Abstract
Despite decades of debate, it remains unclear whether human bipedalism evolved from a terrestrial knuckle-walking ancestor or from a more generalized, arboreal ape ancestor. Proponents of the knuckle-walking hypothesis focused on the wrist and hand to find morphological evidence of this behavior in the human fossil record. These studies, however, have not examined variation or development of purported knuckle-walking features in apes or other primates, data that are critical to resolution of this long-standing debate. Here we present novel data on the frequency and development of putative knuckle-walking features of the wrist in apes and monkeys. We use these data to test the hypothesis that all knuckle-walking apes share similar anatomical features and that these features can be used to reliably infer locomotor behavior in our extinct ancestors. Contrary to previous expectations, features long-assumed to indicate knuckle-walking behavior are not found in all African apes, show different developmental patterns across species, and are found in nonknuckle-walking primates as well. However, variation among African ape wrist morphology can be clearly explained if we accept the likely independent evolution of 2 fundamentally different biomechanical modes of knuckle-walking: an extended wrist posture in an arboreal environment (Pan) versus a neutral, columnar hand posture in a terrestrial environment (Gorilla). The presence of purported knuckle-walking features in the hominin wrist can thus be viewed as evidence of arboreality, not terrestriality, and provide evidence that human bipedalism evolved from a more arboreal ancestor occupying the ecological niche common to all living apes.
Keywords: bipedalism, development, hominoid, homoplasy, wrist
Since Darwin first discussed pathways of human evolution in The Descent of Man, there has been an ongoing and often rancorous debate over the nature of locomotion in our prebipedal human ancestor. The debate can be summarized with 2 competing models. One model envisions the prehuman ancestor as a terrestrial knuckle-walker, a behavior frequently used by our closest living relatives, the African apes (1–6). In the alternative model, early human bipedalism is seen as having evolved from a more generalized arboreal, climbing-oriented ancestor, a mode of locomotion that is used by all living apes (7–10). Each scenario has important and profoundly different implications for understanding the evolution of ape and human locomotion.
If early human bipedalism evolved from an arboreal ancestor, current ape-human phylogeny showing chimpanzees and bonobos as the sister taxa of humans (supporting information (SI) Fig. S1) logically implies that knuckle-walking evolved independently in both African ape lineages (Gorilla and Pan). In contrast, proponents of a terrestrial knuckle-walking hypothesis of human locomotor evolution hypothesize that African apes and humans share a common knuckle-walking ancestor. Advocates for this model support their claims by arguing that there are specific morphological features, particularly of the wrist and hand, that reflect this behavior in all living African apes and that can also be found in fossil and living humans (2, 4–6).
Studies advocating a terrestrial knuckle-walking ancestor and arguing for a clear form-function relationship in the primate wrist have neglected to consider variation within and across species and age classes. Previous analyses of African ape hand morphology have documented variation in metacarpal knuckle-walking features (11–13) and allometric growth of the phalanges (14) and carpals (15) between Pan and Gorilla. Building upon this work, we test the specific hypotheses that posited knuckle-walking features of the wrist are consistently found in both Pan and Gorilla using novel ontogenetic data. The presence of such morphology across all African apes would provide strong evidence that these features are indeed knuckle-walking adaptations. However, variation in the developmental timing, frequency, and/or expression of these features between Pan and Gorilla may suggest a more complicated pathway for the evolution of knuckle-walking behavior which, in turn, has profound implications for the locomotor origin of human bipedalism. This study provides detailed data on variation in putative knuckle-walking features of the wrist in both adult and juvenile African apes and other primates and we interpret our results in both a biomechanical and behavioral context.
The features most commonly thought to reflect knuckle-walking behavior in the African ape wrist are listed in Table 1. It has been argued that these features reflect the need for increased stability and limited extension* at the radiocarpal and midcarpal joints during the stance phase of knuckle-walking locomotion (2, 4–6, 16–19). However, these conclusions have been based on small sample sizes, often of chimpanzee morphology alone (16, 17), and have been made in the absence of specific biomechanical data on hand posture or context of locomotion across taxa. Not only is it imperative to analyze these wrist features in all species of African apes, but an analysis of the development of these features throughout ontogeny is also necessary to understand fully the functional significance of morphological variation between Pan and Gorilla. Bony morphology is thought to reflect at least to some extent function during development (20–22). For example, the degree of phalangeal curvature is positively correlated with increased arboreality in African apes during ontogeny (23, 24). The absence of some knuckle-walking features in hominins has been attributed to lack of function during development (5). Thus, ontogenetic analyses can provide a critical insight into the homology and function of morphological features that cannot be gained from adult morphology alone (25, 26).
Table 1.
List of commonly discussed knuckle-walking features of the African ape wrist and their proposed adaptive function
| Feature | Hypothesized function |
|---|---|
| Scaphoid dorsal concavity & | together accommodate the dorsal extension of the distal radius, |
| Scaphoid beak | limiting extension at the radiocarpal joint (4–6,17) |
| Capitate distal concavity & | receives scaphoid between concavity and trapezoid and together |
| Capitate waisting | they limit extension of capitate-scaphoid joint (12, 19) |
| Capitate dorsal ridge & | together limit extension of the proximal carpal row on the distal |
| Hamate dorsal ridge | carpal row (5) |
| Hamate distal concavity | limits extension at the triquetro-hamate joint (19) |
Most of these knuckle-walking features are small, irregular-shaped, morphological outgrowths that cannot be accurately measured quantitatively. Therefore, the timing of appearance, frequency and expression of these features on the scaphoid, capitate and hamate was best assessed qualitatively (rather than quantitatively) throughout juvenile and adult ontogeny.
Knuckle-walking is rare among mammals and unique to African apes among primates (1, 16). Previously researchers have always treated knuckle-walking in Pan and Gorilla as a unified biomechanical phenomenon (1–14, 16–19). This assumption persists despite well-known variation in substrate use among African apes (27–30), as well as known differences in hand posture across species (16, 31, 32) and substrates (33). When considered without respect to important confounding variables like substrate and hand posture, variation in morphology across species of knuckle-walking apes is difficult to interpret and leads to overly complex evolutionary scenarios. However, when considered in an appropriate biomechanical and ecological context, that same variation between Gorilla and Pan is more interpretable and the insights derived from such analyses provide a novel foundation for comparative tests concerning the functional significance of posited knuckle-walking morphology.
As adults, all African apes engage in the same amount of knuckle-walking (27, 30), but they do not do so in the same ecological setting; chimpanzees and bonobos spend significantly more time in the trees than do gorillas (28, 29). In addition, juvenile chimpanzees and bonobos engage in less knuckle-walking than do juvenile gorillas (34, 35).
Therefore, if features of the African ape wrist are to be considered reflective of knuckle-walking behavior as some have suggested (2, 4–6, 18, 19), we should find the following patterns. First, these features should be common to all African apes. Second, these features should be equally, if not more, pronounced in gorillas given their more frequent knuckle-walking behavior and increased load associated with a larger body mass. Finally, compared to Pan, these features should appear relatively earlier in ontogeny in gorillas given their faster growth rate in body mass (36) and the fact that they engage in an adult-like frequency of knuckle-walking behavior at a much earlier age as a consequence of their larger size (28, 34, 35). If the hypothesis that features of the wrist are functionally related to knuckle-walking cannot be supported by these types of analyzes, then the notion that human bipedalism evolved from a terrestrial knuckle-walking ancestor must also be reevaluated and possibly rejected.
Results and Discussion
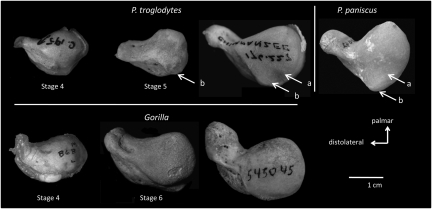
Analysis of the timing of appearance, frequency, and expression of the posited knuckle-walking features (Table 1) in adult and juvenile apes and monkeys (Table S1) supports previous analyses (5, 6, 18) that African ape carpal morphology on the whole can be distinguished from that of Asian apes, arboreal, and terrestrial monkeys, and humans. However, this analysis also reveals a previously unrecognized and surprisingly high amount of morphological variation in adult African ape carpal features that casts doubt on the assumed functional link between specific aspects of wrist morphology and knuckle-walking behavior. We detected significant (P < 0.05 using a χ2 goodness-of-fit test; Table S2) differences in the presence (defined generously to include even weakly developed features) or absence of knuckle-walking features among African apes. Gorillas are significantly different from both chimpanzees and bonobos in their low frequency of all but 2 of the knuckle-walking features analyzed here. For example, the dorsal concavity and beak of the scaphoid, are found in almost all (96%) adult Pan troglodytes in this sample (n = 32) and are fully developed in 76% of Pan paniscus (n = 21). Yet these same features are found together in only 6% of Gorilla (n = 45), regardless of sex or size (Table 2; Fig. 1). Furthermore, both features are also commonly found in nonknuckle-walking arboreal palmigrade (80%, n = 15) monkeys and terrestrial palmigrade (73%, n = 11) and digitigrade (57%, n = 14) monkeys (Table S2).
Table 2.
Qualitative analysis of the ontogenetic timing, frequency and morphology of putative knuckle-walking carpal features in African apes. Frequency of posited knuckle-walking features in African apes evaluated as either present (including poorly developed features) or absent. Juvenile stages divided into stages based on dental eruption and roughly correspond to the following chronological ages (in years) in Pan and Gorilla, respectively (see Table S1): Stage 3: 0.8–4 and 1–3.5; Stage 4: 4–6 and 3.5–4.75; Stage 5: 6–8 and 4.75–7; Stage 6: 8–10 and 7–9; Stage 7: 10–12 and 9–11. (*) Juvenile morphology describes both P. troglodytes and P. paniscus
| Taxon | Stage | Hypothesized knuckle-walking features |
|||||
|---|---|---|---|---|---|---|---|
| Scaphoid |
Capitate |
Hamate |
|||||
| Beak | Dorsal concavity | Distal concavity | Dorsal ridge | Distal concavity | Dorsal ridge | ||
| P. troglodytes | Juv.* | Appears at Stage 5 in 38% of n = 21 | Appears at Stage 6 in 21% of n = 29 | Appears at Stage 4 and in 90% of specimens from Stage 5–7 (n = 61) | Appears at Stage 4 in 27% (n = 15) and 77% of specimens from Stage 5–7 (n = 60) | Appears at Stage 4 in 90% of n = 10 | Appears at Stage 5 and found in 51% of specimens from Stage 5–7 (n = 74) |
| Adult | Both traits well-developed and present in 96% of adults (n = 32) | well-developed and present in all specimens | well-developed and present in 81% of adults (n = 32) | deeper than Gorilla and present in all | well-developed and present in 84% of adults (n = 32) | ||
| P. paniscus | Adult | Both traits present in 76% of adults (n = 21) | well-developed and present in all specimens | well-developed and present in 81% of adults (n = 21) | similar to P. troglodytes and deeper than Gorilla | well-developed and present in 86% of adults (n = 21) | |
| Gorilla | Juv. | Appears at Stage 5 in 17% of n = 6 | Appears at Stage 7 in 13% of n = 8 | Appears at Stage 5 in 29% of n = 7 and 64% of specimens from Stages 6–7 (n = 11) | Appears at Stage 6 in 14% of n = 7 | Appears at Stage 3 but shallower than Pan | Appears at Stage 4 and is found in 20% of juvenile stages (n = 30) |
| Adult | poorly developed, decoupled or absent; found alone in 11% of n = 45 | poorly developed, decoupled or absent; found alone in 16% of n = 45 | shallower than Pan or absent, present in 75% of n = 36 | poorly developed or absent, found in only 53% of n = 36 | shallower than Pan but present in all specimens | poorly developed or absent, found in only 39% of adults (n = 36) | |
| Both traits found together in only 6% of n = 45 | |||||||
Fig. 1.
Ontogenetic morphology of putative knuckle-walking features in African ape scaphoids. Proximolateral view of left scaphoids. Juvenile specimens are labeled by developmental stage (Table S1) and all other specimens are adult. Both the dorsal concavity (A) and beak (B) of the scaphoid's radial facet appear earlier and are more common and accentuated throughout ontogeny in P. troglodytes and P. paniscus. These features are rarely found in Gorilla and instead the corresponding area is round and convex (all specimens to 1 cm scale).
Thus, these results show (i) that most gorillas lack key features that have been assumed to be critical for limiting extension of the wrist during knuckle-walking (4, 17), and (ii) these features are found in monkeys that use a variety of different hand postures and substrates. In addition, the ontogenetic analysis shows that the features of the scaphoid that are assumed to be essential for knuckle-walking (2, 4–6) are not only inconsistently developed in Gorilla, but, when present, do not appear relatively earlier in development in gorillas (Table 2). Therefore, using the traditional functional interpretation of these features (17), it would appear that the Gorilla radiocarpal joint may be actually less, rather than more, stable in extension throughout ontogeny compared to Pan.
A similar lack of correlation is found between knuckle-walking behavior and the ontogeny of scaphoid-centrale fusion (37). This feature is the most commonly discussed knuckle-walking feature in African ape and human wrists (2, 4–6). The strong heterochronic pattern found in the increasingly earlier timing of this fusion among hominoids, in addition to the occurrence of fusion in several nonknuckle-walking, highly arboreal strepsirrhine primates, suggests that this trait may be more clearly linked to phylogeny than to function (37).
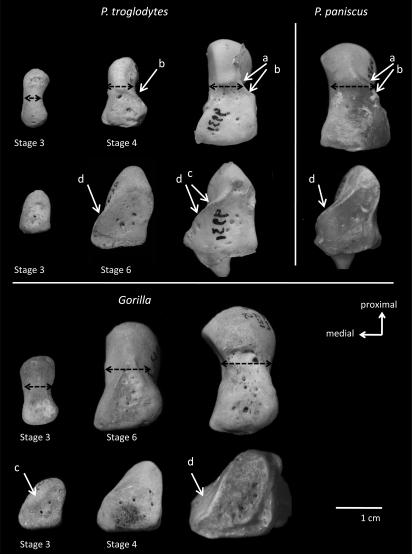
The same pattern is found repeatedly in other carpal bones as well. For example, the morphology of the capitate and hamate has also traditionally been used to diagnose knuckle-walking behavior (5, 6, 18, 19). Compared to other primates, the capitate and hamate in African apes are said to have extension-limiting ridges and concavities that have been interpreted as important features that enhance stability during weight-bearing in knuckle-walking postures (5, 6, 19) (Fig. 2). As expected, these features are common in adult Pan (81–100%). But they are also fully developed in several nonknuckle-walking monkeys (17, 19, 38) (Table S2). However, these ridges and concavities are found at a much lower frequency in Gorilla (as low as 39%) and, when present, are less accentuated than in Pan (Table 2).
Fig. 2.
Ontogenetic morphology of putative knuckle-walking features in African ape capitates and hamates. Dorsal view of right capitate (Above) and hamate (bBelow). Juvenile stages are labeled and all other specimens are adult. Black dashed line shows less capitate waisting at all stages of ontogeny in Gorilla compared to Pan (see Fig. S2). The capitate's distal concavity (A) and dorsal ridge (B) appear earlier and are more common and accentuated in Pan but are frequently absent in Gorilla. In Gorilla the hamate's distal concavity (C) is shallower and the dorsal ridge (D) appears earlier in ontogeny but is again less frequent and accentuated in adults compared to Pan (all specimens to 1 cm scale).
Narrowing or “waisting” of the capitate body, a feature that has been interpreted as limiting extension at the midcarpal joint, is also less accentuated in Gorilla compared to Pan (14) and even compared to some arboreal and terrestrial monkeys (39) (Fig. 2). A ratio of capitate neck width to length of the capitate body in adult African apes and monkeys reveals that Gorilla has significantly less waisting of the capitate compared to P. paniscus (P = 0.00), P. troglodytes (P = 0.00), and terrestrial monkeys (P = 0.01) (Fig. S2). As with the scaphoid, many of these capitate and hamate features also develop later and are less accentuated or absent throughout ontogeny in Gorilla compared to Pan (Table 2; Fig. 2). Thus, the absence or minimal expression of these features in Gorilla reflects more, not less, extension and mobility at the midcarpal joint compared to that of other knuckle-walking African apes.
This research shows that none of the carpal features discussed here that have traditionally been used to diagnose knuckle-walking can be considered clear functional adaptations to knuckle-walking behavior in all African apes. Contrary to functional predictions, the pattern of development, expression, and frequency of the putative knuckle-walking features listed in Table 1 are not the same in all African apes; they are not more frequent or accentuated in gorillas and are not correlated with increased knuckle-walking behavior or body size. The difference between Pan and Gorilla in the presence or absence of carpal bone morphology thought to limit wrist motion is confirmed by reported patterns of joint flexibility in the 2 genera. Gorilla have a much larger range of wrist extension (58°) (40) compared to that of P. troglodytes (30–42°) (19, 40). It remains challenging to explain this difference if it is assumed that knuckle-walking in Pan and Gorilla is biomechanically similar. The morphological and range of motion data demand a new perspective on knuckle-walking that leads to a reevaluation of long established models of human evolution.
One possible explanation for the disparity in carpal morphology among knuckle-walking apes is that Gorilla compensates for the lack of extension-limiting osteological features with a stronger ligamentous system. Although this remains to be tested in detail, the same relative size of forearm flexor musculature among African apes and the recruitment pattern of these muscles during knuckle-walking locomotion (41, 42) suggest that soft tissues are not counteracting this increased mobility in Gorilla.
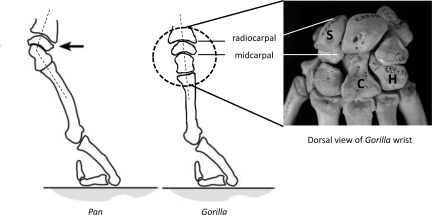
The rejection of these possibilities leads to an alternative hypothesis: that knuckle-walking is not the same biomechanical phenomenon in Pan and Gorilla. Previous researchers have noted postural (16, 31, 32) and biomechanical (13, 14) differences in knuckle-walking behavior among African apes and our hypothesis expands upon this foundation of work to suggest that knuckle-walking is a fundamentally different mode of locomotion in Pan and Gorilla. This hypothesis is supported by existing African ape locomotor data (17, 27–35). As with other forms of locomotion, limb posture has a profound influence the load experienced by the wrist and digits in a knuckle-walking animal. We propose that Gorilla uses a relative “columnar” forelimb posture during knuckle-walking in which the hand and wrist joints are aligned in a relatively straight, neutral posture compared to the more extended postures adopted by Pan (Fig. 3). Animals using a relatively columnar wrist and hand posture would have carpal joints that are in line with the hand and forearm, similar to limb joint position in large graviportal animals such as elephants (43). Supporting loads directly over more vertically-oriented forelimb joints during support phase explains the absence of posited bony adaptations to bending loads in gorillas and also permits more mobility at the joint (43). By contrast, Pan, which exhibit extended wrist postures (Fig. 3), will experience higher bending loads. Thus Pan carpal bones have relatively prominent osteological features that have traditionally, but mistakenly, been interpreted simply as features associated with knuckle-walking in general rather than with a specific posture. The notion that Pan and Gorilla use different wrist postures is consistent with the morphometric data presented here. Although this hypothesis has yet to be explored in detail with videographic data, this idea is further supported by previous research showing that Gorilla exhibits increased wrist mobility compared to Pan (40), a more hyperextended elbow joint (31) and relatively equal length of rays 2 through 4, which creates a larger, more stable area over which to disperse axial loads (32).
Fig. 3.
Hypothesized biomechanical hand posture models describing morphological variation found between Gorilla and Pan and Gorilla carpus in doral view. Lateral view of wrist and hand postures in Pan and Gorilla adapted from ref. 4. In Pan, the wrist (and carpometacarpal) joints are held in an extended posture (dotted line) such that extension-limiting morphological features are required for stability. In contrast, we hypothesize that Gorilla uses a columnar, neutral wrist and hand posture with axial loading (dotted line) such that stabilizing features are not necessary and generally absent (see text for discussion). Radiocarpal and midcarpal joints are labeled in lateral and dorsal views of Gorilla carpus. “S,” scaphoid; “C,” capitate; “H,” hamate.
The variation in African ape hand posture may be more completely understood through a consideration of differences in substrate use, which in turn may explain the high frequency of posited knuckle-walking features in Pan and several nonknuckle-walking quadrupedal primates reported in this study. Specifically, we propose that features traditionally associated with knuckle-walking may actually reflect the habitual loading of the wrist in an extended posture on arboreal substrates (Fig. 3). Compared to gorillas, chimpanzees, and bonobos more frequently use both knuckle-walking and palmigrady on arboreal substrates throughout ontogeny (28, 30, 34). To maintain balance and security in an arboreal setting, primates may adopt a more extended wrist posture (17), more flexed elbow joint (44), and more variable hand postures (16, 31–33) compared to terrestrial locomotion. Deeper limb joint angles lower the animal's center of mass relative to the substrate (45), improving balance, but will also increase the moment arm on the wrist (and elbow) joints. In addition, it is worth noting that Pan have significantly longer metacarpals compared to Gorilla (46), and this increased length could potentially further increase the bending load arm in Pan. We hypothesize that the high frequency of posited extension-limiting bony morphology shared between Pan and several other primates that use relatively extended wrist postures (e.g., palmigrady in monkeys) are in fact features that are better described as simply reflecting habitual loading of the wrist in an extended position, rather than features that limit extension per se. If, as appears to be the case, wrist extension increases during arboreal locomotion, then these bony features can be seen as reflecting the arboreal hand postures of Pan rather than indication knuckle-walking itself.
Thus, a novel understanding of the functional significance of what had previously been viewed as features reflecting knuckle-walking comes to light when we (i) interpret them in only the taxa for which they are present (Pan) and (ii) recognize the likelihood of 2 biomechanically different types of knuckle-walking in Pan and Gorilla. Functional interpretations of these features fail when knuckle-walking among all African apes is considered a single, unified behavior. Although there are other putative knuckle-walking features of the wrist and forelimb (5, 6) that have not been addressed in this report and that could potentially hold a stronger functional signal of knuckle-walking behavior, the data presented here reveal that the correlation between these features and knuckle-walking locomotion should not be presumed.
The results of this study show that researchers need to reevaluate all posited knuckle-walking features and reconsider their efficacy as indicators of knuckle-walking behavior in extant and extinct primates. In this context, the absence of several posited knuckle-walking features in extant knuckle-walkers (and the presence of some of these features in nonknuckle-walkers) makes it difficult to argue that there is unambiguous evidence that bipedalism evolved from a terrestrial knuckle-walking ancestor. Instead, our data support the opposite notion, that features of the hand and wrist found in the human fossil record that have traditionally been treated as indicators of knuckle-walking behavior are in fact evidence of arboreality and not terrestriality.
The data presented here and in other studies of variation in African ape morphology (11, 12, 14, 15) and behavior (30–32, 35) support a hypothesis of independent evolution of knuckle-walking behavior in the 2 African ape lineages. Our data cannot reject the hypothesis that knuckle-walking evolved only once at the base of the African ape and human clade and that these differences evolved after the Gorilla and Pan split (Fig. S1). Without fully understanding the evolutionary and ontogenetic plasticity of these osteological features or the affect on wrist morphology of other locomotor behaviors in which Pan and Gorilla engage, it is difficult to be certain about the evolution of nonhomologous knuckle-walking behavior in African apes. However, in absence of clear evidence for a terrestrial knuckle-walking origin for human bipedalism, we suggest that the independent evolution of a generalized locomotor adaptation that simply allows large-bodied apes to retain highly-arboreal morphology while also moving effectively on the ground is a reasonable and likely evolutionary scenario. The increasing climatic and ecological instability that typified much of the Miocene (47) may have forced the hominoid lineage to transition through several independent stages of terrestrial and arboreal locomotor behaviors. The Miocene hominoid fossil record strongly supports the independent evolution of specialized suspensory adaptations (e.g., Morotopithecus, Oreopithecus, or Pongo) (48) and the same may be true for knuckle-walking. There are few, if any, wrist similarities shared among all African apes and humans that can be directly related to terrestrial knuckle-walking locomotion. Thus the independent evolution of this behavior among African apes requires less homoplasy than previously proposed (4–6). Features found in the hominin fossil record that have traditionally been associated with a broad definition of knuckle-walking are more likely reflecting the habitual Pan-like use of extended wrist postures that are particularly advantageous in an arboreal environment. This, in turn, suggests that human bipedality evolved from a more arboreal ancestor occupying a generalized locomotor and ecological niche common to all living apes (7, 8, 10).
Materials and Methods
Qualitative and quantitative comparisons were made to an ontogenetic sample of largely wild-caught hominoids and cercopithecoids listed in Table S1. Sample sizes for juvenile stages are relatively small due to late ossification of carpal bones in some taxa and a general paucity of juvenile specimens in osteological collections. Juvenile stages (pooled sexes) were based on eruption of teeth into full occlusion and were defined as the following: Stage 1, deciduous dentition with less than dP3 fully erupted; Stage 2, dP3 fully erupted; Stage 3, dP4 fully erupted; Stage 4, mixed dentition ranging between permanent M1 fully erupted to crown of M2 fully exposed in alveolus and Stage 5 ranging between M2 erupted just past alveolar surface to an almost fully erupted M2; Stage 6, mixed dentition ranging from an M2 fully erupted to crown of M3 exposed in alveolus and Stage 7 ranging between M3 erupted just past alveolar surface to an almost fully erupted M3; Adult, any specimens with a fully erupted M3. Dental stages were correlated with chronological ages (in years) for Gorilla (49) and wild Pan (50).
Frequency of putative knuckle-walking features were scored as a “present,” even when weakly developed, or “absent” throughout all ontogenetic stages in all taxa, including cercopithecoids (Table S2b). Significant differences (P < 0.05) in frequency among African ape groups were tested using a χ2 goodness-of-fit test for all pairwise comparisons and results are given in Table S2a.
Morphometric variables of the adult capitates were measured using digital calipers. Proximodistal length of the capitate was defined as the maximum distance from the head of the capitate to the metacarpal articular surface. Breadth of the capitate neck was measured in dorsal view and at the narrowest point just distal to the head. The degree of capitate waisting was quantified as a ratio of capitate neck breadth divided by the length of the capitate body. Differences in the capitate waisting ratio between groups were tested using Mann-Whitney U test with significance at P < 0.05 (Fig. S2).
Supplementary Material
Acknowledgments.
We gratefully acknowledge the assistance of curators at the numerous institutions that provided access to specimens in their care. We also thank D. Begun, E. Fiume, J. Hanna, B. Hare, J. Horvath, C. Orr, B. Richmond, M. Rose, M. Tocheri, C. Wall, R. Wunderlich, Animal Locomotion Lab members, 2 anonymous reviewers and the editor for providing valuable comments and discussion on our manuscript. This research was supported by Natural Sciences and Engineering Research Council of Canada, General Motors Women in Science and Mathematics and the University of Toronto to T.L.K.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901280106/DCSupplemental.
For clarity in this paper, we use “neutral” when referring to a posture in which the wrist in held in line with the radius and ulna of the forearm. Deviations from neutral in which the angle of the wrist relative to the anterior forearm is greater than 180 degrees is referred to as “extension” (see Fig. 3).
References
- 1.Washburn SL. Behavior and the origin of man. Proc R Anthropol Inst Gr Br Ireland. 1967;3:21–27. [Google Scholar]
- 2.Begun DR. Miocene fossil hominids and the chimp-human clade. Science. 1992;257:1929–1933. doi: 10.1126/science.1411507. [DOI] [PubMed] [Google Scholar]
- 3.Gebo DL. Climbing, brachiation and terrestrial quadrupedalism: Historical precursors of hominid bipedalism. Am J Phys Anthropol. 1996;101:55–92. doi: 10.1002/(SICI)1096-8644(199609)101:1<55::AID-AJPA5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Richmond BG, Strait DS. Evidence that humans evolved from a knuckle-walking ancestor. Nature. 2000;404:382–385. doi: 10.1038/35006045. [DOI] [PubMed] [Google Scholar]
- 5.Richmond BG, Begun DR, Strait DS. Origin of human bipedalism: The knuckle-walking hypothesis revisited. Yearb Phys Anthropol. 2001;44:70–105. doi: 10.1002/ajpa.10019.abs. [DOI] [PubMed] [Google Scholar]
- 6.Begun DR. In: From Biped to Strider: The Emergence of Modern Human Walking, Running, Resource Transport. Meldrum DJ, Hilton CE, editors. New York: Kluwer; 2004. pp. 9–33. [Google Scholar]
- 7.Stern JTJ. Before bipedality. Yearb Phys Anthropol. 1975;19:59–68. [Google Scholar]
- 8.Rose MD. In: Origine(s) de la Bipedie Chez les Hominides. Coppens Y, Senut B, editors. Paris: Centre National de la Recherche Scientique; 1991. pp. 37–48. [Google Scholar]
- 9.Schmitt D. Insights into the evolution of human bipedalism from experimental studies of humans and other primates. J Exp Biol. 2003;206:1437–1448. doi: 10.1242/jeb.00279. [DOI] [PubMed] [Google Scholar]
- 10.Thorpe SKS, Holder RL, Crompton RH. Origin of human bipedalism as an adaptation for locomotion on flexible branches. Science. 2007;316:1328–1331. doi: 10.1126/science.1140799. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SE. Variation in the presence and development of the dorsal ridge of the metacarpal head in African apes. Am J Phys Anthropol. 1990;81:243. [Google Scholar]
- 12.Zylstra M. PhD dissertation. Toronto: Univ of Toronto; 1999. Functional Morphology of the Hominoid Forelimb: Implications for Knuckle-walking and the Origin of Hominid Bipedalism. [Google Scholar]
- 13.Inouye SE, Shea BT. The implications of variation in knuckle-walking features for models of African hominoid locomotor evolution. J Anthropol Sci. 2004;82:67–88. [Google Scholar]
- 14.Inouye SE. Ontogeny and allometry of African ape manual rays. J Hum Evol. 1992;23:107–138. [Google Scholar]
- 15.Dainton M, Macho GA. Did knuckle walking evolve twice? J Hum Evol. 1999;36:171–194. doi: 10.1006/jhev.1998.0265. [DOI] [PubMed] [Google Scholar]
- 16.Tuttle RH. Knuckle-walking and the evolution of hominoid hands. Am J Phys Anthropol. 1967;26:171–206. [Google Scholar]
- 17.Jenkins FAJ, Fleagle JG. In: Primate Functional Morphology and Evolution. Tuttle RH, editor. The Hague: Mouton; 1975. pp. 213–231. [Google Scholar]
- 18.Corruccini RS. Comparative osteometrics of the hominoid wrist joint, with special reference to knuckle-walking. J Hum Evol. 1978;7:307–321. [Google Scholar]
- 19.Richmond BG. In: Human Origins and Environmental Backgrounds. Ischida H, Pickford M, Tuttle R, Ogihara N, Nakatsukasa M, editors. Chicago: Springer; 2006. pp. 105–122. [Google Scholar]
- 20.Frost HM. A chondral theory of modeling. Calcif Tissue Int. 1979;28:181–200. doi: 10.1007/BF02441236. [DOI] [PubMed] [Google Scholar]
- 21.Hamrick MW. A chondral modeling theory revisited. J Theor Biol. 1999;201:201–208. doi: 10.1006/jtbi.1999.1025. [DOI] [PubMed] [Google Scholar]
- 22.Pearson OM, Lieberman DE. The aging Wolff's “Law”: Ontogeny and responses to mechanical loading in cortical bone. Yearb Phys Anthropol. 2004;47:63–99. doi: 10.1002/ajpa.20155. [DOI] [PubMed] [Google Scholar]
- 23.Richmond BG. PhD dissertation. Stony Brook: State University of New York; 1998. Ontogeny and Biomechanics of Phalangeal Form in Primates. [Google Scholar]
- 24.Paciulli LM. Ontogeny of phalangeal curvature and positional behavior in chimpanzees. Am J Phys Anthropol. 1995;20:165. [Google Scholar]
- 25.Raff RA. The Shape of Life: Genes, Development, and the Evolution of Animal Form. Chicago: University of Chicago Press; 1996. [Google Scholar]
- 26.Hall BK. Homoplasy and homology: Dichotomy or continuum? J Hum Evol. 2007;52:473–479. doi: 10.1016/j.jhevol.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Tuttle RH, Watts DP. Primate Morphophysiology, Locomotor Analysis and Bipedalism. Tokyo: University of Tokyo Press; 1985. pp. 261–288. [Google Scholar]
- 28.Doran DM. Comparative locomotor behavior of chimpanzees and bonobos: The influence of morphology on locomotion. Am J Phys Anthropol. 1993;91:83–98. doi: 10.1002/ajpa.1330910106. [DOI] [PubMed] [Google Scholar]
- 29.Remis M. Effects of body size and social context on the arboreal activities of lowland gorillas in the Central African Republic. Am J Phys Anthropol. 1995;97:413–433. doi: 10.1002/ajpa.1330970408. [DOI] [PubMed] [Google Scholar]
- 30.Doran DM. In: Great Ape Societies. McGrew WC, Merchant LF, Nishida T, editors. Cambridge: Cambridge Univ Press; 1996. pp. 213–224. [Google Scholar]
- 31.Inouye SE. Variability of hand postures in the knuckle-walking behavior in African apes. Am J Phys Anthropol S. 1989;78:245. [Google Scholar]
- 32.Inouye SE. Ontogeny of knuckle-walking hand postures in African apes. J Hum Evol. 1994;26:459–485. [Google Scholar]
- 33.Wunderlich RE, Jungers WL. Manual digital pressures during knuckle-walking in chimpanzees (Pan troglodytes) Am J Phys Anthropol. 2009;139:394–403. doi: 10.1002/ajpa.20994. [DOI] [PubMed] [Google Scholar]
- 34.Doran DM. The ontogeny of chimpanzee and pygmy chimpanzee locomotor behavior: A case study of paedomorphism and its behavioral correlates. J Hum Evol. 1992;23:139–197. [Google Scholar]
- 35.Doran DM. Ontogeny of locomotion in mountain gorillas and chimpanzees. J Hum Evol. 1997;32:323–344. doi: 10.1006/jhev.1996.0095. [DOI] [PubMed] [Google Scholar]
- 36.Leigh SR, Shea BT. Ontogeny and the evolution of adult body size dimorphism in apes. Am J Primatol. 1995;36:37–60. doi: 10.1002/ajp.1350360104. [DOI] [PubMed] [Google Scholar]
- 37.Kivell TL, Begun DR. Frequency and timing of scaphoid-centrale fusion in hominoids. J Hum Evol. 2007;52:321–340. doi: 10.1016/j.jhevol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Beard KC, Teaford MF, Walker A. New wrist bones of Proconsul africanus and P. nyanzae from Rusinga Island, Kenya. Folia Primatol. 1986;47:97–118. doi: 10.1159/000156268. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor BL. Functional morphology of the cercopithecoids wrist and inferior radioulnar joints, and their bearing on some problems in evolution of Hominoidea. Am J Phys Anthropol. 1975;43:113–121. doi: 10.1002/ajpa.1330430115. [DOI] [PubMed] [Google Scholar]
- 40.Tuttle RH. Quantitative and functional studies on the hands of Anthropoidea. I. The Hominoidea. J Morphol. 1969;128:309–364. doi: 10.1002/jmor.1051280304. [DOI] [PubMed] [Google Scholar]
- 41.Tuttle RH, Basmajian JV. Electromyography of brachial muscles in Pan gorilla and hominoid evolution. Am J Phys Anthropol. 1974;41:71–90. doi: 10.1002/ajpa.1330610108. [DOI] [PubMed] [Google Scholar]
- 42.Susman RL, Stern JTJ. Telemetered electromyography of flexor digitorum profundus and flexor digitorum superficialis in Pan troglodytes and implications for interpretation of the O.H. 7 hand. Am J Phys Anthropol. 1979;50:565–574. doi: 10.1002/ajpa.1330500408. [DOI] [PubMed] [Google Scholar]
- 43.Ren L, et al. The movements of limb segments and joints during locomotion in African and Asian elephants. J Exp Biology. 2008;211:2735–2751. doi: 10.1242/jeb.018820. [DOI] [PubMed] [Google Scholar]
- 44.Tuttle RH, Velte MJ, Basmajian JV. Electromyography of brachial muscles in Pan troglodytes and Pongo pygmaeus. Am J Phys Anthropol. 1983;61:75–83. doi: 10.1002/ajpa.1330610108. [DOI] [PubMed] [Google Scholar]
- 45.Cartmill M. In: Functional Vertebrate Morphology. Hildebrand M, Bramble DM, Leim KF, Wake DB, editors. Cambridge: Belknap Press of Harvard Univ Press; 1985. pp. 73–88. [Google Scholar]
- 46.Drapeau MSM, Ward CV. Forelimb segment length proportions in extant hominoids and Australopithecus afarensis. Am J Phys Anthropol. 2007;132:327–343. doi: 10.1002/ajpa.20533. [DOI] [PubMed] [Google Scholar]
- 47.Hill A. In: Paleoclimate and Evolution with Emphasis on Human Origins. Vrba ES, Denton GH, Partridge TC, Burckle LH, editors. New Haven: Yale Univ Press; 1995. pp. 178–193. [Google Scholar]
- 48.Begun DR. How to identify (as opposed to define) a homoplasy: Examples from fossil and living great apes. J Hum Evol. 2007;52:559–572. doi: 10.1016/j.jhevol.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 49.Smith BH, Crummett TL, Brandt KL. Ages of eruption in primate teeth: A compendium for aging individuals and comparing life histories. Yearb Phys Anthropol. 1994;37:177–231. [Google Scholar]
- 50.Zihlman A, Bolter D, Boesch C. Wild chimpanzee dentition and its implications for assessing life history in immature hominin fossils. Proc Natl Acad Sci USA. 2004;101:10541–10543. doi: 10.1073/pnas.0402635101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.