Abstract
Children reared in unfavorable socioeconomic circumstances show increased susceptibility to the chronic diseases of aging when they reach the fifth and sixth decades of life. One mechanistic hypothesis for this phenomenon suggests that social adversity in early life programs biological systems in a manner that persists across decades and thereby accentuates vulnerability to disease. Here we examine the basic tenets of this hypothesis by performing genome-wide transcriptional profiling in healthy adults who were either low or high in socioeconomic status (SES) in early life. Among subjects with low early-life SES, there was significant up-regulation of genes bearing response elements for the CREB/ATF family of transcription factors that conveys adrenergic signals to leukocytes, and significant down-regulation of genes with response elements for the glucocorticoid receptor, which regulates the secretion of cortisol and transduces its antiinflammatory actions in the immune system. Subjects from low-SES backgrounds also showed increased output of cortisol in daily life, heightened expression of transcripts bearing response elements for NF-κB, and greater stimulated production of the proinflammatory cytokine interleukin 6. These disparities were independent of subjects' current SES, lifestyle practices, and perceived stress. Collectively, these data suggest that low early-life SES programs a defensive phenotype characterized by resistance to glucocorticoid signaling, which in turn facilitates exaggerated adrenocortical and inflammatory responses. Although these response patterns could serve adaptive functions during acute threats to well-being, over the long term they might exact an allostatic toll on the body that ultimately contributes to the chronic diseases of aging.
Keywords: cortisol, inflammation, NF-kappa B, socioeconomic status, stress
Mounting evidence suggests that early-life socioeconomic status (SES) is a determinant of susceptibility to the chronic diseases of adulthood. To the extent that children spend the first years of their lives in unfavorable socioeconomic conditions, they show increased vulnerability to infectious, respiratory, and cardiovascular diseases in adulthood, as well as some forms of cancer (1–3). These disparities are generally independent of SES in adulthood. For example, the Precursors Study followed 1,131 graduates of Johns Hopkins Medical School over 40 years and found that even among this highly educated, affluent cohort of physicians, low early-life SES conferred a 2.4-fold risk of incident coronary heart disease by age 50 years (4).
These findings raise a challenging mechanistic question: How does a child's SES modulate his or her biology in a fashion that persists across multiple decades and eventually heightens susceptibility to the chronic diseases of adulthood? One plausible answer to this question comes from the literature on the biological programming of early social experience (5–7). This work indicates that animals that receive inadequate parental nurturance or experience prolonged maternal separations can show permanent alterations in stress-related outflow of the autonomic nervous system (ANS) and the hypothalamic–pituitary–adrenocortical (HPA) axis (8–10). These effects are partially mediated by diminished expression of and signaling by the glucocorticoid receptor (GR), a ligand-activated transcription factor that regulates HPA outflow through a hippocampal negative-feedback circuit (11, 12). The effects of early social adversity also extend to the immune system (13). For example, mice that are repeatedly separated from their mothers early in life show an excessive inflammatory response and reduced viral clearance after influenza infection in adulthood (14), an effect that is partially mediated by impaired glucocorticoid regulation of cytokine release.
Collectively, these data suggest that repeated social adversity in early life can program a “defensive” phenotype, which is marked by exaggerated adrenocortical and inflammatory responses to challenge (15). Some evidence indicates that this phenotype involves the development of a functional resistance to GR-mediated signaling, which allows cortisol to partially escape inhibition by negative feedback and facilitates the synthesis of proinflammatory mediators by leukocytes. In social contexts where threats like crowding, predation, and conflict are common, these response patterns might confer a survival advantage by helping organisms to rapidly mobilize energy for fighting and fleeing and to mount vigorous immune responses to infection and injury (15). However, in the context of late-life chronic illnesses, dysregulated GR signaling may enable exaggerated inflammatory responses that contribute to the pathogenesis of cardiovascular disease, some types of cancer, and respiratory conditions.
The current project examines the possibility that a GR-related defensive phenotype emerges in humans who have been reared in the adverse social conditions of a low-SES household. In a sample of healthy young adults who varied in early-life SES, we conducted genome-wide transcriptional surveys on peripheral blood mononuclear cells (PBMCs). When used in concert with bioinformatic techniques (16), these analyses provide an in vivo marker of ANS and HPA signaling to the genome via receptor-mediated signal transduction pathways. Based on the defensive-programming hypothesis, we expected that low early-life SES would be associated with later-life gene transcription profiles characterized by heightened expression of genes responsive to ANS signaling and, because of glucocorticoid resistance, diminished expression of genes responsive to GR-mediated signaling. The latter profile should manifest in reduced expression of transcripts bearing response elements for GR, as well as increased expression of proinflammatory transcripts that are tonically inhibited by GR. For example, NF-κB is a key proinflammatory transcription factor that is subject to potent counterregulation by GR (17). Finally, we expected that any functional resistance to GR-mediated signaling in low-SES subjects would also manifest in greater diurnal output of cortisol and more systemic inflammatory activity.
Results
Sample Characteristics.
This project enrolled 103 healthy adults ages 25–40 years. They were recruited to be either low or high in early-life SES as defined by parental occupation during the first 5 years of life. Data in Table 1 show that the 2 groups differed markedly in this regard, with mean parental occupational status ratings of 1.5 and 6.0 on a 1–8 scale (t = 22.78, P < 0.001). However, because the project's sampling strategy involved balancing the groups with respect to the number of subjects who were low vs. high in current SES, they had identical average occupational status ratings at the time of assessment (t = 0.02, P = 0.98). Thus, any biological disparities between the groups cannot be attributed to subjects' current SES. As Table 1 shows, the groups were also similar on a variety of other demographic (age, sex, ethnicity) and biobehavioral characteristics (smoking, body mass, physical activity, alcohol use, sleep quality, perceived stress) that might provide alternative explanations for an association between early-life SES and the project's biologic outcomes.
Table 1.
Characteristics of the sample
| Low SES in early life (n = 53) | High SES in early life (n = 50) | P | |
|---|---|---|---|
| Age, y, mean ± SD | 34.0 ± 7.0 | 32.2 ± 5.1 | 0.14 |
| Sex, female, n (%) | 34 (64.2) | 29 (58) | 0.55 |
| Descent, European, n (%) | 32 (60.4) | 38 (76) | 0.09 |
| Early SES: parent occupation (1–7), mean ± SD* | 6.0 ± 1.3 | 1.5 ± 0.6 | 0.001 |
| Current SES: own occupation (1–7), mean ± SD* | 4.1 ± 2.5 | 4.0 ± 2.6 | 0.98 |
| Current family income >$50,000, n (%) | 28 (53.8) | 28 (56) | 0.85 |
| Perceived stress (0–40), mean ± SD | 14.1 ± 6.6 | 14.6 ± 7.3 | 0.71 |
| Daily cigarette smoker, n (%) | 5 (9.4) | 2 (4) | 0.44 |
| Body mass index, kg/m2, mean ± SD | 24.7 ± 4.2 | 23.3 ± 3.8 | 0.11 |
| Physical activity, hr/wk, mean ± SD | 2.7 ± 2.6 | 2.5 ± 2.9 | 0.83 |
| Alcohol use, drinks per week, mean ± SD | 2.9 ± 5.6 | 2.6 ± 4.2 | 0.77 |
| Subjective sleep difficulties (0–3), mean ± SD | 1.0 ± 0.8 | 0.9 ± 0.8 | 0.53 |
*Lower values signify higher occupational status.
Transcriptional Dynamics.
To evaluate the defensive programming hypothesis at the functional genomic level, we measured expression of 18,630 transcripts in PBMCs by using Illumina HumanRef-8 microarrays. [These analyses were conducted on a randomly selected subgroup of 60 subjects who were low (n = 30) or high (n = 30) in early-life SES.] After age, sex, and ethnicity had been statistically controlled, a total of 110 transcripts showed a 1.20-fold or larger difference in expression (corresponding to a false-discovery rate of 5%; ref. 17). Table S1 and Table S2 list these genes. Of the genes that were differentially expressed, 73 were up-regulated (66.4%) and 37 were down-regulated (33.6%) in low-SES subjects.
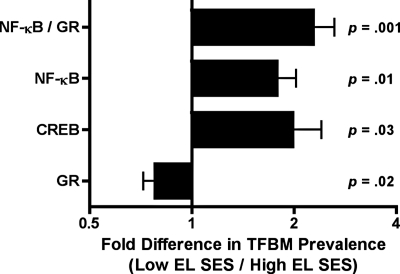
To examine the principal hypotheses, we used the Transcription Element Listening System (TELiS; ref. 17) bioinformatics analysis to quantify the prevalence of transcription factor-binding motifs (TFBMs) in the promoters of differentially expressed genes. These values facilitate inferences about the upstream signaling pathways that give rise to transcriptional disparities between groups (16). As Fig. 1 illustrates, the TELiS results were consistent with all 3 elements of the defensive-programming hypothesis. First, among subjects with low early-life SES, there was a significant up-regulation of genes bearing response elements for CREB/ATF transcription factors that convey adrenergic signals to the leukocyte transcriptome (1.98-fold difference in V$CREB_04 prevalence; SE = 0.43; P = 0.03). Second, there was a relative down-regulation of genes with response elements for the GR (0.77 fold-difference in V$GR_Q6 prevalence; SE = 0.06; P = 0.02), which transduces cortisol's antiinflammatory actions in the immune system. Finally, and consistent with the expectation that diminished GR signaling would facilitate expression of proinflammatory genes, TELiS identified a significant up-regulation of genes bearing NF-κB response elements (1.78-fold difference in V$NFKAPPAB_01 prevalence; SE = 0.25; P = 0.01). Indications of greater NF-κB activity (1.78-fold change) and reduced GR activity (0.77-fold change) resulted in a net 2.31-fold skew (1.78/0.77) toward a proinflammatory phenotype in subjects who were low in early-life SES (P < 0.001).
Fig. 1.
Transcriptional activity of CREB, GR, and NF-κB signaling pathways. TELiS bioinformatics analysis compared the prevalence of response elements in the promoters of differentially expressed genes. Data represent the ratio (±SE) of response element prevalence in promoters of genes up-regulated in subjects with low early-life SES relative to genes up-regulated in subjects with high early-life SES.
To verify the results of the microarray expression analysis, we used quantitative real-time RT-PCR (qRT-PCR) to assess relative quantities of mRNA for 7 of the genes that were identified as up-regulated in subjects low in early-life SES. The results were concordant in all instances (Fig. S1). General linear models confirmed that among subjects with low early-life SES, there was a relative up-regulation of genes coding for the inflammatory mediators IL1A, CCL2, CXCL2, and CCL20 (P < 0.01). Among these subjects, there was also a relative up-regulation of OLR1 and GPR132, which facilitate macrophage uptake of oxidized low-density lipoproteins, and of ADM, which regulates vascular tone (P < 0.01).
To ensure that variation in the distribution of leukocyte subsets within the PBMC pool did not contribute to the observed disparities, we performed complete blood counts on subjects' whole blood. The groups displayed similar percentages of monocytes (6.6% vs. 6.8%; P = 0.55), lymphocytes (31.0% vs. 31.9%; P = 0.51), and granulocytes (62.4% vs. 61.3%; P = 0.43), and when gene expression profiles were statistically adjusted to control for individual differences in leukocyte subset distributions (18), TELiS analyses again indicated significant disparities in GR, CREB, and NF-κB activity as a function of early-life SES (P < 0.05).
We also used qRT-PCR to examine whether reduced GR activity in low-SES subjects was secondary to down-regulation of GR itself. The GR has both an α-isoform, which transduces cortisol signals, and a β-isoform, which is unable to bind ligand but can inactivate α. The groups expressed similar levels of mRNA for both molecules (P > 0.35), suggesting that differences in GR availability per se were not the underlying mechanism. (Low- and high-SES groups also had similar quantities of total GR mRNA on microarray, P = 0.97.)
Immune Activation.
To evaluate whether the transcriptional disparities gave rise to differential immune activation, we assessed serum levels of the inflammatory biomarker C-reactive protein (CRP) and PBMC production of the inflammatory cytokine interleukin 6 (IL6) after ex vivo stimulation with ligands to Toll-like receptors (TLRs; Table S3). Although the groups had similar amounts of CRP (0.90 vs. 0.78 mg/L; P = 0.93), their PBMCs responded differently to TLR stimulation. After statistically controlling for age, sex, and ethnicity, PBMCs from subjects with low early-life SES produced 51% more IL6 in response to TLR3 stimulation with the ligand poly(I:C) than did those with high early-life SES [F(1,98) = 5.07; P = 0.03; Fig. 2A]. PBMCs from these subjects also produced 35% more IL6 in response to TLR5 stimulation with flagellin [F(1,98) = 3.97; P = 0.05; Fig. 2B]. There were no differences in IL6 response to the other TLR ligands (P > 0.39).
Fig. 2.
IL6 production by early-life SES. PBMCs were cultured for 24 h with ligands against TLRs. After supernatants were harvested, the proinflammatory cytokine IL6 was measured by ELISA. Low early-life SES was associated with increased production of IL6 in cultures stimulated with (A) the TLR3 ligand poly(I:C) (P = 0.03) and (B) the TLR5 ligand flagellin (P = 0.05).
Cortisol Output.
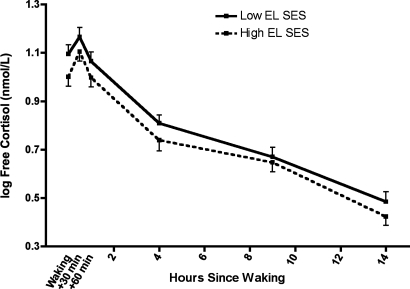
To examine whether diminished GR signaling was also apparent in adrenocortical regulation, salivary cortisol levels were assessed 6 times daily over 3 days according to a schedule that captures the hormone's diurnal rhythm. After statistically controlling for age, sex, and ethnicity, analyses showed that subjects with low early-life SES had greater overall cortisol output across the sampling period than did those with high early-life SES [0.90 ± 0.006 vs. 0.88 ± 0.006; F(1,82) = 4.53, P = 0.04; Fig. 3]. However, the groups had similar cortisol-awakening responses and similar rhythms of diurnal output across the day (P > 0.77).
Fig. 3.
Diurnal cortisol output by early-life SES. Subjects collected saliva 6 times daily for 3 days, and cortisol was measured by immunoassay. Low early-life SES was associated with a relative increase in overall cortisol output across the monitoring period (P = 0.04). There were no SES differences in magnitude of the waking response or the shape of the diurnal rhythm across the day (P > 0.77).
Biobehavioral Contributors.
We considered the possibility that low early-life SES might place subjects on a trajectory to experience more psychological stress or engage in unhealthy lifestyles. As shown in Table 1, the groups reported similar levels of perceived stress over the last month (P = 0.77) and similar levels of smoking, adiposity, exercise, alcohol use, and sleep quality (P ≥ 0.11). In analyses adjusting for these potential mediators, the early-SES differences in overall cortisol output and PBMC IL-6 production in response to TLR3 and TL5 stimulation persisted (P < 0.05), as did the disparities in expression of CREB-responsive, NF-κB-responsive, and GR-responsive genes (P < 0.05), and the net proinflammatory skew in the ratio of the NF-κB/GR response elements in differentially expressed promoters (P = 0.04).
Exploratory Analyses.
In addition to testing the study's primary hypotheses regarding CREB/ATF, GR, and NF-κB activity, we performed exploratory TELiS analyses to evaluate whether early-life SES was associated with altered activity of any other transcription-control pathways. Results linked low early-life SES to up-regulated expression of genes bearing TFBMs for GATA family transcription factors (P < 0.001) and Sp1 (P = 0.003), and a significant down-regulation of genes responsive to heat shock factors 1 and 2 (P < 0.02).
Discussion
Mounting evidence indicates that children who are reared in low-SES households have increased susceptibility to many of the chronic diseases of aging (1–3). One mechanistic hypothesis for this phenomenon suggests that early social adversity programs biological systems in a manner that persists across decades, and thereby accentuates vulnerability to disease later in life (5, 6). The present results are consistent with this hypothesis in demonstrating that low early-life SES is associated with disparities in PBMC gene expression profiles at 25–40 years of age. These disparities appear to be structured by altered activity of neuroendocrine transcriptional control circuits, including the catecholamine-mediated CREB/ATF pathway and the glucocorticoid-mediated GR pathway. Consistent with previous studies linking social stress to GR desensitization and consequent increases in inflammatory gene expression (18–20), this phenotype was manifest in down-regulation of transcripts bearing GR response elements in their promoters, increased output of cortisol in daily life, heightened expression of genes controlled by the proinflammatory transcription factor NF-κB, and greater stimulated production of the cytokine IL6.
Because the project's design involved balancing groups on current SES, it is highly unlikely that their social standing at the time of biological assessment contributed to these disparities. We considered the possibility that low early-life SES might place subjects on a trajectory to experience more psychological stress or engage in unhealthy lifestyles, which in turn might affect neuroendocrine and inflammatory biology. However, covariance analyses offered little support for this alternate explanation. How, then, might early-life SES come to be associated with biological processes that are occurring 20–30 years later?
We speculate that the early years of life are a sensitive period during which the operating dynamics of the GR can be shaped by signals from the environment. If a child is reared in a setting with limited social and/or economic resources, GR expression or functioning may be programmed in a fashion that diminishes its sensitivity to cortisol-mediated signaling. If this process occurred in the hippocampal centers that regulate HPA outflow, it might enable cortisol to partially escape negative feedback inhibition, and lead to the relative increase in diurnal cortisol output seen in our subjects low in early-life SES. A similar dynamic operating in cells of the immune system, particularly monocytes, could diminish GR's capacity to inhibit NF-κB, AP-1, and other proinflammatory transcriptional-control pathways, which in turn could result in the heightened IL6 responses to TLR stimulation seen in subjects low in early-life SES (20). We recognize that several elements of this hypothesis were not directly measurable in the present study; e.g., we did not assess defensive responses after a metabolic stressor or psychosocial challenge and did not directly interrogate GR activity through methods such as dexamethasone suppression. However, their existence would be consistent with animal studies showing that signals from the social world can “cue” long-term alterations in HPA axis function, as well as other circuits in the endocrine, immune, and metabolic systems (7, 9, 10, 13). These changes are thought to endow an animal with a defensive phenotype that is well suited to surviving in difficult social conditions (15, 21). The same kinds of changes may be cued in humans by early social adversity, and enable them to mount vigorous adrenocortical and inflammatory responses to challenges from the social world (e.g., conflict, predation, crowding). Much like fight-or-flight, this pattern of exaggerated responding could serve adaptive functions in the context of acute threats to well-being, but if activated chronically may exact an allostatic toll on the body that ultimately contributes to the chronic diseases of aging (22, 23).
Our study does not provide evidence about the molecular processes that could initiate and sustain this defensive programming. Because levels of mRNA for GR, NF-κB, and CREB were similar across low- and high-SES subjects, it is unlikely that a difference in the bioavailability of these molecules per se is responsible. However, all of these molecules are subject to remodeling mechanisms that can bring about long-term alterations in their functions. For example, the GR can be phosphorylated by MAP kinases, which desensitizes it to cortisol-mediated signaling and, in doing so, facilitates proinflammatory activity by NF-κB (24). And the activity of NF-κB itself can be markedly altered by posttranslational modifications, such as acetylation, ubiquitination, and phosphorylation (25). In future research, it will be important to determine what role these remodeling mechanisms play in the formation of the transcriptional phenotype we have observed.
Our study also does not address whether expression of this phenotype can be modified by social mobility over the life course. For example, it is possible that a dampening of adrenocortical and inflammatory responsivity would occur in low-SES children if they transitioned into higher social classes upon reaching adulthood. Whether this would have positive or negative consequences for health remains unclear. Evidence from the fetal origins literature suggests that organisms may be especially vulnerable to disease when there is a “mismatch” between their developmental and subsequent environments (7). Future research will need to embrace a life-course perspective on these issues and evaluate the consequences of social mobility vs. stability on adrenocortical and inflammatory biology.
The findings here converge with other studies that have reported increased cortisol output and heightened inflammatory activity in humans exposed to social adversity in early life (26–32). They extend this line of work by suggesting a potential underlying genomic mechanism, in which early-life experience blunts GR-mediated and heightens CREB-mediated and NF-κB-mediated transcription. This dynamic has also been observed in human adults exposed to enduring interpersonal difficulties—e.g., chronic feelings of loneliness, inadequate social support, or terminal illness in a family member (18, 20, 33)—as well as experimental animal models of social stress (19). As such, a syndrome of GR desensitization and reciprocally increased proinflammatory signaling might represent a common biological response to threats from the social world, and a mechanism by which they potentially contribute to the development and progression of medical illness.
Several limitations of this study should be considered. First, the assessment of SES in early life was retrospective. Although this precludes inferences about the direction of causality, it is difficult to conceive of reverse directionality explanations for the findings, particularly because early SES was indexed by parent occupational status, and we obtained this information directly from subjects' mothers or fathers. Second, although we used covariance analyses to rule out a variety of potential demographic, behavioral, and biomedical confounds, other factors we did not assess (e.g., food-intake patterns) may have contributed to the observed associations. Thus, the findings need to be considered preliminary until they have been substantiated in future studies that have larger samples, more rigorous prospective designs, and more thorough assessment of potential confounders. Third, the transcriptional profiling assessments were done on PBMCs, leaving it unclear which leukocyte subpopulations contributed to the findings. However, the present results are consistent with data from previous studies focusing specifically on monocytes (20), suggesting that this cell population could play a significant role. Fourth, because we defined early SES as corresponding to the first 5 years of life, it is not possible to establish whether there is a critical period in that window during which SES most potently shapes biology, and whether experiences that occur later in childhood or adolescence can be embedded in a similar fashion. Finally, SES is a broad construct with a pervasive influence. It not only shapes the social conditions that children are reared in but also may influence exposure to chemical, nutritional, or infectious agents that can modify biology for the long term. We are unable to determine the relative importance of these exposures here, but doing so needs to be one of the top priorities for future research in this area. In the meantime, these findings provide some insights into the mechanisms through which SES in the early years of life might shape risk for chronic diseases of aging that emerge many decades later.
Methods
Sample and Design.
The subjects were 103 adults recruited from Vancouver, BC, Canada, through postings in local media and public transit. To be eligible, they had to be 25–40 years of age and in good health, defined as being free of infection the past 4 weeks, and without a history of chronic disease. Subjects also had to be reared in either a low-SES or high-SES household in early life (details below). To prevent early SES and later SES from becoming confounded, we balanced the number of participants in each group who were low vs. high in current SES. This process ensured that although the groups differed in early-life SES, they had equivalent average SES at the time of participation.
SES was defined according to occupational status by using the United Kingdom's National Statistics Socioeconomic Classification (34). This study focused on subjects who fell into the low-SES category, based on their parents having routine, manual, or lower supervisory occupations, and the high-SES category, based on their parents having managerial or professional occupations. For classification of early-life SES, we contacted each subject's mother or father by telephone and inquired about his or her occupation (and his or her spouse's) during their child's first 5 years of life (including the in utero period). For current SES, we interviewed the subject about his or her primary occupation in the past 5 years, as well as that of his or her romantic partner, if one existed. Given that social status is often ascribed to households rather than individuals, we used the highest occupation in the family to categorize SES.
The project was approved by the University of British Columbia's Research Ethics Board, and all subjects gave written consent before participating.
Salivary Cortisol.
Diurnal cortisol output was assessed by having subjects collect saliva as they went about 3 days of normal activities. Each day, saliva was collected at waking and at 0.5, 1, 4, 9, and 14 h later. To collect saliva, the subjects chewed on a cotton dental roll for 1 min (Salivette; Sarstedt). MEMS 6 TrackCap monitors (Aardex) were used to monitor compliance. Cortisol was measured with a commercially available chemiluminescent technique (IBL-Hamburg) at the Technical University of Dresden (Dresden, Germany). This assay has a sensitivity of 0.16 ng/mL, and intraassay and interassay coefficients of variation less than 12%. After cortisol values had been log-transformed, area under the curve calculations were performed on each day's data to form indices of total daily hormone secretion and morning rise relative to waking. An index of diurnal rhythm was also computed by linear regression of cortisol onto time since waking. Values were averaged across days
Markers of Inflammation.
Systemic inflammation was assessed through serum levels of CRP. A high-sensitivity, chemiluminescent assay was performed on an IMMULITE 2000 (Diagnostic Products). This assay has an interassay coefficient of variation of 2.2% and a lower detection threshold of 0.19 mg/L. To model the dynamics of inflammatory signaling pathways under immune challenge, we quantified PBMC production of IL6 after stimulation with TLR ligands (35). Whole blood was drawn into Vacutainer Cell Preparation Tubes (Becton Dickinson), and PBMCs were isolated through centrifugation, washed, and resuspended in R10 medium (Sigma). The cells were then dispensed into flat-bottom tissue-culture plates. Each well contained 5 × 105 PBMCs in 500 μL of R10 and one of the TLR ligands at a dosage listed in Table S3 (purchased from Cedarlane). Cells were incubated for 24 h at 37 °C in a 5% CO2 atmosphere, after which supernatants were harvested and frozen at −80 °C until analysis. The samples were later assayed in duplicate for the inflammatory cytokine IL6 by using commercially available ELISA Development kits from R&D Systems (no. DY206E). These kits have detection thresholds of 5 pg/mL, and intraassay and interassay coefficients of variation <5%.
Transcriptional Profiles.
After PBMCs were isolated from whole blood through density-gradient centrifugation, they were lysed and then homogenized in QiaShredder Spin Columns (Qiagen). Lysates were frozen at −80 °C until RNA was extracted by using AllPrep DNA/RNA kits (Qiagen). RNA purity and integrity were verified by using an Agilent 2100 bioanalyzer (Agilent Technologies). A total of 50 ng of RNA was later assayed on an Illumina Beadstation 500 using HumanRef-8 v3.0 Expression Beadchips (Illumina). The assays were performed at the Génome Québec Innovation Centre (Montréal, Canada). The raw data were log-2 transformed, and differentially expressed genes were identified as those showing ≥20% difference in mean expression levels between low- and high-SES groups (corresponding to a false-discovery rate of <5%; ref. 36). The data were deposited in Gene Expression Omnibus (accession no. GSE15180).
To identify the upstream signal transduction pathways that underlie differential gene expression, we used a 2-sample variant of the TELiS (www.telis.ucla.edu; ref. 16). TELiS analyzes differential gene expression data in terms of the prevalence of transcription factor-binding motifs (TFBMs) within the promoters of differentially expressed genes. This approach can accurately identify the activation of specific hormone or cytokine signaling pathways based on the resulting pattern of gene induction, which occurs selectively in genes bearing TFBMs responsive to transcription factors activated through that pathway. The present analyses assessed the activity of GR by using the TRANSFAC V$GR_Q6 DNA motif, CREB by using the V$CREB_04 motif, and NF-κB by using the V$NFKAPPAB_01 motif. P values were calculated by using independent-sample t tests with Welch's correction for heteroscedasticity (37) Analyses used aggregate indices that had been pooled across 9 different technical specifications involving variations of promoter length and TFBM match stringency.
Confirmation of Microarray Findings.
A subset of transcripts that were identified as up-regulated on microarray was independently assayed by qRT-PCR using TaqMan Gene Expression Assays (Applied Biosystems). Seven genes were analyzed: IL1A, CCL2, CXCL2, CCL20, OLR1, GPR132, and ADM. Assays for each sample were carried out in triplicate by using an Applied Biosystems Prism 7000 Sequence Detection System according to the manufacturer's recommended 1-step thermal cycling protocol. Threshold cycle numbers for each analyte were normalized to β-actin before analysis.
Potential Mediators.
We also collected information on lifestyle practices and perceived stress as potential mediators. Lifestyle practices included smoking status, alcohol use, body mass, hours per week of brisk physical activity (38), and subjective sleep quality (39). Stress was measured with the extensively validated Perceived Stress Scale (40).
Supplementary Material
Acknowledgments.
This study was supported by National Institute of Child Health and Human Development Grant HD0058502; the Heart and Stroke Foundation of Canada; the British Columbia Ministry of Child and Family Development via the Human Early Learning Partnership; and the Allergy, Genes and Environment Research Network. This study was also supported by Career Scholar Awards from the Michael Smith Foundation for Health Research (to G.E.M., E.C., and M.S.K.). M.S.K. is a Scholar of the Canadian Institute for Advanced Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE15180).
This article contains supporting information online at www.pnas.org/cgi/content/full/0902971106/DCSupplemental.
References
- 1.Galobardes B, Lynch JW, Davey Smith G. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: Systematic review and interpretation. Epidemiol Rev. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosom Med. 2004;66:553–558. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- 3.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 4.Kittleson MM, et al. Association of childhood socioeconomic status with subsequent coronary heart disease in physicians. Arch Intern Med. 2006;166:2356–2361. doi: 10.1001/archinte.166.21.2356. [DOI] [PubMed] [Google Scholar]
- 5.Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann NY Acad Sci. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 6.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dial Clin Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 9.Newport DJ, Stowe ZN, Nemeroff CB. Parental depression: Animal models of an adverse life event. Am J Psychiatry. 2002;159:1265–1283. doi: 10.1176/appi.ajp.159.8.1265. [DOI] [PubMed] [Google Scholar]
- 10.Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 12.Szyf M, et al. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol. 2005;26:139–162. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Coe CL, Lubach GR. In: Psychoneuroimmunology. 4th Ed. Ader R, editor. Boston: Elsevier; 2007. pp. 455–474. [Google Scholar]
- 14.Avitsur R, Hunzeker J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain Behav Immun. 2006;20:339–348. doi: 10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Zhang TY, et al. Maternal programming of defensive responses through sustained effects on gene expression. Biol Psychol. 2006;73:72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Cole SW, Yan W, Galic Z, Arevalo J, Zack JA. Expression-based monitoring of transcription factor activity: The TELiS database. Bioinformatics. 2005;21:803–810. doi: 10.1093/bioinformatics/bti038. [DOI] [PubMed] [Google Scholar]
- 17.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 18.Cole SW, et al. Social regulation of gene expression: Inflammation and the human transcriptional response to loneliness. Genome Biol. 2007;8:r89. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stark JL, et al. Social stress induces glucocorticoid resistance in macrophages. Am J Physiol. 2001;280:1799–1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- 20.Miller GE, et al. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-κB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluckman P, Hanson M. In: Developmental Origins of Health and Disease. Gluckman P, Hanson M, editors. New York: Cambridge Univ Press; 2006. pp. 33–50. [Google Scholar]
- 22.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S, Janicki-Deverts DL, Miller GE. Psychological stress and disease. J Am Med Assoc. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 24.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene. 2006;25:6713–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 26.Heim C, et al. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 27.Heim C, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. J Am Med Assoc. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Power C, Kelly S, Kirschbaum C, Hertzman C. Life-time socio-economic position and cortisol patterns in mid-life. Psychoneuroendocrinology. 2007;32:824–833. doi: 10.1016/j.psyneuen.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Pace TW, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 30.Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller G, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosom Med. 2007;69:402–409. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- 33.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid resistance model. Health Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 34.Office of National Statistics. The National Statistics Socio-Economic Classification. Hampshire, UK: Palgrave Macmillan; 2005. [Google Scholar]
- 35.Hirschfeld AF, et al. Prevalence of Toll-like receptor signalling defects in apparently healthy children who developed invasive pneumococcal infection. Clin Immunol. 2007;122:271–278. doi: 10.1016/j.clim.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Cole SW, Galic Z, Zack JA. Controlling false-negative errors in microarray differential expression analysis: A PRIM approach. Bioinformatics. 2003;19:1808–1816. doi: 10.1093/bioinformatics/btg242. [DOI] [PubMed] [Google Scholar]
- 37.Miller RG. Beyond ANOVA. New York: Wiley; 1986. [Google Scholar]
- 38.Paffenbarger RS, Blair SN, Lee I, Hyde RT. Measurement of physical activity to assess health effects in a free-living population. Med Sci Sport Exer. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Buysse DJ, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 40.Cohen S, Kamarck TW, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.