellular functions (34–36).
We have identified a protein, Alix, that galectin-3 binds to and is a likely link of its function in T cells. This protein is known to interact with a number of intracellular regulators that are involved in endocytosis and down-regulation of certain cell surface receptors, as well as signal transduction (reviewed in ref. 37). For example, it binds to SETA (CIN85) (38) and endophilin (39), which are involved in receptor endocytosis and down-regulation (40, 41); Src, which is involved in activation of cellular signaling cascade (42); and FAK and PYK-2, which are involved in focal adhesion (43). In particular, Alix has been shown to antagonize the formation of SETA-endophilin-Cbl complex that facilitates down-regulation of epidermal growth factor receptor (41). Interestingly, a SETA homologue, CD2AP, was observed to promote TCR down-regulation, possibly through cSMAC (25). Thus, Alix may antagonize TCR down-regulation, through binding to CD2AP at the IS, and, moreover, galectin-3 may promote TCR down-regulation by attenuating Alix's function.
In studying the function of a glycosyltransferase, Mgat5, other investigators found that TCR is modified by Mgat5 and TCR complex components bind to extracellular galectin-3 (44). Treatment of Mgat5+/+ T cells with lactose (to elute lactose-binding proteins off the cell surface) enhanced TCR lateral motility and the T-cell response. These authors proposed a model in which extracellular galectin-3 forms lattices with TCR, thereby restricting TCR lateral mobility and suppressing the T-cell response. Our results, however, indicate that endogenous galectin-3 suppresses TCR signaling by facilitating receptor down-regulation through interactions with intracellular regulatory proteins.
Although galectins are demonstrated to function extracellularly, where their glycan ligands predominantly reside, accumulating evidence points to their intracellular roles (45, 46). We have previously shown that galectin-3 regulates phagocytosis by macrophages (10) and mediator release/cytokine production by mast cells (9), through its intracellular actions. Other investigators have reported that galectin-3 forms complexes with oncogenic K-Ras (47). In addition, galectin-3's functions appear to be related to its tendency to translocate to intracellular membranous structures. For example, galectin-3 has been shown to translocate to the mitochondria in cells treated with apoptotic stimuli (48), as well as to the phagosomes in macrophages undergoing phagocytosis (10), and be located in the lipid rafts on the membrane of dendritic cells (49).
In summary, we have identified a function of galectin-3 in CD4+ T cells and established that this protein serves as a negative regulator in the TCR-mediated T-cell response. We found that galectin-3 is recruited intracellularly to the IS and promotes TCR down-regulation at the pSMAC. Our studies suggest an important role of galectin-3 in regulation of the T-cell response and the associated mechanism. The findings also strengthen the existence of intracellular actions of the galectin family members and provide insight into the functions of these proteins.
Materials and Methods
For materials, please see SI Text.
Signaling.
Purified CD4+ T cells were stimulated with plate-bound anti-CD3/CD28 and the activation was stopped at different time points by using SDS sample buffer. The total cell lysates were subjected to immunoblotting analysis by using antibodies specific for phospho-PLCγ, phospho-Zap 70, and phosphostyrosine.
Immunofluorescence Staining of the IS.
Jurkat cells transfected with galectin-3 or control transfecants were mixed with SEE-pulsed RPMI8866 B cells attached on polyL-lysine-coated coverslides on ice for 15 min. The coverslides were then transferred to 37 °C for 5 min and subsequently fixed with 2% paraformaldehyde to stop the reaction. The cells were stained with rabbit anti-Zap70 and biotinylated goat anti-human galectin-3, followed by staining with Rhodamine-conjugated goat anti-rabbit IgG and Alexa fluor 488 (AF-488)-conjugated streptavidin, respectively. The stained cells were observed with a deconvolution fluorescence microscope. Jurkat T cells were also stimulated by goat anti-mouse IgG.Fc coated latex beads followed by mouse anti-human CD3 mAb on cold coverslides and stained as above. Alternatively, CD4+ T cells activated by Con A and IL-2 were exposed to beads coated with hamster anti-mouse CD3. The mixtures were then stained by rabbit anti-rat galectin-3 followed by Rhodamine-conjugated goat anti-rabbit IgG, AF-488-phalloidins and Hoechst 33258. For immunofluorescence staining of Alix, B-T cell conjugates were stained by rabbit anti-LCK, mouse anti-Alix (AbD Serotec) and biotinylated goat anti-galectin-3 followed by fluorescence labeled secondary antibodies.
Transmigration Assay.
In vitro activated, purified and IL-2-maintained gal3−/− and gal3+/+ CD4+ OTII T cells (14 days) were placed onto the upper wells of transwell plates (Millipore Multiscreen-MIC plates, MAMIC5S10, 3-μm pore size), which were precoated with ICAM-1-GPI (50 mol/μm2) and subsequently with serially diluted pMHC. The cells in the upper wells were cultured in RPMI-1640/1% FBS and incubated at 37 °C and 5% CO2 for 2 h. The transmigrated cells in the lower wells were counted by FACS with 3-μm beads as a reference.
Adhesion Assay.
Gal3−/− and gal3+/+ CD4+ OTII T blast cells were labeled with membrane dye DiO per the manufacturer's instructions (Molecular Probes) and individually mixed with DiI-labeled BMDC as APCs in the absence or presence of 1 μg/mL OVA323–339 peptide. The cell mixtures were cultured at 37 °C and 5% CO2 for 0.5, 1, and 2 h. Cells were fixed at each time point with 2% paraformaldehyde and subjected to flow cytometric analysis. The percentage of cell conjugates were calculated by dividing the double-fluorescence labeled cell counts over the total stained T-cell counts.
Induction of the IS on Supported Lipid Bilayers.
Please see SI Text.
Assays for TCR Down-Regulation.
Jurkat cells transfected with galectin-3 or control transfectants were stimulated by goat anti-mouse IgG.Fc coated latex beads that were premixed with mouse anti-human CD3. The cells were placed in a 96-well U bottom plate, cultured for 1 h, and then stained with PC5-conjugated anti-CD3ε at 4 °C. The cells were analyzed by flow cytometry and the percentage of mean fluorescence intensity (MFI) decrease was calculated as [(MFIno anti-CD3-MFIanti-CD3)/MFIno anti-CD3]. For the CD3ζ levels, Jurkat transfectants or mouse CD4+ T cells were stimulated with anti-CD3/CD28 overnight in the presence of 10 μg/mL cycloheximide and subsequently subjected to immunoblotting analysis with anti-CD3ζ antibody (Santa Cruz Biotechnology).
Yeast Two-Hybrid Screening for Galectin-3-Binding Partners.
Please see SI Text.
Co-Immunoprecipitation of Galectin-3 and Alix.
HEK-293T were co-transfected with plasmid DNA coding for Alix (cat # MC201554, Origene) and plasmid DNA coding for galectin-3. The cells were washed with PBS and treated with 1 mM DSP (Pierce) at room temperature for 30 min and lysed with buffer containing 1% TX-100, 0.5% deoxylcholate, 0.1% SDS, and protease inhibitor (Sigma). The lysates were immunoprecipitated with rabbit anti-Alix serum, anti-galectin-3, or control serum and protein A-Sepharose beads. The lysates were immunoblotted with antibodies against Alix or galectin-3.
Supplementary Material
Acknowledgments.
We thank Lan Yu, Rajat Varma, Tom Cameron, and Yang Dai for technical assistances and discussion. This work is supported by National Institutes of Health Grant 2R01 AI020958.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903497106/DCSupplemental.
References
- 1.Yang RY, Rabinovich GA, Liu FT. Galectins: Structure, function, and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 2.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: A small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 3.Garner OB, Baum LG. Galectin-glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem Soc Trans. 2008;36:1472–1477. doi: 10.1042/BST0361472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 5.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 6.Liu FT. Regulatory roles of galectins in the immune response. Int Arch Allergy Immunol. 2005;136:385–400. doi: 10.1159/000084545. [DOI] [PubMed] [Google Scholar]
- 7.Nakahara S, Oka N, Raz A. On the role of galectin-3 in cancer apoptosis. Apoptosis. 2005;10:267–275. doi: 10.1007/s10495-005-0801-y. [DOI] [PubMed] [Google Scholar]
- 8.Hsu DK, Yang RY, Liu FT. Galectins in apoptosis. Methods Enzymol. 2006;417:256–273. doi: 10.1016/S0076-6879(06)17018-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen HY, et al. Role of galectin-3 in mast cell functions: Galectin-3-deficient mast cells exhibit impaired mediator release and defective JNK expression. J Immunol. 2006;177:4991–4997. doi: 10.4049/jimmunol.177.8.4991. [DOI] [PubMed] [Google Scholar]
- 10.Sano H, et al. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest. 2003;112:389–397. doi: 10.1172/JCI17592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacKinnon AC, et al. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008;180:2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- 12.Joo HG, et al. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001;69:555–564. [PubMed] [Google Scholar]
- 13.Hsu DK, et al. Human T lymphotropic virus-I infection of human T lymphocytes induces expression of the beta-galactoside-binding lectin, galectin-3. Am J Pathol. 1996;148:1661–1670. [PMC free article] [PubMed] [Google Scholar]
- 14.Stillman BN, et al. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- 15.Fukumori T, et al. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63:8302–8311. [PubMed] [Google Scholar]
- 16.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA. 1996;93:6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol Rev. 2008;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 18.Yokosuka T, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 19.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monks CR, et al. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 21.Grakoui A, et al. The immunological synapse: A molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 22.Freiberg BA, et al. Staging and resetting T cell activation in SMACs. Nat Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 23.Varma R, et al. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaizuka Y, et al. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc Natl Acad Sci USA. 2007;104:20296–20301. doi: 10.1073/pnas.0710258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KH, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 26.Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: A role for the ESCRT machinery. Science. 2007;316:1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 27.Fujii K, Hurley JH, Freed EO. Beyond Tsg101: The role of Alix in ‘ESCRTing’ HIV-1. Nat Rev Microbiol. 2007;5:912–916. doi: 10.1038/nrmicro1790. [DOI] [PubMed] [Google Scholar]
- 28.Valitutti S, Muller S, Salio M, Lanzavecchia A. Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J Exp Med. 1997;185:1859–1864. doi: 10.1084/jem.185.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alcover A, Alarcon B. Internalization and intracellular fate of TCR-CD3 complexes. Crit Rev Immunol. 2000;20:325–346. [PubMed] [Google Scholar]
- 30.Yamasaki S, Saito T. Inhibitory adaptors in lymphocytes. Semin Immunol. 2004;16:421–427. doi: 10.1016/j.smim.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Fackler OT, Alcover A, Schwartz O. Modulation of the immunological synapse: A key to HIV-1 pathogenesis? Nat Rev Immunol. 2007;7:310–317. doi: 10.1038/nri2041. [DOI] [PubMed] [Google Scholar]
- 32.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huflejt ME, et al. L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. J Biol Chem. 1993;268:26712–26718. [PubMed] [Google Scholar]
- 34.Mazurek N, et al. Phosphorylation of the beta-galactoside-binding protein galectin-3 modulates binding to its ligands. J Biol Chem. 2000;275:36311–36315. doi: 10.1074/jbc.M003831200. [DOI] [PubMed] [Google Scholar]
- 35.Yoshii T, et al. Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. J Biol Chem. 2002;277:6852–6857. doi: 10.1074/jbc.M107668200. [DOI] [PubMed] [Google Scholar]
- 36.Takenaka Y, et al. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol Cell Biol. 2004;24:4395–4406. doi: 10.1128/MCB.24.10.4395-4406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odorizzi G. The multiple personalities of Alix. J Cell Sci. 2006;119:3025–3032. doi: 10.1242/jcs.03072. [DOI] [PubMed] [Google Scholar]
- 38.Chen B, et al. The glioma-associated protein SETA interacts with AIP1/Alix and ALG-2 and modulates apoptosis in astrocytes. J Biol Chem. 2000;275:19275–19281. doi: 10.1074/jbc.M908994199. [DOI] [PubMed] [Google Scholar]
- 39.Chatellard-Causse C, et al. Alix (ALG-2-interacting protein X), a protein involved in apoptosis, binds to endophilins and induces cytoplasmic vacuolization. J Biol Chem. 2002;277:29108–29115. doi: 10.1074/jbc.M204019200. [DOI] [PubMed] [Google Scholar]
- 40.Soubeyran P, et al. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 2002;416:183–187. doi: 10.1038/416183a. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt MH, et al. Alix/AIP1 antagonizes epidermal growth factor receptor downregulation by the Cbl-SETA/CIN85 complex. Mol Cell Biol. 2004;24:8981–8993. doi: 10.1128/MCB.24.20.8981-8993.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt MH, Dikic I, Bogler O. Src phosphorylation of Alix/AIP1 modulates its interaction with binding partners and antagonizes its activities. J Biol Chem. 2005;280:3414–3425. doi: 10.1074/jbc.M409839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt MH, Chen B, Randazzo LM, Bogler O. SETA/CIN85/Ruk and its binding partner AIP1 associate with diverse cytoskeletal elements, including FAKs, and modulate cell adhesion. J Cell Sci. 2003;116:2845–2855. doi: 10.1242/jcs.00522. [DOI] [PubMed] [Google Scholar]
- 44.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 45.Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263–273. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 46.Wang JL, Gray RM, Haudek KC, Patterson RJ. Nucleocytoplasmic lectins. Biochim Biophys Acta. 2004;1673:75–93. doi: 10.1016/j.bbagen.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Elad-Sfadia G, Haklai R, Balan E, Kloog Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J Biol Chem. 2004;279:34922–34930. doi: 10.1074/jbc.M312697200. [DOI] [PubMed] [Google Scholar]
- 48.Yu F, Finley RL, Jr, Raz A, Kim HR. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J Biol Chem. 2002;277:15819–15827. doi: 10.1074/jbc.M200154200. [DOI] [PubMed] [Google Scholar]
- 49.Hsu DK, et al. Endogenous galectin-3 is localized in membrane lipid rafts and regulates migration of dendritic cells. J Invest Dermatol. 2009;129:573–583. doi: 10.1038/jid.2008.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.