Abstract
We demonstrate that accurate values of mass-per-length (MPL), which serve as strong constraints on molecular structure, can be determined for amyloid fibrils by quantification of intensities in dark-field electron microscope images obtained in the tilted-beam mode of a transmission electron microscope. MPL values for fibrils formed by residues 218–289 of the HET-s fungal prion protein, for 2-fold- and 3-fold-symmetric fibrils formed by the 40-residue β-amyloid peptide, and for fibrils formed by the yeast prion protein Sup35NM are in good agreement with previous results from scanning transmission electron microscopy. Results for fibrils formed by the yeast prion protein Rnq1, for which the MPL value has not been previously reported, support an in-register parallel β-sheet structure, with one Rnq1 molecule per 0.47-nm β-sheet repeat spacing. Since tilted-beam dark-field images can be obtained on many transmission electron microscopes, this work should facilitate MPL determination by a large number of research groups engaged in studies of amyloid fibrils and similar supramolecular assemblies.
Keywords: Alzheimer's disease, molecular structure, prions, solid state NMR
The mass-per-length (MPL) of an amyloid fibril is an important constraint on its molecular structure. Given that amyloid fibrils contain β-sheets with cross-β alignment relative to the long fibril axis (1, 2) and that the spacing between β-strands in β-sheets is always 0.47 ± 0.01 nm, one expects the MPL to be nearly η×MW/0.47 kDa/nm, where MW is the amyloid-forming polypeptide's molecular weight and η is the number of molecules in each β-sheet spacing. MPL measurements were the first indication that fibrils formed by the 40-residue β-amyloid peptide (Aβ1–40) might have both 2-fold-symmetric (η ≈ 2) and three-fold-symmetric (η ≈ 3) polymorphs (3–6). MPL measurements support a two-fold-symmetric structural model for amylin fibrils with a specific morphology (7) and provide evidence for polymorphism similar to that of Aβ1–40 fibrils (8). MPL measurements (9) provide support for a β-helix-like structure (10, 11) for fibrils formed by residues 218–298 of the HET-s prion protein (HET-s218–289), with each molecule spanning two turns of the β-helix (η ≈ 0.5). MPL measurements on fibrils formed by the prion domains of the yeast prion proteins Ure2p (12) and Sup35 (13) support in-register parallel β-sheet structures (14–16), with backbone hydrogen bonds in the β-sheets being purely intermolecular (η ≈ 1). Molecular models corresponding to these various values of η are given in the relevant papers (4, 7, 10, 16).
MPL data in these cases comes from quantification of intensities in images obtained with scanning transmission electron microscopy (STEM). STEM images of unstained samples are dark-field images, in the sense that the background has low intensity (arising from weak electron scattering when the rastering electron beam strikes the thin carbon film on which fibrils are adsorbed), while the fibrils have higher intensity (arising from stronger electron scattering when the beam strikes the fibrils themselves). As shown by earlier studies (17–20), STEM image intensities are proportional to mass densities (per unit area), so that MPL values can in principle be determined quantitatively in either of two ways: (i) by measurement of incident electron beam flux, knowledge of electron scattering cross-sections and detector geometry, and calibration of detector sensitivity; (ii) by comparison of fibril image intensities with image intensities of reference objects with known mass densities, typically tobacco mosaic virus (TMV) rods codeposited with the fibrils. In practice, the second method has been used in studies of amyloid fibrils. Although dedicated STEM instruments have been used in most MPL studies, MPL determination using the STEM mode of a commercial transmission electron microscope at 300 kV has also been demonstrated (21). As an alternative to STEM, energy-filtered transmission electron microscopy (EF-TEM) has been used to measure mass densities (22, 23) and amyloid fibril MPL values (24) in the same manner. EF-TEM also produces dark-field images of unstained samples, but by uniform illumination and image formation from inelastically scattered electrons using electron optics, rather than by rastering of a narrow electron beam and collection of primarily elastically scattered electrons as in STEM.
Dark-field images of unstained samples can also be obtained with a conventional transmission electron microscope (TEM) by tilting the incident electron beam by a small angle so that it is blocked by the objective aperture after passing through the sample. The image is then formed from scattered electrons that pass through the aperture, using the same electron optics as in bright-field TEM imaging of stained samples. This is a dark-field mode available on many TEM instruments, which we call tilted-beam TEM (TB-TEM). TB-TEM images of amyloid fibrils are superficially quite similar to STEM and EF-TEM images, suggesting that MPL values might also be obtainable from TB-TEM images. In this paper, we demonstrate that accurate MPL values for amyloid fibrils can indeed be obtained by quantification of intensities in TB-TEM images, so that MPL measurements can be carried out with widely available instrumentation and relative ease.
Results
Fig. 1 shows examples of bright-field TEM and dark-field TB-TEM images of amyloid fibrils formed by HET-s218–289 (Fig. 1A), Aβ1–40 (Fig. 1 B and C), and Sup35NM (Fig. 1D). Aβ1–40 fibrils have predominantly two-fold-symmetric (2f-Aβ1–40, Fig. 1B) or three-fold-symmetric (3f-Aβ1–40, Fig. 1C) structures, depending on growth conditions as previously described (3, 4, 25). Sup35NM (residues 1–253 of Sup35p) includes the N-terminal prion (N) and middle (M) domains (26) and is often used in studies of the [PSI+] prion (27). Bright-field images in Fig. 1 are negatively stained with uranyl acetate. Dark-field images are unstained and also contain TMV rods. All images were obtained at 80 kV electron beam energy.
Fig. 1.
TEM images of amyloid fibrils. Bright-field images of negatively stained samples are in the upper left and dark-field TB-TEM images are in the remaining three sections of each panel. (Scale bars, 100 nm and 200 nm for bright-field and dark-field images, respectively.) Single-headed arrows indicate examples of fibrils analyzed for MPL determinations. Double-headed arrows indicate TMV rods. (A) HET-s218–289 fibrils. Lower two sections show examples of MPL values (kDa/nm) determined for segments enclosed in rectangles. (B) 2f-Aβ40 fibrils. Lower left section shows examples of MPL values along the length of a single fibril. Upper right section shows examples of MPL values for segments of TMV rods, calibrated by assuming the average TMV MPL value to be 131 kDa/nm. (C) 3f-Aβ40 fibrils. Lower left and upper right sections show examples of MPL values for 3f-Aβ40 fibrils and TMV rods, respectively. (D) Sup35NM fibrils. Lower left section shows examples of MPL values along the length of a single fibril.
MPL values were extracted from TB-TEM images as previously described for STEM (8, 28): (i) image intensities were integrated over rectangular areas centered on fibril segments (IF) and over equal areas of background on either side of each fibril segment (IB1 and IB2); (ii) similarly, image intensities were integrated over rectangular areas centered on TMV segments (ITMV) and over equal areas of background on either side of each TMV segment (IB3 and IB4); (iii) for each image, the quantity 〈ITMV〉 was calculated as the average of the quantities ITMV–(IB3 + IB4)/2 within the image; (iv) MPL values were calculated as MPL = 131×[IF − (IB1 + IB2)/2]/〈ITMV〉, based on the known 131 kDa/nm MPL of TMV (29). All rectangles were 80 nm in length. Rectangle widths were adjusted to include the fibril or TMV width in each image. Except as noted below, all intensities used to calculate a given MPL value were taken from the same image, as intensities from different images were not necessarily directly comparable due to variations in incident beam intensity and other factors.
Fig. 2 shows MPL histograms, extracted from 30 or more TB-TEM images of each fibril sample. MPL peak positions were determined by fitting the histograms to one or more Gaussian functions. For HET-s218–289 fibrils, the histogram in Fig. 2A shows a single peak at 8.3 kDa/nm, in good agreement with the value 9.2 kDa/nm determined previously by STEM (9) and the value 9.20 kDa/nm predicted by the β-helix-like molecular structural model of Meier and coworkers (10, 11) (MW = 8.65 kDa, η = 0.5). For 2f-Aβ1–40, the histogram in Fig. 2B shows a major peak at 17.4 kDa/nm, in good agreement with the value of approximately 21 kDa/nm determined previously by STEM (3) and the value 18.4 kDa/nm predicted by the two-fold-symmetric structural model of Petkova et al. (25) (MW = 4.33 kDa, η = 2). For 3f-Aβ1–40, the histogram in Fig. 2C shows a major peak at 27.7 kDa/nm, in good agreement with the value of 26–29 kDa/nm determined previously by STEM (3, 4) and the value 27.6 kDa/nm predicted by the three-fold-symmetric structural model of Paravastu et al. (3, 4) (MW = 4.33 kDa, η = 3). Goldsbury et al. have also reported that MPL values for Aβ1–40 fibrils derived from STEM images are either 17 ± 2 kDa/nm or 28 ± 4 kDa/nm (5).
Fig. 2.
MPL histograms extracted from TB-TEM images. Solid curves are fits to one or more Gaussian functions. Vertical dashed lines indicate ideal MPL values predicted by experimentally-based structural models, as discussed in the text. (A) HET-s218–289 fibrils, fit to one peak at 8.3 kDa/nm, with 4.7 kDa/nm full width at half maximum (FWHM). (B) 2f-Ab40 fibrils, fit to three peaks at 6.6, 17.4, and 28.0 kDa/nm, with 1.9, 9.3, and 11.0 kDa/nm FWHM, respectively. Relative areas are 0.03, 1.00, and 0.21. (C) 3f-Ab40 fibrils, fit to four peaks at 8.2, 20.1, 27.7, and 34.9 kDa/nm, with 6.4, 6.3, 6.4, and 5.8 kDa/nm FWHM, respectively. Relative areas are 0.18, 0.13, 1.00, and 0.09. (D) Sup35NM fibrils, fit to one peak at 60.8 kDa/nm, with 18.2 kDa/nm FWHM.
The histogram in Fig. 2B shows a minor peak at 28.0 kDa/nm, attributable to a minor population of 3f-Aβ1–40 fibrils in the 2f-Aβ1–40 sample. The histogram in Fig. 2C shows minor peaks at 20.1 kDa/nm and 34.9 kDa/nm, attributable to minor populations of single and paired 2f-Aβ1–40 fibrils in the 3f-Aβ1–40 sample. Both histograms show minor peaks near 9 kDa/nm, arising from rare occurrences of fibrils with η = 1 (see Fig. S1). Such fibrils were not observed in previous STEM studies (3–6,28) and may represent “immature” structures, especially since Aβ1–40 fibril samples for our TB-TEM measurements were incubated for 24 h or less after seeding.
Our assignment of minor peaks in Fig. 2 B and C to specific minority structures is supported by the observation of multiple, nearly equal MPL counts along the lengths of individual fibrils (such as fibrils in Fig. S1 A and C), as well as by the inability to fit the MPL histogram to single Gaussian function, as in Fig. 2 B and C.
For Sup35NM fibrils, the histogram in Fig. 2D shows a single peak at 60.8 kDa/nm, consistent with the value 62.6 kDa/nm predicted for MW = 29.4 kDa if η = 1, as in the in-register parallel β-sheet structure indicated by solid state NMR measurements (14, 16). Although we are not aware of STEM measurements on Sup35NM fibrils, STEM measurements for fibrils formed by residues 1–65 of Sup35p fused to green fluorescent protein (GFP-Sup35p1–65) indicate MPL values ranging from 72.5 kDa/nm to 95.4 kDa/nm, depending on the exact fibril morphology (13), in approximate agreement with the value 79.2 kDa/nm predicted for MW = 37.2 kDa and η = 1. Thus, the TB-TEM results in Fig. 2D suggest that Sup35NM fibrils and certain classes of GFP-Sup35p1–65 fibrils have a common structural organization. Interestingly, fibrils formed in vitro by both Sup35NM (30) and GFP-Sup35p1–65 (31) have been shown to be capable of inducing the [PSI+] prion phenotype in yeast.
As an initial application to a system for which no MPL data are available from STEM or other techniques, we performed the measurements shown in Fig. 3 on fibrils formed by the prion domain of Rnq1, the protein that determines the [PIN+] prion of yeast. As shown by Liebman and coworkers, the Rnq1 prion domain is contained in residues 153–405 (32). Our sample contained a mixture of residues 153–405 and residues 216–405 (see Materials and Methods). The MPL histogram in Fig. 4B shows a peak centered at 46.2 kDa/nm, in good agreement with the value 46.6 kDa/nm predicted for a cross-β structure formed by residues 216–405 (MW = 21.9 kDa) with η = 1. A small number of counts were also observed near MPL approximately 59.8 kDa/nm, the value predicted for residues 153–405 (MW = 28.1 kDa) with η = 1. These results are consistent with the earlier demonstration by solid state NMR that Rnq1 prion domain fibrils have an in-register parallel β-sheet structure (33).
Fig. 3.
MPL determination for Rnq1 prion domain fibrils. (A) Bright-field image of negatively stained sample. (B) MPL histogram determined from TB-TEM images, fit to one Gaussian peak at 46.2 kDa/nm, with 13.2 kDa/nm FWHM. (C and D) Examples of TB-TEM images, with TMV rods indicated by double-headed arrows. MPL values (kDa/nm) are shown for fibril segments enclosed in rectangles.
Fig. 4.
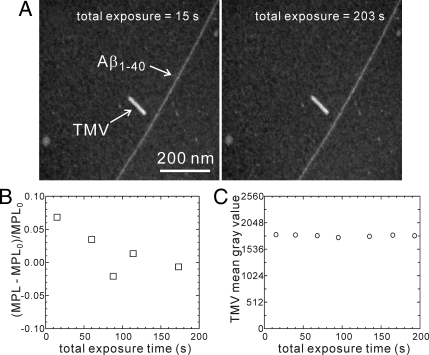
Assessment of electron beam damage during MPL measurements. Electron flux density is approximately 2.4 × 103 nm−2s−1. (A) TB-TEM images of a 3f-Aβ40 fibril, obtained after 15 s of exposure to the electron beam (first image in a series at the same location on the sample grid) and after 203 s of exposure (final image in a series). (B) Fractional variations in the apparent MPL of the fibril in panel A, calibrated against the TMV rod in the same set of images, with MPL0 = 27 kDa/nm. (C) Average gray scale value for a single TMV rod in a series of images, under continuous exposure to the electron beam.
Discussion
Reliability and Precision of MPL Determinations by TB-TEM.
Results in Figs. 1 and 2 demonstrate empirically that accurate MPL values for amyloid fibrils can be determined from TB-TEM images over an MPL range from 10 kDa/nm (or possibly less) to 60 kDa/nm (or probably more). These experimental results allay concerns that MPL determination might be precluded by damage to the fibrils induced by the electron beam, non-uniform illumination of the image field by the electron beam, nonlinearities in the electron detector or camera system, imperfections in the electron optics, multiple scattering, or other considerations. Aspects of our experimental protocol that we believe to be essential for accurate MPL determination are described below (see Materials and Methods).
The precision of the MPL values, estimated from the uncertainty in the major Gaussian peak positions in Fig. 2, is not as high as in some STEM studies of amyloid fibrils (5, 9, 13, 28) but is in all cases sufficient to determine η, the important structural parameter, to within ± 0.3 (e.g., η = 1.9 ± 0.3 for 2f-Aβ40 fibrils). In the specific context of amyloid fibrils, the demonstrated precision is sufficient to distinguish among plausible candidate structural models. For HET-s218–289, the precision is apparently limited by the signal-to-noise of the TB-TEM images, that is, the low fibril image intensity relative to fluctuations in background intensity. Fluctuations in background intensity in measurements on HET-s218–289 fibrils, measured as 131× IB1 − IB2/〈ITMV〉, have a Gaussian distribution with FWHM = 5.6 kDa/nm (Fig. S2A). These fluctuations should produce a FWHM of × 5.6 kDa/nm in the MPL histogram for HET-s218–289 fibrils when background subtraction is carried out as described above, in reasonable agreement with the 4.7 kDa/nm FWHM in Fig. 2A.
Fig. 1 B and C (lower left) show series of MPL values along the lengths of single 2f-Aβ40 and 3f-Aβ40 fibrils. The random scatter among these values indicates that non-uniform illumination by the electron beam within a single image is not a major cause of variations in the apparent MPL values, as non-uniform illumination would be expected to produce monotonic variations. Fig. 1 B and C (upper right) show MPL values for TMV rods within a single image, derived by setting the average of these values to 131 kDa/nm. Again, the random scatter argues against non-uniform illumination as a source of imprecision. A histogram of TMV MPL values (Fig. S3) indicates that structural variations in TMV rods (34) contribute less than 10% to imprecision in MPL.
The MPL histogram for Sup35NM fibrils (Fig. 2D) shows the broadest peak. Background intensity fluctuations in TB-TEM images of Sup35NM fibrils have FWHM = 10.8 kDa/nm (Fig. S2B), greater than in TB-TEM images of HET-s218–289 fibrils due the presence of more extraneous material on the sample grids and the use of wider rectangular integration areas. Background intensity fluctuations may then contribute roughly 13 kDa/nm to the observed 18.2 kDa/nm FWHM in Fig. 2D. Additional contributions to MPL variations apparently arise from structural nonuniformity of the fibrils, as shown in Fig. 1D where MPL values along the length of a single fibril have relatively large scatter. Structural nonuniformity may include unresolved breaks in the fibrils and adhesion of nonfibrillar material to the fibrils. We conclude that the most important limitation on the precision of MPL determination is sample quality, at least for MPL values above 10 kDa/nm. Similar issues can affect STEM measurements.
Perhaps surprisingly, damage to the fibrils and TMV by the electron beam plays no discernible role in our TB-TEM measurements. Previous studies indicate that a total electron dose of 104 e/nm2 at 80 keV produces a mass loss of roughly 30% for typical protein assemblies (including TMV) in STEM measurements at room temperature (18, 34). Similar mass losses have been measured in EF-TEM studies (22, 23). We estimate the typical electron dose for a single TB-TEM image, acquired in 10 s under our experimental conditions, to be 0.6–2.5 × 104 e/nm2 (see Materials and Methods). Fig. 4A shows TB-TEM images of 3f-Aβ1–40 and TMV obtained at the beginning and end of a 200-s period of continuous exposure to the electron beam. No degradation of the image is seen. Fig. 4 B and C shows MPL values and TMV gray scale intensities determined from images of a single field during a 200-s period. No systematic changes in these quantities are observed (other than a possible 5% reduction in 3f-Aβ1–40 fibril MPL over 200 s). Comparison of TMV images recorded with total beam exposure times as short as 1 s (i.e., roughly 2.5 × 103 e/nm2) with images at longer exposure times shows no evidence for rapid mass loss.
Theoretical Justification for Quantification of TB-TEM Images.
As for STEM and EF-TEM imaging (18, 21, 23), the integrated intensity within an area A of the TB-TEM image is proportional to the number of collected, scattered electrons Ns from that area. After background subtraction, this quantity can be expressed as
 |
where Ne is the number of incident electrons on A, nk is the number of atoms of type k within A, σk is the scattering cross-section, and fk is the fraction of scattered electrons that are collected and focused to form the image. This expression assumes that no multiple scattering events occur, that unscattered electrons are entirely blocked by the objective aperture, that the microscope's camera system is linear, and that diffraction effects (i.e., interference among scattering amplitudes from different atoms in the sample) are negligible. The mass per area of the sample is
 |
where Mk is the atomic mass. Consequently, NS = μNeQ, with
 |
being the effective scattering area per mass within A. The value of Q depends on the elemental composition and elemental densities as well as the microscope geometry. Q is expected to be approximately the same for all proteinaceous materials (18, 23). Therefore, TB-TEM image intensities can be used to determine μ/A, and hence MPL.
Both elastic and inelastic scattering contribute to σk. Given a 1.5-mm distance from the sample grid to the objective aperture, a 1.2° (21 mrad) beam tilt, and a 50-μm objective aperture diameter, electrons with scattering angles in the 4.3–37.6 mrad range contribute to the TB-TEM image. Although the total inelastic scattering cross-section is greater than the total elastic scattering cross-section, the majority of inelastically scattered electrons have scattering angles less than 4 mrad under our experimental conditions, while the majority of elastically scattered electrons have scattering angles greater than 8 mrad (35). Our TB-TEM images are therefore formed primarily from elastically scattered electrons, with a minor contribution from inelastic scattering. Since previous work has shown that MPL values can be determined from either elastic scattering, as in STEM studies, or inelastic scattering, as in EF-TEM studies, images that result from a combination of elastic and inelastic scattering should also permit MPL determination.
Materials and Methods
Sample Preparation.
HET-s218–289 was expressed in E. coli with a C-terminal hexa-His tag and purified as described by Dos Reis et al. (36). Briefly, cells were lysed by sonication and the protein was extracted from the insoluble pellet fraction with 8 M guanidine-HCl, purified on a Talon (Clontech) column, and eluted in 8 M urea. Denaturant was removed using a HiTrap column GE Healthcare) in 175 mM acetic acid (37). The eluate containing 1.4 mM protein was neutralized with Tris base and incubated at 4 °C without agitation for 7 days to allow fibril formation.
Aβ1–40 was synthesized and purified as previously described (3, 4). Approximately 1 mg of lyophilized peptide was dissolved initially in dimethyl sulfoxide at 8 mM concentration, then diluted to 230 μM in 10 mM phosphate buffer, pH 7.4. For seeded fibril growth, preexisting 2f-Aβ1–40 or 3f-Aβ1–40 fibrils were added in a 1:20 molar ratio of fibrillar peptide to fresh peptide, the solution was sonicated to break the preexisting fibrils into short fragments (Branson model 250 sonifier, lowest power, 10% duty cycle, 30 s), and fibrils were allowed to grow at 24 °C without mixing or agitation of the solution. Sup35NM was expressed with a C-terminal hexa-His tag and purified under denaturing conditions as previously described (14). Sup35NM was then dialyzed into non-denaturing buffer (5 mM phosphate, pH 7.4, and 150 mM NaCl) and incubated for 7 days at 4 °C to permit fibril formation. Fibrils were collected by centrifugation and resuspended in deionized water.
The prion domain of Rnq1p was expressed with a C-terminal hexa-His tag and partially purified under denaturing conditions by binding to a Ni-NTA column and eluting with imidazole (33). Electrospray-ionization mass spectrometry showed our sample to contain proteins with MW = 28.102 kDa (corresponding to residues 153–405 of Rnq1 with the hexa-His tag) and MW = 21.931 kDa (corresponding to residues 216–405 of Rnq1p with the hexa-His tag). Proteins were precipitated with cold methanol, dried, and dissolved in 4 M urea, 5 mM potassium phosphate, pH 7.4, and 150 mM NaCl. Fibrils then formed at room temperature with gentle agitation (38).
For TB-TEM, fibrils were adsorbed to carbon films on lacey carbon supports on 300 mesh copper grids. Films were 4–10 nm thick, estimated from the volume of evaporated carbon and geometry of our carbon deposition chamber. Grids were glow-discharged immediately before use. A 5-μL aliquot of fibril solution and a 1-μL aliquot of TMV solution were applied simultaneously to the grid. Fibril solutions were diluted as required to produce suitable coverages in TB-TEM images. TMV concentration was 0.08–0.23 mg/mL. After a 5-min adsorption period, solutions were blotted, washed three times with deionized water (5 μL aliquot, 10 s each), blotted, and dried in air. Grids of 2f-Aβ1–40 and 3f-Aβ1–40 fibrils were prepared 8 h and 24 h after seeding, respectively, to minimize lateral association of fibrils.
For TEM images of negatively stained samples, grids were prepared by the same procedure, but without adsorption of TMV and with a 60-s period of staining with 5 μL of 3% uranyl acetate before final blotting and drying.
Image Acquisition and Processing.
Images were acquired with an FEI Morgagni TEM, operating at 80 kV, equipped with a side-mounted, 1 megapixel AMT Advantage HR CCD camera. Bright-field images were acquired at 140,000× magnification. TB-TEM images were acquired at 56,000× magnification, using a beam tilt angle of 1.2°, a 50-μm diameter objective aperture, and a 300-μm condenser aperture. Smaller tilt angles did not produce adequate blocking of the unscattered electron beam. Other microscope settings included spot 5, bias 3, and filament current in the 10–20 μA range. Before recording TB-TEM images, condenser stigmators were carefully adjusted to give a circular beam profile when the beam was viewed on the carbon film in TB-TEM mode (at lower magnification), and the beam was carefully centered and spread to produce uniform illumination over the field of view, as indicated by uniform background intensity from the carbon film in the final images. The sample grid was scanned manually for promising areas at lower magnifications and lower beam intensities (≈100 e/nm2-s), using the survey mode of the AMT software with a camera gain value of 8. Once an area that apparently contained both TMV rods and amyloid fibrils was identified, the magnification and beam intensity were increased (to ≈600–2,500 e/nm2-s), the focus was quickly adjusted to maximize the clarity of the TMV rods, and final TB-TEM images were recorded. Each image was the average of 8 acquisitions, each with a 1.2-s exposure time and a camera gain value of 1. Images were stored either as 8-bit tiff files, after automatic linear rescaling of image intensities by the camera software to fill the 8-bit range (threshold = 10 and tail = 5 in the AMT software), or as raw 16-bit tiff files without rescaling (threshold = 0 and tail = 0). Equivalent MPL results were obtained in the two cases. Electron doses were estimated from our own calibration of the camera gray scale in images of an empty sample grid with no beam tilt against direct measurements of current from the TEM viewing screen. For our system, 1 gray scale unit in a 16-bit image corresponds to roughly 0.040 electrons per pixel.
For Rnq1 prion domain fibrils, we had difficulty finding grid areas that contained both fibrils and TMV rods within the same field at 56,000× magnification. Therefore, images of many non-overlapping fields within a promising grid area were recorded under identical beam conditions and saved as 16-bit tiff files without rescaling. We found that TMV intensities in these images were identical to within the usual uncertainty (e.g., as in Fig. S2), and that the average TMV intensity across these images could be used to derive MPL values for fibrils within the same set of images, even though most images contained only fibrils or only TMV rods.
TB-TEM images were analyzed with ImageJ software (available at http://rsbweb.nih.gov/ij/). Only fibrils that appeared to be single filaments, rather than pairs or higher-order bundles, were selected for MPL measurements. Thus, as with STEM measurements, the relative areas of peaks in MPL histograms do not necessarily reflect the true populations of fibrils with different MPL values in the sample.
The 80-nm length of rectangular areas was chosen to allow several hundred MPL counts for each sample in Fig. 2 and for consistency among samples. In principle, longer rectangles might produce more precise final MPL values, subject to restrictions imposed by fibril curvature and overlap of fibrils in the TB-TEM images. The same considerations apply in STEM measurements.
Supplementary Material
Acknowledgments.
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health. We thank Dr. D. Eric Anderson of NIDDK for mass spectrometry of Rnq1 prion domain fibrils, and Prof. Gerald Stubbs and Dr. Amy Kendall of Vanderbilt University for providing TMV.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907821106/DCSupplemental.
References
- 1.Sunde M, Blake CCF. From the globular to the fibrous state: Protein structure and structural conversion in amyloid formation. Q Rev Biophys. 1998;31:1–39. doi: 10.1017/s0033583598003400. [DOI] [PubMed] [Google Scholar]
- 2.Tycko R. Molecular structure of amyloid fibrils: Insights from solid state NMR. Q Rev Biophys. 2006;39:1–55. doi: 10.1017/S0033583506004173. [DOI] [PubMed] [Google Scholar]
- 3.Petkova AT, Leapman RD, Guo ZH, Yau WM, Mattson MP, Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer's β-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 4.Paravastu AK, Leapman RD, Yau WM, Tycko R. Molecular structural basis for polymorphism in Alzheimer's β-amyloid fibrils. Proc Natl Acad Sci USA. 2008;105:18349–18354. doi: 10.1073/pnas.0806270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldsbury C, Frey P, Olivieri V, Aebi U, Muller SA. Multiple assembly pathways underlie amyloid-β fibril polymorphisms. J Mol Biol. 2005;352:282–298. doi: 10.1016/j.jmb.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Goldsbury CS, et al. Studies on the in vitro assembly of Aβ1–40: Implications for the search for Aβ fibril formation inhibitors. J Struct Biol. 2000;130:217–231. doi: 10.1006/jsbi.2000.4259. [DOI] [PubMed] [Google Scholar]
- 7.Luca S, Yau WM, Leapman R, Tycko R. Peptide conformation and supramolecular organization in amylin fibrils: Constraints from solid state NMR. Biochemistry. 2007;46:13505–13522. doi: 10.1021/bi701427q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldsbury CS, et al. Polymorphic fibrillar assembly of human amylin. J Struct Biol. 1997;119:17–27. doi: 10.1006/jsbi.1997.3858. [DOI] [PubMed] [Google Scholar]
- 9.Sen A, et al. Mass analysis by scanning transmission electron microscopy and electron diffraction validate predictions of stacked β-solenoid model of HET-s prion fibrils. J Biol Chem. 2007;282:5545–5550. doi: 10.1074/jbc.M611464200. [DOI] [PubMed] [Google Scholar]
- 10.Wasmer C, Lange A, Van Melckebeke H, Siemer AB, Riek R, Meier BH. Amyloid fibrils of the HET-s(218–289) prion form a β-solenoid with a triangular hydrophobic core. Science. 2008;319:1523–1526. doi: 10.1126/science.1151839. [DOI] [PubMed] [Google Scholar]
- 11.Ritter C, et al. Correlation of structural elements and infectivity of the HET-s prion. Nature. 2005;435:844–848. doi: 10.1038/nature03793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baxa U, et al. Architecture of Ure2p prion filaments: The N-terminal domains form a central core fiber. J Biol Chem. 2003;278:43717–43727. doi: 10.1074/jbc.M306004200. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Avalos R, King CY, Wall J, Simon M, Caspar DLD. Strain-specific morphologies of yeast prion amyloid fibrils. Proc Natl Acad Sci USA. 2005;102:10165–10170. doi: 10.1073/pnas.0504599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shewmaker F, Ross ED, Tycko R, Wickner RB. Amyloids of shuffled prion domains that form prions have a parallel in-register β-sheet structure. Biochemistry. 2008;47:4000–4007. doi: 10.1021/bi7024589. [DOI] [PubMed] [Google Scholar]
- 15.Baxa U, et al. Characterization of β-sheet structure in Ure2p1–89 yeast prion fibrils by solid state nuclear magnetic resonance. Biochemistry. 2007;46:13149–13162. doi: 10.1021/bi700826b. [DOI] [PubMed] [Google Scholar]
- 16.Shewmaker F, Wickner RB, Tycko R. Amyloid of the prion domain of Sup35p has an in-register parallel β-sheet structure. Proc Natl Acad Sci USA. 2006;103:19754–19759. doi: 10.1073/pnas.0609638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wall JS, Hainfeld JF. Mass mapping with the scanning transmission electron microscope. Ann Rev Biophys Biophys Chem. 1986;15:355–376. doi: 10.1146/annurev.bb.15.060186.002035. [DOI] [PubMed] [Google Scholar]
- 18.Engel A. Mass determination by electron scattering. Micron. 1982;13:425–436. [Google Scholar]
- 19.Engel A. Molecular weight determination by scanning transmission electron microscopy. Ultramicroscopy. 1978;3:273–281. doi: 10.1016/s0304-3991(78)80037-0. [DOI] [PubMed] [Google Scholar]
- 20.Zeitler E, Bahr GF. Photometric procedure for weight determination of submicroscopic particles by quantitative electron microscopy. J Appl Phys. 1962;33:847–853. [Google Scholar]
- 21.Sousa AA, Leapman RD. Quantitative STEM mass measurement of biological macromolecules in a 300 kV TEM. J Microsc-Oxf. 2007;228:25–33. doi: 10.1111/j.1365-2818.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 22.Feja B, Aebi U. Molecular mass determination by STEM and EFTEM: A critical comparison. Micron. 1999;30:299–307. [Google Scholar]
- 23.Feja B, Durrenberger M, Muller S, Reichelt R, Aebi U. Mass determination by inelastic electron scattering in an energy-filtering transmission electron microscope with slow-scan CCD camera. J Struct Biol. 1997;119:72–82. [Google Scholar]
- 24.Paravastu AK, Qahwash I, Leapman RD, Meredith SC, Tycko R. Seeded growth of β-amyloid fibrils from Alzheimer's brain-derived fibrils produces a distinct structure. Proc Natl Acad Sci USA. 2009;106:7443–7448. doi: 10.1073/pnas.0812033106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petkova AT, Yau WM, Tycko R. Experimental constraints on quaternary structure in Alzheimer's β-amyloid fibrils. Biochemistry. 2006;45:498–512. doi: 10.1021/bi051952q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teravanesyan MD, Dagkesamanskaya AR, Kushnirov VV, Smirnov VN. The Sup35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [PSI+] in the yeast Saccharomyces cerevisiae. Genetics. 1994;137:671–676. doi: 10.1093/genetics/137.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glover JR, Kowal AS, Schirmer EC, Patino MM, Liu JJ, Lindquist S. Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell. 1997;89:811–819. doi: 10.1016/s0092-8674(00)80264-0. [DOI] [PubMed] [Google Scholar]
- 28.Antzutkin ON, Leapman RD, Balbach JJ, Tycko R. Supramolecular structural constraints on Alzheimer's β-amyloid fibrils from electron microscopy and solid state nuclear magnetic resonance. Biochemistry. 2002;41:15436–15450. doi: 10.1021/bi0204185. [DOI] [PubMed] [Google Scholar]
- 29.Namba K, Stubbs G. Structure of tobacco mosaic virus at 3.6 Å resolution: Implications for assembly. Science. 1986;231:1401–1406. doi: 10.1126/science.3952490. [DOI] [PubMed] [Google Scholar]
- 30.Sparrer HE, Santoso A, Szoka FC, Weissman JS. Evidence for the prion hypothesis: Induction of the yeast [PSI+] factor by in vitro-converted Sup35 protein. Science. 2000;289:595–599. doi: 10.1126/science.289.5479.595. [DOI] [PubMed] [Google Scholar]
- 31.King CY, Diaz-Avalos R. Protein-only transmission of three yeast prion strains. Nature. 2004;428:319–323. doi: 10.1038/nature02391. [DOI] [PubMed] [Google Scholar]
- 32.Vitrenko YA, Pavon ME, Stone SI, Liebman SW. Propagation of the [PIN+] prion by fragments of Rnq1 fused to GFP. Curr Genet. 2007;51:309–319. doi: 10.1007/s00294-007-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wickner RB, Dyda F, Tycko R. Amyloid of Rnq1p, the basis of the [PIN+] prion, has a parallel in-register β-sheet structure. Proc Natl Acad Sci USA. 2008;105:2403–2408. doi: 10.1073/pnas.0712032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller SA, Engel A. Structure and mass analysis by scanning transmission electron microscopy. Micron. 2001;32:21–31. doi: 10.1016/s0968-4328(00)00022-6. [DOI] [PubMed] [Google Scholar]
- 35.Wall J, Isaacson M, Langmore JP. Collection of scattered electrons in dark field electron microscopy. 2. Inelastic scattering. Optik. 1974;39:359–374. [Google Scholar]
- 36.Dos Reis S, Coulary-Salin B, Forge V, Lascu I, Begueret J, Saupe SJ. The HET-s prion protein of the filamentous fungus Podospora anserina aggregates in vitro into amyloid-like fibrils. J Biol Chem. 2002;277:5703–5706. doi: 10.1074/jbc.M110183200. [DOI] [PubMed] [Google Scholar]
- 37.Benkemoun L, et al. Methods for the in vivo and in vitro analysis of [HET-s] prion infectivity. Methods. 2006;39:61–67. doi: 10.1016/j.ymeth.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Sondheimer N, Lindquist S. Rnq1: An epigenetic modifier of protein function in yeast. Mol Cell. 2000;5:163–172. doi: 10.1016/s1097-2765(00)80412-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.