Abstract
Dominantly inherited mutations in leucine-rich repeat kinase 2 (LRRK2) are a common genetic cause of Parkinson's disease (PD). The importance of the R1441 residue in the pathogenesis is highlighted by the identification of three distinct missense mutations. To investigate the pathogenic mechanism underlying LRRK2 dysfunction, we generated a knockin (KI) mouse in which the R1441C mutation is expressed under the control of the endogenous regulatory elements. Homozygous R1441C KI mice appear grossly normal and exhibit no dopaminergic (DA) neurodegeneration or alterations in steady-state levels of striatal dopamine up to 2 years of age. However, these KI mice show reductions in amphetamine (AMPH)-induced locomotor activity and stimulated catecholamine release in cultured chromaffin cells. The introduction of the R1441C mutation also impairs dopamine D2 receptor function, as suggested by decreased responses of KI mice in locomotor activity to the inhibitory effect of a D2 receptor agonist, quinpirole. Furthermore, the firing of nigral neurons in R1441C KI mice show reduced sensitivity to suppression induced by quinpirole, dopamine, or AMPH. Together, our data suggest that the R1441C mutation in LRRK2 impairs stimulated dopamine neurotransmission and D2 receptor function, which may represent pathogenic precursors preceding dopaminergic degeneration in PD brains.
Keywords: Parkinson's disease, knockin, dopamine D2 receptor, amphetamine, quinpirole
Parkinson's disease (PD) is the most common neurodegenerative movement disorder, characterized by resting tremor, slow movement, muscular rigidity, and postural instability. The clinical symptoms of PD are thought to result from reduced dopamine input to the striatum due to the severe loss of dopaminergic (DA) neurons in the pars compacta of the substantia nigra (SNpc). Although most PD cases occur sporadically, several genes associated with monogenetic forms of the disease mimicking clinical symptoms of sporadic PD have been identified. The leucine-rich repeat kinase 2 (LRRK2) gene on chromosome 12 (chromosome 15 in mice) harbors 5 pathogenic mutations, which segregate with the disease, and many more amino acid residue substitutions have also been found to be associated with the disease (1–3). LRRK2 contains 51 exons and encodes a large 2527-aa protein, which consists of several functional domains, including a Ras-like small GTPase domain and a MAP kinase-like domain (1, 2). The pathogenic mutations all affect highly conserved amino acid residues, and are collectively the most common genetic cause of the late-onset PD (≈7% familial and 3% sporadic PD) (1–7).
Interestingly, multiple amino acid substitutions of the same residue R1441 (R1441C, R1441G, and R1441H) in the highly conserved GTPase domain and multiple mutations (I2012T, G2019S, and I2020T) in the kinase domain have been identified, whereas no multiplication or exonic deletion of LRRK2 has been reported (3). Most LRRK2 mutations cause clinically typical PD, but the neuropathology varies ranging from pure nigral degeneration without Lewy bodies (LBs) to nigral degeneration with brainstem or widespread LBs, or neurofibrillary tau-positive tangles (2, 8); thus, confirming that a single gene disorder can have multiple pathological consequences.
Recent in vitro studies suggest that mutations in LRRK2 (R1441C, G2019S) cause increases in its kinase activity (9, 10), which mediates neuronal toxicity and is regulated by the GTPase domain in a GTP-dependent manner (10–13). It has also been reported that expression of pathogenic LRRK2 mutants (R1441C, Y1699C, or G2019S) results in neuronal degeneration and protein aggregation or inclusions in SH-SY5Y cells and cultured cortical neurons (11, 14, 15). LRRK2 has also been implicated in the biogenesis and/or regulation of intracellular membrane structures (16), synaptic vesicle endocytosis (17), and neurite outgrowth (15). However, the physiological function of mammalian LRRK2 is unknown. Recently, Lee et al. (18) reported that loss-of-function mutants for LRRK, the sole Drosophila ortholog of human LRRK2, exhibited severely impaired locomotor activity and reduced tyrosine hydroxylase (TH) immunoreactivity in DA neurons, but transgenic (Tg) expression of pathogenic mutant or WT LRRK did not result in any significant defects. However, in another study, Tg expression of human WT or G2019S LRRK2 led to adult-onset selective loss of DA neurons, locomotor dysfunction, and early mortality in Drosophila (19).
To investigate the pathogenic mechanism by which mutations in LRRK2 cause PD, we generated a LRRK2 knockin (KI) mouse by introducing the R1441C mutation into exon 31 and allowing its expression under the control of the endogenous regulatory elements. This R1441C mutation is of particular interest, because three distinct mutations have been identified in this arginine residue, and diverse neuropathology has been identified in families carrying this R1441C mutation (1–3, 20). Although LRRK2 R1441C KI mice are viable and appear grossly normal, our multidisciplinary analysis has suggested that the R1441C mutation causes abnormal regulation of activity-dependent dopamine neurotransmission, which may be pathogenic precursors preceding to frank DA degeneration in PD patients.
Results
Generation and Molecular Characterization of R1441C KI Mice.
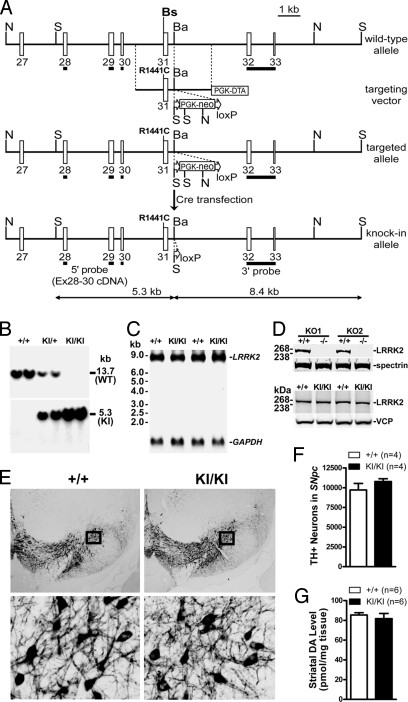
To generate LRRK2 KI mice, the R1441C mutation was introduced into exon 31 of the LRRK2 gene by homologous recombination in mouse ES cells (Fig. 1A). The floxed PGK-neo selection cassette was removed in the R1441C-targeted ES clone by transient transfection of a Cre-expressing plasmid to avoid any possible interference with LRRK2 expression (Fig. 1A). The resulting ES clones were further analyzed using Southern blotting and PCR followed by DNA sequencing to confirm the removal of the PGK-neo cassette, and then injected into C57/BL6 blastocysts. The chimeric offspring were crossed to F1 hybrids of 129 and C57/BL6 mice to obtain heterozygous mutant mice. The germ-line transmission of the R1441C mutation and the absence of the floxed PGK-neo cassette were confirmed in these heterozygous mutant mice by Southern blotting using the 5′ (Fig. 1B) and 3′ probes and PCR followed by DNA sequencing. The heterozygous KI (KI/+) mice were crossed together to obtain homozygous KI/KI mice, which are viable, fertile, and appear grossly normal. Normal LRRK2 mRNA expression level in KI/KI mice was confirmed by Northern blotting using a probe specific for exons 1–5 (Fig. 1C). We also generated a rabbit polyclonal antibody against the recombinant protein of the kinase domain of LRRK2. The specificity of the LRRK2 antibody was verified by Western blotting using the brain homogenates from our two distinct lines of LRRK2 KO mice (Fig. 1D). Using this antibody, we confirmed normal LRRK2 protein levels in KI/KI mice (Fig. 1D). The presence of the R1441C mutation and the correct mRNA splicing around the mutation were further confirmed using RT-PCR followed by DNA sequencing.
Fig. 1.
Normal DA neurons and striatal dopamine levels in R1441C KI mice. (A) Targeting strategy. The R1441C mutation was introduced into exon 31. The locations of the 5′ and 3′ probes used for Southern blotting are indicated. Restriction sites: N, NcoI; S, SpeI, Bs, BssSI; Ba, BamHI are indicated. (B) Southern blotting of F2 mice using the 5′ probe shows the germ-line transmission of the KI allele. Tail genomic DNAs were digested with SpeI. The 13.7-kb band represents the WT allele, whereas the 5.3-kb band represents the KI allele. (C) Northern blotting of total RNAs from KI/KI brains. A 406-bp cDNA fragment spanning exons 1–5 of LRRK2 was used as a probe. (D) Western blotting of brain samples indicates normal protein levels of LRRK2 in KI/KI mice. The specificity of our LRRK2 antibody is confirmed by the absence of LRRK2 in the brain of our two distinct lines of LRRK2 KO mice, KO1 and KO2. Antibodies specific for valosin containing protein (VCP) or spectrin were used to normalize the amounts of protein in each lane for KI or for KO samples, respectively. (E) TH staining of DA neurons in the SNpc. A series of 16-μm coronal sections from 4 KI/KI and 4 WT littermate (+/+) mice at ≈2 years of age were stained with a TH antibody. The representative comparable sections are shown. (Lower) Higher magnification views of the boxed areas. (F) Quantification of TH-immunoreactive neurons in the SNpc by unbiased stereological counting. (G) Levels of striatal DA measured by HPLC in the 2-year-old mice (n = 6 each genotype). Data in all panels are expressed as mean ± SEM.
Normal DA Neurons and Striatal Catecholamine Levels in R1441C KI Mice.
To determine whether R1441C KI mice develop degeneration of DA neurons, we performed immunohistochemical analysis of the KI/KI brains at the ages of 3, 12, and 22 months using an antibody specific for TH, the rate-limiting enzyme in dopamine synthesis. The morphology of DA neurons and their projections appears grossly normal in KI/KI mice (Fig. 1E). Quantification of TH-immunoreactive neurons in the SNpc using stereological methods revealed no significant difference in the number of DA neurons between KI/KI and WT littermate controls at 12 and 22 months of age (Fig. 1F). Because degeneration of noradrenergic neurons in the locus coeruleus (LC) has also been reported in PD patients (21), we similarly examined the morphology and quantified TH-immunoreactive neurons in the LC, and found no significant alteration in the morphology and number of noradrenergic neurons in KI/KI mice (WT, 494 ± 36; KI/KI, 459 ± 63; P > 0.05). Our initial analysis of KI/+ and KI/KI mice, including morphological analysis of TH-immunoreactive neurons, immunohistochemistry for protein aggregation, measurement of striatal DA and its metabolites, and behavioral analysis (spontaneous locomotor activity and motor coordination) of the mice at 3 months of age, revealed phenotypes essentially identical to WT controls. We then focused on analysis of only WT and KI/KI mice with the anticipation that KI/KI mice would have more dramatic phenotypes than KI/+ mice.
To look for any alteration in steady-state dopamine levels, which may occur before the frank loss of DA neurons, we measured the levels of striatal dopamine and its major metabolites using HPLC. The striatal dopamine level is not significantly different in KI/KI mice at the ages of 3, 12, and 23 months (Fig. 1G), compared with their respective WT littermate controls. There was also no significant alteration in the striatal level of major metabolites of dopamine, dihydroxylphenylacetic acid (DOPAC; WT, 11.9 ± 0.8 pmol/mg; KI/KI, 14.0 ± 1.2 pmol/mg; P > 0.05) and homovanillic acid (HVA; WT, 7.7 ± 1.3 pmol/mg; KI/KI, 7.9 ± 0.4 pmol/mg; P > 0.05) in KI/KI mice by the age of ≈2 years, suggesting that DA turnover is normal in KI/KI mice.
Besides the common DA neuronal loss in the SNpc, members of the families carrying the R1441C mutation exhibited diverse protein aggregation-related neuropathology, ranging from α-synuclein-immunoreactive LBs or Lewy neurites, ubiquitin-immunoreactive neuronal cytoplasmic or nuclear inclusions, to tau-immunoreactive neurofibrillary tangles (2). Therefore, we performed immunohistochemical analysis using antibodies specific for α-synuclein, phospho-α-synuclein (Ser-129), ubiquitin, tau, phospho-tau (Ser-396/404 or Ser-202), or GFAP. There was no obvious accumulation or abnormal phosphorylation of α-synuclein, ubiquitin, or tau in the SNpc and LC of KI/KI mice at the ages of 3, 12, and 22 months. This result is further confirmed by Western blotting, showing normal levels of α-synuclein, phospho-α-synuclein (Ser-129), ubiquitin, and tau and phospho-tau (Ser-396/404 or Ser-202) in KI/KI mice at 6 and 12 months. GFAP immunoreactivity was also similar between KI/KI and control brains at the ages of 12 and 22 months, indicating the absence of gliosis.
Reduced Response to Amphetamine (AMPH) in Locomotor Activity of R1441C KI Mice.
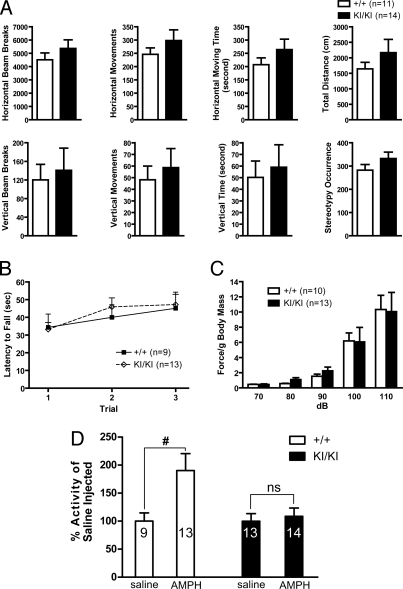
We examined LRRK2 KI mice in various behavioral paradigms for abnormalities in general reflexes, movements, and posture. In the open field test, KI/KI mice at the ages of 3, 12 (Fig. 2A), and 24 months exhibited normal spontaneous locomotor activity. Involuntary movement of KI/KI mice at the ages of 3 and 12 months (+/+, n = 9; KI/KI, n = 13; Fig. 2B) assessed by the rotarod test is also not significantly changed. Acoustic startle reflex, which has been shown to be sensitive to alterations in dopamine (22), was similar between KI/KI mice and their WT controls at the age of 1 year (Fig. 2C). Acoustic startle response can be inhibited by a preceding weaker stimulus, a process termed prepulse inhibition (PPI), which is thought to be modulated by the central noradrenergic neurotransmission. PPI was also normal in KI/KI mice.
Fig. 2.
Reduced response to AMPH in locomotor activity of R1441C KI mice. (A) Spontaneous activity of KI/KI (n = 14) and +/+ (n = 11) mice at 1 year of age in the open field during 1-h test. Two infrared light arrays measured horizontal movements, and one array measured vertical movements (rearing on hind legs). Repeated sequential breakings of the same beam were scored as occurrences of stereotyped behaviors (scratching, grooming, etc.). (B) Rotarod test. KI/KI and +/+ mice show similar latencies to fall off an accelerating rotating rod. The average time before falling off the rod is shown for each of three consecutive trials. (C) KI/KI and +/+ mice show similar acoustic startle responses at each decibel of loud noise tested. Each mouse was tested 10 times at each decibel level in a semirandom order with a 30-s interstimulus interval, and the startle response was averaged and normalized to body mass. Body mass does not differ between the genotypes. (D) AMPH injection (i.p., 2.0 mg/kg body weight) dramatically stimulates the locomotor activity of WT (+/+), but not that of KI/KI mice during the 1-h test (#, P < 0.05; ns, not significant). The numbers shown in the bars indicate the numbers of mice used for each experiment. Data in all panels are expressed as mean ± SEM.
Because the spontaneous locomotor activity of LRRK2 KI/KI mice is normal, we examined whether there is any abnormality in drug-stimulated locomotor activity in KI/KI mice. We monitored the locomotor activity of KI/KI mice in the open field test for 1 h, after injection with a psychostimulant AMPH, which is known to stimulate locomotor activity by inducing synaptic dopamine release via dopamine transporter (DAT) and vesicular monoamine transporter 2 (VMAT2) (23). Compared with the saline control, AMPH injection (2 mg/kg) resulted in markedly increased activity in WT mice, whereas it failed to increase the locomotor activity of KI/KI mice (Fig. 2D). Similar results were obtained when a higher dose of AMPH (10 mg/kg) was used. Thus, the reduced response of LRRK2 KI/KI mice to AMPH stimulation in locomotor activity may be due to impairment in AMPH-induced dopamine release.
Reduced Catecholamine Release in Cultured R1441C KI Chromaffin Cells.
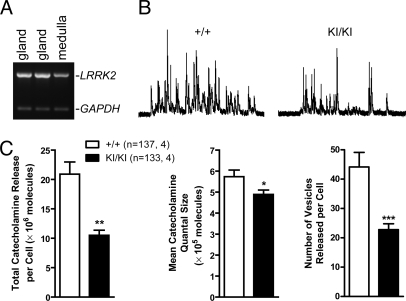
To determine whether mutations in LRRK2 affect release of all catecholamine, we used adrenal chromaffin cells isolated from LRRK2 KI/KI mice as a model to study exocytotic catecholamine release. Adrenal chromaffin cells are neuroendocrine cells in the medulla of the adrenal gland, derived from the same embryonic origin as sympathetic neurons, and are responsible for the “fight or flight” response of the body by releasing catecholamines epinephrine and norepinephrine into the blood stream (24). These cells are thought to share the same exocytosis mechanism as neurons, and have been widely used as a model system to study catecholamine release, including measurement of total catecholamine released per cell, quantal size (number of catecholamine molecules released per vesicle during exocytosis), and number of vesicles releasable from each cell (24, 25). Amperometric recordings showed significant reductions in the total catecholamine released per cell (≈50%), quantal size, and frequency of released events (≈50%) in cultured chromaffin cells isolated from the KI/KI mice, when stimulated by high K+ (picospritzing for 6 s with an 80 mM K+ solution) (Fig. 3). The results show that evoked release of catecholamines in real time is compromised in KI/KI mice, and it is likely mediated through two mechanisms: a decrease in catecholamine quantal size per vesicle and a reduction in the number of vesicles releasing catecholamines.
Fig. 3.
Reduced catecholamine release in cultured chromaffin cells from R1441C KI mice. (A) Expression of LRRK2 mRNAs, shown by RT-PCR, in medulla of adrenal glands, which is composed mainly of chromaffin cells. (B) The representative traces for high-K+-stimulated catecholamine release from KI/KI and +/+ controls. (C) Reduced total catecholamine released per cell, quantal size, and numbers of vesicles released per cell in chromaffin cells of KI/KI mice. The number of cells recorded (Left) and mice used (Right) is indicated in the parenthesis. Data in all panels are expressed as mean ± SEM. Asterisk denotes statistical significance (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Impaired D2 Receptor Function in R1441C KI Mice.
We further evaluated dopamine D2 receptor function or signaling in LRRK2 KI/KI mice, which is impaired in DJ-1 KO mice (26), and is involved in feedback regulation of dopamine release (27). We assessed locomotor activity of KI/KI mice in the open field test after injection with quinpirole, a D2 receptor agonist, at a low (0.05 mg/kg) or high dose (1.0 mg/kg). As expected, the injection of quinpirole caused significant dose-dependent inhibition of the locomotor activity in WT mice (Fig. 4). However, the extent of inhibition by quinpirole in locomotor activity is decreased in KI/KI mice in horizontal and vertical movements (Fig. 4). In particular, the injection of the low-dose quinpirole (0.05 mg/kg) only caused marginal inhibition of the locomotor activity in KI/KI mice, whereas it resulted in significant reduction in WT controls. These results indicate that KI/KI mice are less sensitive to quinpirole treatment, suggesting that D2 receptor-mediated functions may be impaired in KI/KI mice.
Fig. 4.
Reduced inhibition of locomotor activity by quinpirole in R1441C KI mice. Intraperitoneal injections of low (0.05 mg/kg body weight) or high (1.0 mg/kg body weight) dose of quinpirole, a D2 receptor agonist, result in significant inhibition of locomotor activity in both KI/KI and +/+ mice in a dose-dependent manner (#, P < 0.05; ##, P < 0.01). However, the extent of the inhibition in KI/KI mice is smaller for both horizontal and vertical movements, compared with the control (*, P < 0.05). The number shown in the bar indicates the number of mice used for each experiment. Data in all panels are expressed as mean ± SEM.
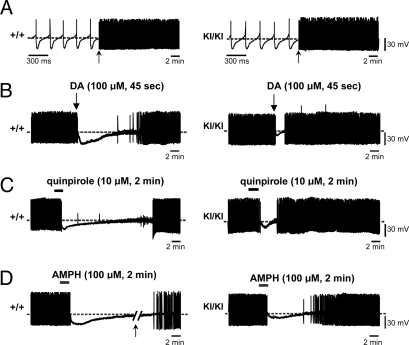
To investigate the possible mechanisms underlying the reduced response of R1441C KI mice to quinpirole and AMPH, we evaluated the function of DA neurons in acute horizontal midbrain slices (300 μm) from KI/KI and WT mice at 6–8 weeks of age by monitoring spontaneous firing activity of DA neurons and their responses to the application of dopamine, quinpirole, or AMPH. The DA neurons of the SNpc were identified according to their electrophysiological and pharmacological criteria (28–30). Spontaneous, rhythmic firing activity of nigral neurons in KI/KI mice was comparable with that recorded from WT controls (WT, 3.56 ± 0.2 Hz; KI/KI, 3.4 ± 0.6 Hz; P > 0.05) (Fig. 5A).
Fig. 5.
Decreased sensitivity to inhibition of firing induced by DA, quinpirole, and AMPH in R1441C KI nigral neurons. (A) Sample traces showing the comparable spontaneous, rhythmic action potentials recorded in DA neurons from +/+ (Left) and KI/KI (Right) mice. Firing activity is shown at different time scales marked by an arrow. (B) In midbrain slices from +/+ mice (left trace), bath-application of DA (100 μM, 45 s) hyperpolarizes the cell membrane and blocks the firing activity. On DA washout, the membrane potential slowly recovers, and the action potential discharge returns to control levels. DA-induced firing cessation is significantly shorter in nigral neurons of KI/KI mice (right trace). (C and D) Sample traces showing the reduced responses in firing of nigral neurons in KI/KI mice to bath-application of quinpirole (10 μM, 2 min) (C) or AMPH (100 μM, 2 min) (D). The arrow in D indicates a 10-min interruption in the trace of +/+ nigral neurons.
Membrane hyperpolarization and blockade of action potentials are typical responses of nigral neurons to dopamine application due to activation of somatodendritic D2 autoreceptors (31). Indeed, dopamine (100 μM, 45 s) inhibited cell firing and induced a membrane hyperpolarization (17.5 ± 2.1 mV; Fig. 5B) in nigral neurons of WT slices. Notably, the response to dopamine was significantly smaller in amplitude (9.5 ± 0.9 mV; P < 0.05) and shorter in duration of hyperpolarization in nigral neurons from KI/KI mice (Fig. 5B). Indeed, on dopamine washout, in WT slices, the membrane potential of nigral neurons slowly recovered, and firing activity resumed to control levels, whereas in KI/KI mice, the time to recovery of predrug firing activity was significantly shorter (WT, 12.9 ± 3.0 min, n = 6; KI/KI, 1.5 ± 0.1 min, n = 7; P < 0.05) (Fig. 5B). The inhibitory effect of dopamine on nigral neurons is mediated specifically by somatodendritic D2 autoreceptors, because it is fully prevented by D2 receptor antagonists (29) and is absent in D2R−/− mice (32). Accordingly, bath-application of the D2 receptor agonist quinpirole (10 μM, 2 min) caused a long-lasting hyperpolarization of the cell membrane (13.5 ± 2.1 mV) and blockade of the firing activity in nigral neurons from WT mice (16.6 ± 0.9 min, time to recovery of firing activity; n = 4) (Fig. 5C). Interestingly, nigral neurons from KI/KI mice (n = 6) showed a dramatic reduction in the sensitivity to quinpirole (8.6 ± 0.7 mV; 3.8 ± 0.9 min; P < 0.05) (Fig. 5C). These data suggest that D2 receptor-mediated functions are impaired in KI/KI mice.
Similarly, in midbrain slices from WT mice, bath-application of AMPH (100 μM, 2 min) hyperpolarized the cell membrane and blocked the firing activity of nigral neurons. On drug washout, the membrane slowly recovered, and action potential discharge was resumed. However, the duration of firing cessation induced by bath-application of AMPH was much shorter in the nigral neurons of KI/KI mice (WT, 38.4 ± 8.3 min; KI/KI, 8.2 ± 2.8 min; n = 6 for each genotype; P < 0.05) (Fig. 5D). The amplitude of the AMPH-induced membrane hyperpolarization was smaller in KI/KI mice than the one observed in their WT controls (WT, 18.0 ± 6.7 mV; KI/KI, 8.6 ± 0.7 mV; P < 0.05) (Fig. 5D). These results suggest the reduced response of KI/KI nigral neurons to AMPH.
Discussion
The importance of the R1441 residue for the normal function of LRRK2 is highlighted by the presence of multiple pathogenic missense mutations at this residue in PD patients. Therefore, we chose to focus on the R1441C mutation and developed a mouse model to study the pathogenic mechanism of LRRK2 dysfunction by creating a KI mouse, in which this commonly found mutant form of LRRK2 is expressed under the control of the endogenous regulatory elements. R1441C KI mice do not develop DA degeneration or alterations in striatal dopamine levels up to 2 years of age (Fig. 1). However, these KI mice show abnormal activity-dependent DA neurotransmission, including impairment in stimulation (e.g., AMPH, high-K+)-induced locomotor activity (Fig. 2) and catecholamine release (Fig. 3), as well as dopamine D2 receptor-mediated functions (Figs. 4 and 5). Thus, our multidisciplinary analysis of R1441C KI mice has revealed an essential role for LRRK2 in the nigrostriatal DA pathway.
Similar to our prior findings in parkin−/− (33), DJ-1−/− (26, 34), and PINK1−/− mice (35), the R1441C KI mouse does not develop nigrostriatal DA degeneration during its lifespan, providing further evidence that genetic recapitulation of pathogenic mutations in mice is insufficient to reproduce this terminal neuropathological hallmark of PD. Interestingly, Tg flies expressing either human WT or mutant G2019S LRRK2 exhibit selective loss of DA neurons, locomotor dysfunction, and early mortality (19), whereas Tg expression of WT or pathogenic equivalent mutant forms of LRRK, the sole Drosophila ortholog of human LRRK2, does not result in any significant defects in flies (18). However, it is unclear why expression of human LRRK2 or fly LRRK has such different effects in flies, and why expression of either WT or mutant human LRRK2 similarly results in loss of DA neurons in flies. Also, our KI mice develop no protein aggregation or inclusions in the brain up to 2 years of age, in contrast to previous findings in cell lines or cultured cortical neurons, (11, 14, 15). Also, we saw no alterations in levels of α-synuclein and tau, as well as their phosphorylated forms measured by immunostaining or Western blotting in our R1441C LRRK2 KI/KI brains up to 2 years of age.
Interestingly, introduction of the R1441C mutation in LRRK2 affects activity-dependent DA neurotransmission, such as reduced responses to AMPH stimulation in locomotor activity (Fig. 2) and significant reduction in stimulation-induced catecholamine release (Fig. 3) in the absence of DA degeneration (Fig. 1). AMPH exerts its effects by stimulating dopamine efflux into the synaptic cleft via multiple mechanisms, including inhibition of VMAT2 and monoamine oxidase activity, which increases cytosolic dopamine available for DAT-mediated reverse transport of dopamine (23). Calcium released from intracellular stores plays a key role in AMPH-mediated dopamine release (36, 37). The reduced response to AMPH stimulation in locomotor activity of R1441C KI mice suggests that AMPH-stimulated dopamine release may be reduced in these mice. This possibility will need to be confirmed by in vivo microdialysis to measure directly extracellular dopamine levels in freely moving mice after AMPH treatment. Consistent with this result, stimulation-induced catecholamine release is decreased in chromaffin cells isolated from R1441C KI mice, as indicated by significant reductions in total catecholamine release, quantal size, and the number of vesicles releasable after high-K+ stimulation (Fig. 3). Identification of a possible common mechanism underlying the impairment of AMPH-induced locomotion in the open field and evoked catecholamine release in cultured chromaffin cells awaits further investigation. Also, parallel studies using cultured DA neurons from postnatal ventral midbrains will provide additional support for this conclusion.
Another interesting phenotype exhibited by R1441C KI mice is the impairment of dopamine D2 receptor-mediated functions, as indicated by reduced responses of KI mice in locomotor activity to the inhibitory effect of a D2 receptor agonist, quinpirole (Fig. 4), and decreased sensitivity of KI nigral neurons in firing activity to suppression induced by quinpirole or dopamine (Fig. 5). Prior studies have shown that these inhibitory effects of quinpirole are abolished in mice lacking all D2 receptors, but are retained in mice expressing only the short isoform, which serves presynaptic autoreceptor functions (32, 38, 39). Interestingly, similar compromises of D2 autoreceptor-mediated functions have been reported in another PD mouse model, DJ-1−/− mice, which also exhibited reduced responses in locomotor activity to quinpirole and reduced responses of nigral neurons to dopamine and quinpirole (26). These results raise the possibility that the D2 autoreceptor-mediated function may be a converging common target of PD mutations, a notion supported by the clinical efficacy of D2 receptor agonists in PD (40). Also, several variants of the dopamine D2 receptor gene have been associated with PD (41). Recent clinical trials have shown that use of D2 receptor agonists ropinirole and pramipexole retards loss of functional nigral projections to the striatum (42, 43). Thus, our KI mouse model provides a tool for the study of the normal physiological role of LRRK2 and its dysfunction in PD pathogenesis, which may yield previously undescribed targets for development of effective therapeutic drugs.
After we submitted our manuscript, Li et al. (44) reported that overexpression of R1441G LRRK2 in BAC Tg mice results in age-dependent striking reductions (80–95% at 10–12 months) of rearing activities measured by a cylinder test, decreases in spontaneous DA release measured by in vivo microdialysis, loss of TH+ DA dendrites near the DA cell body in the SNpc at 9–10 months, and elevated levels of tau and phospho-tau. Although the dopamine release defect appears to be shared between our R1441C KI and their R1441G BAC Tg mice, KI mice do not exhibit defects in locomotion, dendritic degeneration of DA neurons, or phosphorylation of tau up to 2 years of age. Possible explanations for the phenotypic variation include differences in expression levels of mutant LRRK2 (endogenous levels in KI/KI vs. 5- to 10-folds overexpression in Tg), the presence of WT LRRK2 (none in KI/KI), genetic background (B6/129 for KI/KI, FVB for Tg), and specific mutations (R1441C for KI/KI vs. R1441G for Tg). The most striking difference between the KI and Tg mice, however, is perhaps their open field activity (quantified as normal in KI/KI vs. immobility in Tg without quantitative data). It was unclear whether the degree of reduction in open field activity and the severity of other phenotypes correlate with the overexpression level of LRRK2 mutant proteins in two independent Tg lines (5- or 10-fold overproduction compared with endogenous mouse LRRK2 measured by a human LRRK2-specific antibody). Studies of additional LRRK2 mutant lines and quantitative comparison of the phenotypes exhibited by different mutant lines will yield further insight into how LRRK2 mutations cause DA dysfunction and degeneration in PD.
Materials and Methods
Generation and Molecular Characterization of R1441C KI Mice.
To generate R1441C KI mice, a 1.7-kb DNA fragment encompassing exon 31 and a 1.6-kb DNA fragment containing part of intron 31 were amplified by PCR using tail genomic DNA of B6 mice as template, and the R1441C mutation was introduced into exon 31 in the 1.7-kb DNA fragment by site-directed mutagenesis. These two DNA fragments were used as 5′ and 3′ homologous arms, respectively, in the targeting vector. Linearized targeting vector was transfected by electroporation into MKV6.5 ES cells derived from B6/129 F1 mice (gift of R. Jaenisch). Desired homologous recombination events were confirmed by Southern blotting with the neo probe and the 5′ and 3′ probes. ES cells from one targeted clone were transiently transfected with a Cre expressing plasmid to excise the floxed PGK-neo selection cassette. The new ES cells were injected into B6 blastocysts. The resulting chimeric mice were mated with B6/129 F1 mice. Neuron counting, behavioral and pharmacological analyses, as well as amperometric and electrophysiological recordings were performed in a genotype blind manner.
Histological and Electrophysiological Analyses of DA Neurons.
Immunohistochemical analysis and stereological DA neuron counting, as well as intracellular recordings of nigral neurons, were performed as previously described (26). For immunohistochemical analysis, coronal brain sections were stained with antibodies to TH (Chemicon), total α-synuclein (4D6; Abcam) or phosphorylated at Ser-129 (Wako), ubiquitin (Abcam), total tau or phosphorylated tau at Ser-396/404 (PHF-1) or at Ser-202 (CP13) (both were provided by Peter Davies), and glial fibrillary acidic protein (GFAP; Sigma).
Striatal Dopamine Measurements by HPLC.
Dorsal striata were dissected, weighed, and stored at −80 °C until use. Frozen striata were sonicated in ice-cold solution (0.1 M perchloric acid/0.2 mM sodium bisulfite) and centrifuged for 10 min at 14,000 × g at 4 °C. The levels of dopamine and its metabolites in the supernatant were measured using HPLC and normalized to tissue weight.
Behavioral Analysis and Ameperometry.
Behavioral tests were performed as previously described (26). To test the responses of R1441C KI mice in locomotor activity to AMPH or quinpirole, mice were injected (i.p.) with saline, AMPH (2 or 10 μg/g body weight), or quinpirole (0.05 or 1.0 μg/g body weight) immediately before placed in the testing chambers. Amperometric recordings of dissociated chromaffin cells were performed as previously described (45).
Acknowledgments.
We thank Dr. Peter Davies for the use of phospho-tau antibodies, and Huailong Zhao and Lan Wang for technical assistance. This work was supported by grants from the National Institute of Neurological Disorders and Stroke and the Michael J. Fox Foundation (to J.S.) and the National Parkinson Foundation (to Y.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Paisan-Ruiz C, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Zimprich A, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Mata IF, et al. Lrrk2 pathogenic substitutions in Parkinson's disease. Neurogenetics. 2005;6:171–177. doi: 10.1007/s10048-005-0005-1. [DOI] [PubMed] [Google Scholar]
- 4.Skipper L, et al. Comprehensive evaluation of common genetic variation within LRRK2 reveals evidence for association with sporadic Parkinson's disease. Hum Mol Genet. 2005;14:3549–3556. doi: 10.1093/hmg/ddi376. [DOI] [PubMed] [Google Scholar]
- 5.Berg D, et al. Type and frequency of mutations in the LRRK2 gene in familial and sporadic Parkinson's disease. Brain. 2005;128:3000–3011. doi: 10.1093/brain/awh666. [DOI] [PubMed] [Google Scholar]
- 6.Taylor JP, Mata IF, Farrer MJ. LRRK2: A common pathway for parkinsonism, pathogenesis and prevention? Trends Mol Med. 2006;12:76–82. doi: 10.1016/j.molmed.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Di Fonzo A, et al. Comprehensive analysis of the LRRK2 gene in sixty families with Parkinson's disease. Eur J Hum Genet. 2006;14:322–331. doi: 10.1038/sj.ejhg.5201539. [DOI] [PubMed] [Google Scholar]
- 8.Funayama M, et al. An LRRK2 mutation as a cause for the parkinsonism in the original PARK8 family. Ann Neurol. 2005;57:918–921. doi: 10.1002/ana.20484. [DOI] [PubMed] [Google Scholar]
- 9.West AB, et al. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci USA. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith WW, et al. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9:1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 11.Greggio E, et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 12.West AB, et al. Parkinson's disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet. 2007;16:223–232. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- 13.Ito G, et al. GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson's disease. Biochemistry. 2007;46:1380–1388. doi: 10.1021/bi061960m. [DOI] [PubMed] [Google Scholar]
- 14.Smith WW, et al. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci USA. 2005;102:18676–18681. doi: 10.1073/pnas.0508052102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacLeod D, et al. The familial Parkinsonism gene LRRK2 regulates neurite process morphology. Neuron. 2006;52:587–593. doi: 10.1016/j.neuron.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Biskup S, et al. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 17.Shin N, et al. LRRK2 regulates synaptic vesicle endocytosis. Exp Cell Res. 2008;314:2055–2065. doi: 10.1016/j.yexcr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Lee SB, Kim W, Lee S, Chung J. Loss of LRRK2/PARK8 induces degeneration of dopaminergic neurons in Drosophila. Biochem Biophys Res Commun. 2007;358:534–539. doi: 10.1016/j.bbrc.2007.04.156. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, et al. A Drosophila model for LRRK2-linked parkinsonism. Proc Natl Acad Sci USA. 2008;105:2693–2698. doi: 10.1073/pnas.0708452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zabetian CP, et al. A clinic-based study of the LRRK2 gene in Parkinson disease yields new mutations. Neurology. 2005;65:741–744. doi: 10.1212/01.wnl.0000172630.22804.73. [DOI] [PubMed] [Google Scholar]
- 21.Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Meloni EG, Davis M. Synergistic enhancement of the acoustic startle reflex by dopamine D1 and 5-HT1A agonists and corresponding changes in c-Fos expression in the dorsal raphe of rats. Psychopharmacology. 2000;151:359–367. doi: 10.1007/s002130000474. [DOI] [PubMed] [Google Scholar]
- 23.Robertson SD, Matthies HJ, Galli A. A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol Neurobiol. 2009;39:73–80. doi: 10.1007/s12035-009-8053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia AG, Garcia-De-Diego AM, Gandia L, Borges R, Garcia-Sancho J. Calcium Signaling and Exocytosis in Adrenal Chromaffin Cells. Physiol Rev. 2006;86:1093–1131. doi: 10.1152/physrev.00039.2005. [DOI] [PubMed] [Google Scholar]
- 25.Neher E. A comparison between exocytic control mechanisms in adrenal chromaffin cells and a glutamatergic synapse. Pflugers Arch. 2006;453:261–268. doi: 10.1007/s00424-006-0143-9. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg MS, et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 27.Starke K, Gothert M, Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989;69:864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- 28.Johnson SW, Seutin V, North RA. Burst firing in dopamine neurons induced by N-methyl-D-aspartate: Role of electrogenic sodium pump. Science. 1992;258:665–667. doi: 10.1126/science.1329209. [DOI] [PubMed] [Google Scholar]
- 29.Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercuri NB, Bonci A, Calabresi P, Stefani A, Bernardi G. Properties of the hyperpolarization-activated cation current Ih in rat midbrain dopaminergic neurons. Eur J Neurosci. 1995;7:462–469. doi: 10.1111/j.1460-9568.1995.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 31.Mercuri NB, Calabresi P, Bernardi G. Responses of rat substantia nigra compacta neurones to L-DOPA. Br J Pharmacol. 1990;100:257–260. doi: 10.1111/j.1476-5381.1990.tb15792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mercuri NB, et al. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience. 1997;79:323–327. doi: 10.1016/s0306-4522(97)00135-8. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg MS, et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi H, Shen J. Absence of dopaminergic neuronal degeneration and oxidative damage in aged DJ-1-deficient mice. Mol Neurodegener. 2007;2:10. doi: 10.1186/1750-1326-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitada T, et al. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci USA. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kantor L, et al. Protein kinase C and intracellular calcium are required for amphetamine-mediated dopamine release via the norepinephrine transporter in undifferentiated PC12 cells. J Pharmacol Exp Ther. 2001;297:1016–1024. [PubMed] [Google Scholar]
- 37.Gnegy ME, et al. Intracellular Ca2+ regulates amphetamine-induced dopamine efflux and currents mediated by the human dopamine transporter. Mol Pharmacol. 2004;66:137–143. doi: 10.1124/mol.66.1.137. [DOI] [PubMed] [Google Scholar]
- 38.Usiello A, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 39.Centonze D, et al. Dopamine D2 receptor-mediated inhibition of dopaminergic neurons in mice lacking D2L receptors. Neuropsychopharmacology. 2002;27:723–726. doi: 10.1016/S0893-133X(02)00367-6. [DOI] [PubMed] [Google Scholar]
- 40.Jenner P. Dopamine agonists, receptor selectivity and dyskinesia induction in Parkinson's disease. Curr Opin Neurol. 2003;16:S3–S7. doi: 10.1097/00019052-200312001-00002. [DOI] [PubMed] [Google Scholar]
- 41.Noble EP. The DRD2 gene in psychiatric and neurological disorders and its phenotypes. Pharmacogenomics. 2000;1:309–333. doi: 10.1517/14622416.1.3.309. [DOI] [PubMed] [Google Scholar]
- 42.Whone AL, et al. Slower progression of Parkinson's disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol. 2003;54:93–101. doi: 10.1002/ana.10609. [DOI] [PubMed] [Google Scholar]
- 43.Kitamura Y, Taniguchi T, Shimohama S, Akaike A, Nomura Y. Neuroprotective mechanisms of antiparkinsonian dopamine D2-receptor subfamily agonists. Neurochem Res. 2003;28:1035–1040. doi: 10.1023/a:1023207222944. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, et al. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson's disease. Nat Neurosci. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pothos EN, et al. Stimulation-dependent regulation of the pH, volume and quantal size of bovine and rodent secretory vesicles. J Physiol. 2002;542:453–476. doi: 10.1113/jphysiol.2002.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]