Abstract
Cancer cells often display defects in mitochondrial respiration, thus the identification of pathways that promote cell survival under this metabolic state may have therapeutic implications. Here, we report that the targeted ablation of mitochondrial respiration markedly increases expression of Polo-like kinase 2 (PLK2) and that it is required for the in vitro growth of these nonrespiring cells. Furthermore, we identify PLK2 as a kinase that phosphorylates Ser-137 of PLK1, which is sufficient to mediate this survival signal. In vivo, knockdown of PLK2 in an isogenic human cell line with a modest defect in mitochondrial respiration eliminates xenograft formation, indicating that PLK2 activity is necessary for growth of cells with compromised respiration. Our findings delineate a mitochondrial dysfunction responsive cell cycle pathway critical for determining cancer cell outcome.
Keywords: cell cycle, respiration, sco2
Mitochondrial dysfunction with increased dependence on glycolysis is frequently observed in cancer cells, and significant advances have been made to clarify the genetic basis for this metabolic phenomenon (1, 2). This increase in glycolysis may represent a compensatory mechanism responding to the bioenergetic stress of decreased oxidative phosphorylation as initially described by Warburg (3). Analogous to the importance of cell cycle checkpoints in determining cell fate after DNA damage, the regulation of the cell cycle by signals initiated by mitochondrial dysfunction may similarly govern outcome (4, 5). Thus, insights into the mediators that enable cell growth after disruption of respiration may help advance our understanding of cancer biology with potential therapeutic applications.
To investigate the adaptive changes associated with mitochondrial dysfunction, we disrupted SCO2, a metallochaperone gene essential for cytochrome c oxidase (complex IV) assembly and respiration (6), in a human colon cancer cell line (SCO2-/- HCT116 cell line). Unlike mitochondrial dysfunction induced by the depletion of the entire mitochondrial genome (mtDNA), somatic cell homologous recombination allowed us to selectively ablate respiration through disruption of a single nuclear gene, providing us with paired isogenic cell lines for comparison. In this respiration-deficient context, Polo-like kinase 2 (PLK2) was the most highly expressed gene in SCO2-/- cells compared to respiring wild-type SCO2+/+ cells. PLK2 belongs to a family of cell cycle Ser/Thr kinases represented by PLK1, the prototypical member overexpressed in many human cancers (7–9). Here, we identify PLK2 as a kinase for PLK1 and report that the phosphorylation of PLK1 Ser-137 is sufficient to mediate the signal necessary for survival of cells with compromised respiration.
Results
Mitochondrial Dysfunction Induces PLK2 Expression.
Using the somatic cell homologous recombination technique (10–13), we disrupted the remaining wild-type allele of the SCO2 gene, known to be essential for mitochondrial respiration (6), using the human colon cancer HCT116 SCO2+/- cell line that we had previously reported (14). We confirmed disruption of both copies of SCO2 allele by genomic PCR and by the absence of significant respiration (Fig. S1). For the described in vitro experiments, one representative SCO2-/- cell line was used. However, all significant findings were reproduced or confirmed using at least one additional SCO2-/- cell line that was obtained by an independent homologous recombination event to rule out clonal variability.
In an attempt to identify genes associated with the cell cycle that may enable the survival of SCO2-/- cells after disruption of respiration, we compared microarray gene expression of respiring SCO2+/+ and nonrespiring SCO2-/- HCT116 human colon cancer cells (Table S1). PLK2 was the most highly expressed gene in the SCO2-/- cells (Table S1), and two features made it stand out as a potential stress-responsive gene for cell survival signaling. PLK2 is essential for cell viability in the setting of DNA damage but not under normal conditions (15), and interestingly, it had also been reported to be regulated by calcium in a model of synaptic plasticity (16). Because calcium is a well-established mitochondrial retrograde signal (17, 18), PLK2 appeared to be a strong candidate gene that could mediate cell survival signaling in mitochondrial dysfunction.
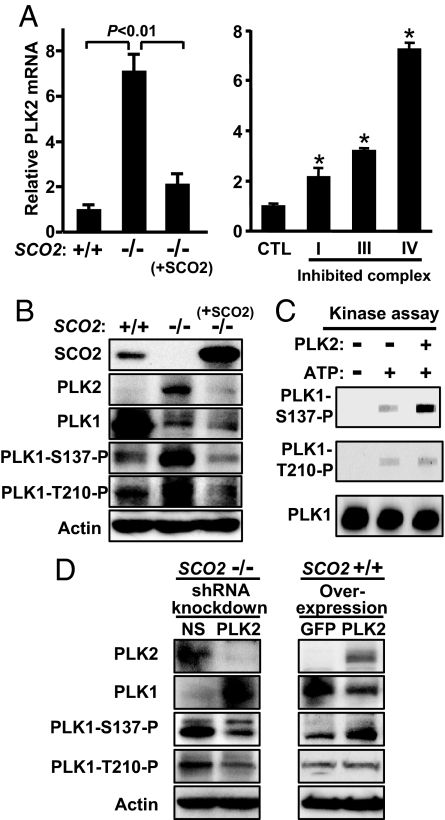
The increased expression of PLK2 in the SCO2-/- cell was confirmed by both mRNA and protein levels (Fig. 1 A and B). We stably re-expressed SCO2 cDNA in SCO2-/- cells to rescue respiration (Fig. S1B) and observed a concomitant decrease in PLK2 expression (Fig. 1 A and B). To test whether PLK2 is generally inducible by disruption of respiration, we added blockers of mitochondrial complex I, III, or IV to SCO2+/+ cells and observed increased PLK2 mRNA in response to each agent compared to control (Fig. 1A). We next considered how disruption of respiration might trigger PLK2 expression. Because of the previous association between PLK2 expression and calcium in neurons (16), we measured cytosolic-free calcium in SCO2-/- cells using the Fluo-4 a.m. calcium indicator. Compared to wild-type cells, nonrespiring SCO2-/- cells had significantly elevated calcium levels (Fig. S2A). When the intracellular calcium was lowered using a membrane-permeable calcium chelator BAPTA/AM, PLK2 mRNA expression decreased in a dose-dependent manner (Fig. S2B). Thus, our data indicate that elevated intracellular calcium associated with the disruption of mitochondrial function in SCO2-/- cells is a major trigger for PLK2 expression.
Fig. 1.
Disruption of mitochondrial respiration increases expression of PLK2 that phosphorylates Ser-137 of PLK1. (A) PLK2 mRNA expression analyzed by RT-PCR in cells with genetic and pharmacologic disruption of respiration. SCO2+/+ cell respiration was inhibited by complex I, III, or IV blockers: Rotenone (100 nM); antimycin A (100 ng/mL); or sodium azide (500 μM), respectively. (*) denotes P < 0.01 versus control (CTL). Mean ± SD, n = 4. (B) Confirmation of SCO2 and PLK2 protein expression in SCO2+/+, SCO2-/-, and after rescue of respiration by re-expression of SCO2 (+SCO2) in SCO2-/- cells. (C) In vitro phosphorylation of PLK1 by PLK2 (slot blot). (D) Effect on PLK1 phosphorylation by PLK2-specific or control nonspecific (NS) shRNA lentiviral knockdown of PLK2 in SCO2-/- cells. Conversely, the effect of PLK2 or control GFP lentivirus overexpression in SCO2+/+ cells.
PLK2 Preferentially Phosphorylates PLK1 Ser-137.
To identify downstream mediators of PLK2 signaling, we performed a kinase substrate screen using purified PLK2. Unexpectedly, the optimal peptide substrate belonged to another PLK family member, specifically, Ser-137 in the catalytic domain of PLK1 (PLK1-S137) (Table S2). Our in vitro phosphorylation result was supported by higher PLK1-S137-P levels despite lower total PLK1 protein in SCO2-/- cells compared to SCO2+/+ cells (Fig. 1B). In contrast, another PLK1 phosphorylation site Thr-210, also in the catalytic domain with similar surrounding amino acid residues, was only modestly phosphorylated in SCO2-/- cells (Fig. 1B). Additionally, when respiration was rescued by stably re-expressing SCO2 cDNA in SCO2-/- cells, there was a parallel decrease in the levels of PLK2 and PLK1-S137-P, further strengthening their association in vivo (Fig. 1B).
We next performed an in vitro kinase assay using full-length recombinant PLK1 and PLK2 proteins to validate the substrate screen. Slot blotting revealed a strong increase in PLK1-S137-P immunoreactivity in the presence of PLK2 over background (Fig. 1C). In contrast, PLK1-T210-P was not significantly changed, demonstrating that PLK2 preferentially phosphorylates the Ser-137 site of PLK1. Thus, PLK2, in the absence of other associated factors, is able to reproduce the PLK1-S137 phosphorylation observed in vivo.
To further confirm that PLK2 phosphorylates PLK1-S137 in cells, we examined the effect of depleting PLK2 in SCO2-/- cells by using shRNA lentivirus or the effect of overexpressing PLK2 in SCO2+/+ cells by using PLK2 lentivirus. We observed good concordance between PLK2 and PLK1-S137-P levels but not to PLK1-T210-P (Fig. 1D). PLK1-S137-P has been reported to have a dominant effect over PLK1-T210-P and to increase the G1 population of cells (19, 20). Indeed, under normal growth conditions, the G1 population was increased in SCO2-/- compared to SCO2+/+ cells in accordance with their slower doubling time, ≈48 h versus ≈24 h, respectively (Fig. S3). The SCO2+/- cells with partial disruption of respiration showed an intermediate cell cycle profile (Fig. S3).
PLK2 Expression Is Essential for SCO2-Deficient Cell Colony Formation.
We next sought to evaluate the functional role of increased PLK2 expression in the respiration-deficient cells. To do this, we stably introduced shRNA targeted against PLK2 and examined colony formation of SCO2+/+, SCO2+/-, and SCO2-/- cells. As expected, knocking down the low basal expression of PLK2 in SCO2+/+ cells did not significantly affect colony formation (Fig. 2 A and B). In contrast, there was a significant correlation between SCO2 gene dose and the reduction in colony formation in SCO2-deficient (SCO2+/- and SCO2-/-) cells, indicating that PLK2 plays an important role in mediating the growth of cells with compromised mitochondrial respiration (Fig. 2 A and B). This observation is consistent with previous reports that PLK2 is not necessary under normal conditions but important for preventing cell death after replication stress (15, 21–23).
Fig. 2.
PLK2 promotes the survival of cells with mitochondrial dysfunction. (A) After being transduced with control nonspecific (NS) or PLK2-specific shRNA lentivirus to knockdown PLK2, equal numbers of cells were cultured and then visualized by crystal violet staining. (B) Quantification of colony formation following PLK2 knockdown in SCO2+/+, SCO2+/-, and SCO2-/- cells. Mean ± SD, n = 3. (C) Cells transduced with the indicated shRNA lentivirus were subcultured in fresh medium. After the indicated culture times, equivalent amounts of culture media and cell lysates were measured for HMGB1 release (representing necrosis) and caspase 3 cleavage products (representing apoptosis), respectively, by western blotting. SCO2+/+ cells were treated for 24 h with 5-fluorouracil as positive control (CTL) for apoptosis.
To clarify the mechanism by which cells with mitochondrial dysfunction may have decreased viability, we synchronized and serum stimulated PLK2 depleted cells. SCO2-deficient cells showed decreased entry into G2/M phase that correlated with their decreased cell growth by colony formation assay in contrast to SCO2+/+ cells, which were not significantly affected (Fig. S4). PLK2 depletion also caused a relatively greater decrease in the G1 population of SCO2-deficient cells corresponding to their higher basal level of PLK2 expression (Fig. S4). Associated with this release of G1 arrested population in the setting of mitochondrial dysfunction was the release of cellular necrosis marker HMGB1 (24) into the medium by the SCO2-/- cells (Fig. 2C). Notably, significant necrosis was also observed in SCO2+/- cells with a more modest degree of mitochondrial dysfunction. However, no apoptosis was detected under these conditions in SCO2-deficient cells as measured by caspase 3 cleavage (Fig. 2C). The reduction in both G1 arrest and G2/M progression and the increase in necrosis after PLK2 knockdown in SCO2-deficient cells were consistent with their decreased viability by colony formation assay.
Phosphorylation of PLK1-S137 Mediates Cell Survival in Mitochondrial Dysfunction.
Both Ser-137 and Thr-210 of PLK1 are located in the kinase domain, but Ser-137 is uniquely located in the short spacer region of a bipartite nuclear localization signal that has been shown to be important for cell cycle progression (25). We hypothesized that the growth of SCO2-deficient cells associated with increased PLK2 may be mediated through the phosphorylation of PLK1-S137. To test this notion, we used a strategy previously used to replace the Ser-137 or Thr-210 of PLK1 with Asp to mimic constitutive phosphorylation that has been shown to increase kinase activity and to specifically regulate the cell cycle (19, 20). We first depleted PLK2 in SCO2-/- cells using shRNA lentivirus (as shown in Fig. 1D) and then cDNA transfected them with negative control (GFP or nonphosphorylatable mutant PLK1-S137A) or phosphorylation-mimic PLK1 (PLK1-S137D or PLK1-T210D) (Fig. 3A). Like the GFP control, PLK1-S137A failed to rescue growth of PLK2-depleted SCO2-/- cells, while PLK1-S137D markedly increased colony formation (Fig. 3 B and C). There was a partial but significant increase in colony formation by introducing PLK1-T210D, suggesting some functional overlap between these two phosphorylation sites (Fig. 3 B and C).
Fig. 3.
Mutant PLK1-S137D that mimicks phosphorylation rescues colony formation in PLK2-depleted SCO2-/- cells. SCO2-/- cells were first transduced with PLK2-specific shRNA lentivirus to knockdown PLK2 (Fig. 1D) and then plasmid-transfected with control (CTL) GFP or the indicated phosphorylation-site mutant PLK1 cDNA. After 24 h, the following experiments were performed. (A) Measurement of PLK1 protein levels relative to GFP control by western blotting. (B) Visualization of colonies by crystal violet staining after culturing at different plating densities. (C) Quantification of colony formation revealed highest viability with PLK1-S137D in SCO2-/- cells. (*) denotes P < 0.01 versus control. Mean ± SD, n = 4.
PLK2 Expression Is Essential for Xenograft Formation of Cells with Compromised Respiration.
We next attempted to use the SCO2-/- cell line for in vivo studies, but were unable to establish xenografts in athymic nude mice. Although the bases for this observation are unclear, we reasoned that the heterozygous (SCO2+/-) cell line with a ≈20% reduction in oxygen consumption compared to SCO2+/+ cells may be more representative of cancer cells which retain some degree of oxidative phosphorylation capacity (14). In fact, the isogenic nature of SCO2+/+ and SCO2+/- cells was ideal for examining the effect of PLK2 on the survival of cells with only a modest decrease in respiration caused by a defined defect in cytochrome c oxidase assembly. Additionally, these paired cell lines grow at virtually identical rates in vitro facilitating their comparison by xenograft formation assay.
Given the modest decrease in respiration of SCO2+/- cells, we were impressed to see a 3-fold increase in PLK2 mRNA level relative to wild-type cells that indicated high sensitivity of PLK2 to alterations in mitochondrial function (Fig. 4A). There were also parallel increases in PLK2 protein and PLK1-S137 phosphorylation by western blotting, consistent with the SCO2-/- cell observations (Fig. 4A). In vitro, we confirmed that, like SCO2-/- cells, the decrease in colony formation after PLK2 knockdown in SCO2+/- cells was rescued by the introduction of PLK1-S137D (Fig. S5 A and B). To test whether increased PLK2 expression in SCO2+/- cells is also necessary for cell survival by xenograft assay, we first transduced the respective cell lines in vitro with either control nonspecific shRNA or PLK2-specific lentivirus and then injected equal numbers of cells into contra-lateral hind limbs of athymic nude mice. shRNA knockdown of PLK2 did not significantly decrease SCO2+/+ xenograft formation, but completely blocked the growth of SCO2+/- cells (Fig. 4 B and C). These in vivo results underscored the importance of the PLK2-PLK1 signaling pathway in the survival of cells with mitochondrial dysfunction.
Fig. 4.
PLK2 expression is essential for xenograft formation of cancer cells with compromised mitochondrial respiration. (A) PLK2 expression is increased in respiration-defective SCO2+/- cells. Left panel, PLK2 mRNA expression analyzed by RT-PCR in SCO2+/+ and SCO2+/- cells. Mean ± SD, n = 3. Right panel, PLK2 protein and PLK1-S137 phosphorylation are increased in SCO2+/- cells. (B) Effect of PLK2 knockdown on xenograft formation of SCO2+/+ cells transduced with either nonspecific shRNA (NS shRNA) or PLK2-shRNA lentivirus in athymic nude mice. Left panel, xenograft pictures of two representative mice at 42 days after s.c. cell injection are shown. Right panel, tumor growth (mm3) over time (d). P = 0.2 at 42 days, mean ± SD, n = 4. (C) Effect of PLK2 knockdown on xenograft formation of SCO2+/- cells. Left panel, xenograft pictures of two representative mice at 42 d after s.c. cell injection are shown. Right panel, tumor growth (mm3) over time (d). P < 0.001 at 42 days, mean ± SD, n = 6.
Discussion
In summary, we have established that PLK2 expression is responsive to genetic or pharmacologic disruption of respiration and that PLK2 can phosphorylate Ser-137 of PLK1, an event that promotes the survival of cells with compromised respiration. Our study also shows that signals generated by disparate stresses of genomic or mitochondrial origin can share critical regulators of the cell cycle. Although the PLK1 mutant mimicking Ser-137 phosphorylation appears to have a dominant cell cycle effect over the better characterized Thr-210 phosphorylation site (19, 20, 26, 27), a kinase for the Ser-137 site had remained elusive. Our current study shows that phosphorylation of PLK1-S137 by PLK2 may be an important adaptive mechanism for cell survival in mitochondrial dysfunction through regulation of G1-S phase checkpoint. This signaling pathway could serve as a potential target for therapeutic intervention as cancer cells often display mitochondrial defects.
Unlike PLK1, which promotes cell proliferation and is often overexpressed in human cancers (28), the function of PLK2 in tumorigenesis is less clear. PLK2 transcription is regulated by p53, but interestingly, the p53 binding sites in the PLK2 gene display both stimulatory and inhibitory elements, suggesting more complex interactions between PLK2 and this well-established tumor suppressor (22, 29, 30). PLK2 has also been shown to be epigenetically silenced in high grade B-cell lymphomas, implicating it as a tumor suppressor; however, this phenomenon appears to be limited to malignant B cells (31). In contrast to some established tumor suppressor genes, somatic mutations in PLK2 have not been identified in primary human cancers, which in some cases express higher levels of PLK2 compared to normal tissues (23, 31, 32). These varying observations suggest that the role of PLK2 in tumorigenesis may depend on cell type and context. Focused studies examining the interaction between PLK2 and PLK1 in additional isogenic human cancer cell lines with the specific disruption of important cell cycle regulatory genes, for example, may help further advance our understanding of these pathways as recently reported for PLK1 in p53-deficient cells (33).
The homeostasis of intracellular calcium and reactive oxygen species, which can serve as retrograde signals, is highly regulated by the mitochondria and is likely to be important for the pathogenesis of various human diseases (17, 18). Given our observation of PLK2 regulation by calcium in human colon cancer cells and the previous work in models of synaptic plasticity (16), it is notable that the promoter region of PLK2 contains consensus sequences for both calcium-dependent cAMP and antioxidant response elements (CRE and ARE, respectively). The growing evidence for the role of mitochondrial dysfunction in neurodegenerative diseases and the recent report of PLK2-mediated α-synuclein phosphorylation, a hallmark of Parkinson's disease and dementia with Lewy bodies, expand the potential role of PLK2 beyond cell cycle regulation and tumorigenesis to diseases of the central nervous system (34).
Materials and Methods
Cell Culture.
The human colon cancer HCT116 cell lines (SCO2+/+ from ATCC) were cultured in McCoy's 5A medium with 10% FBS. The remaining wild-type allele of the SCO2 gene in the previously reported heterozygous HCT116 SCO2+/- cell line (14) was targeted by AAV-mediated homologous recombination to generate the SCO2-/- cell line, confirmed by absence of wild-type SCO2 alleles by genomic PCR and by disruption of respiration (SI Text and Fig. S1). Cells were treated with the indicated concentrations of respiratory blockers for 24 h.
Lentivirus for Gene Knockdown and Overexpression.
We obtained plasmids containing the sequences for nonspecific or PLK2 shRNA (Sigma-Aldrich) for knockdown experiments and pLEX-MCS PLK2 cDNA plasmid (OpenBiosystems) for overexpression experiments. Using these plasmids, lentivirus for the PLK2 shRNA knockdown and lentivirus for PLK2 overexpression were prepared according to manufacturer's protocol (Sigma-Aldrich). Using the pLEX-MCS PLK1 plasmid (OpenBiosystems), point mutations to change PLK1 Ser-137 to nonphosphorylatable Ala or constitutive kinase active Asp and PLK1 Thr-210 to Asp were performed using the QuikChange II kit (Stratagene) according to the manufacturer's protocol. The primer sequences used for the site-directed mutagenesis, as well as the shRNA, are provided in the SI Text section. Cells were incubated with virus (MOI ≈1) for 24 h followed by 2 μg/mL puromycin selection.
Colony Formation Assay.
shRNA lentivirus-transduced cells were selected for 3 d with puromycin, plated at a density of 2,000 cells/well (6-well), and cultured for equivalent periods of time (≈7 d for SCO2+/+ and SCO2+/-, and ≈14 d for SCO2-/- cells). For rescue experiments after PLK2 knockdown by shRNA lentivirus, GFP or mutant PLK1 cDNA was transfected using Effectene (Qiagen) for 24 h, replated at equal density, and stained with crystal violet solution at 7–14 days.
Microarray Analysis.
Total RNA was in vitro transcribed with biotin label using Affymetrix IVT kit. Biotinylated cRNA (20 μg) was fragmented and hybridized to Affymetrix U133A Plus 2.0 chips. Affymetrix GCOS version 1.4 was used to calculate signal intensity and determine percent present calls. To address the issue of multiple comparisons, fold-cutoff filters and false discovery rate (FDR) analysis filters were applied as indicated.
Real-Time (RT)-PCR.
RT-PCR was performed and normalized by measuring average cycle threshold (Ct) ratios between PLK2 and the housekeeping gene TIF using an ABI HT7900 real-time PCR thermal cycler (Applied Biosystems) (35). Primer sequences are provided in SI Text.
In Vitro Kinase Substrate Screening.
PLK2 substrate screening was performed using the Kinase Substrate Screening kit (10 μM substrates; Cell Signaling) and baculovirus-expressed PLK2 (10 nM; Cell Signaling) according to manufacturer's protocol. Femto Maximum Sensitivity Substrate (Pierce) ELISA chemiluminescence signals were detected using a Victor-3 96-well plate reader (Perkin-Elmer).
In Vitro Kinase Assay.
Recombinant human PLK1 and PLK2 (1 ng/μL each) from Sf9 cells (Cell Signaling) were incubated in kinase buffer (60 mM HEPES, pH 7.5, 3 mM MgCl2, 3 mM MnCl2, 3 μM Na-orthovanadate, 1.2 mM DTT, 2.5 μg/50 μL PEG20,000) with 10 μM ATP for 30 min at 30 °C. PLK1 (100 ng protein/well, Bio-Dot SF Slot Blot; Bio-Rad Laboratories) was applied and detected using standard immunoblotting techniques.
Necrosis and Apoptosis Assays.
The cells were transduced with nonspecific or PLK2 shRNA lentivirus and selected in the presence of 2 μg/mL puromycin for 3 days. These cells were then subcultured in fresh growth medium containing puromycin for 0, 2, and 4 days, respectively. The media from the cultured cells at the indicated times were first concentrated using Centricon YM-10 (Millipore) then size fractionated using Ultrafree-MC 100-kDa (Millipore) filters. Equivalent volumes of concentrated media were subjected to SDS-PAGE and western blotting for HMGB1. Cell lysates were subjected to western blotting of both pro- and cleaved-caspase 3. As a positive control for apoptosis (caspase 3 cleavage), SCO2+/+ cells were treated with 50 μg/mL 5-fluorouracil for 24 h.
Xenograft Assay.
The respective cell lines were first transduced with lentivirus and selected with puromycin for 3 days. Tumors were established in athymic nude mice by the s.c. injection of 10 × 106 cells resuspended in Hank's Balanced Salt Solution (HBSS) into both hind limbs using a 27-gauge needle and 1-cc syringe. Tumor growth rate was determined by measuring tumor dimensions twice a week with a digital caliper and estimating tumor volume using the following equation, volume = (width2 × length)/2, as previously described (36).
Antibodies and Western Blotting.
Antibody sources were as follows: PLK1 208G4 and PLK1-S137-P (Cell Signaling), PLK1-T210-P (Abcam), PLK2 SNK N-17 (Santa Cruz Biotechnology), actin C-2 (Santa Cruz Biotechnology), α-tubulin (Invitrogen), SCO2 (14), rabbit polyclonal HMGB1antibody (Abcam), monoclonal antibody recognizing both pro- and cleaved-caspase 3 (IMGENEX). Standard ECL Western blotting was performed (GE Healthcare).
Supplementary Material
Acknowledgments.
We thank N. Raghavachari and X. Xu of the National Heart, Lung, and Blood Institute (NHLBI) microarray core for technical assistance, L. Samsel for cell sorting, P. Dagur for calcium measurements, J.G. Kang for technical support, and T. Finkel for helpful suggestions and critical reading of this manuscript. This work was supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), and a postdoctoral fellowship to T. Matsumoto from the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904229106/DCSupplemental.
References
- 1.Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 2.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 4.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 5.Schieke SM, Finkel T. Mitochondrial signaling, TOR, and life span. Biol Chem. 2006;387:1357–1361. doi: 10.1515/BC.2006.170. [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulou LC, et al. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat Genet. 1999;23:333–337. doi: 10.1038/15513. [DOI] [PubMed] [Google Scholar]
- 7.Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24:267–276. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- 8.van de Weerdt BC, Medema RH. Polo-like kinases: A team in control of the division. Cell Cycle. 2006;5:853–864. doi: 10.4161/cc.5.8.2692. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Lei M, Erikson RL. Normal cells, but not cancer cells, survive severe Plk1 depletion. Mol Cell Biol. 2006;26:2093–2108. doi: 10.1128/MCB.26.6.2093-2108.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirata R, Chamberlain J, Dong R, Russell DW. Targeted transgene insertion into human chromosomes by adeno-associated virus vectors. Nat Biotechnol. 2002;20:735–738. doi: 10.1038/nbt0702-735. [DOI] [PubMed] [Google Scholar]
- 11.Kohli M, Rago C, Lengauer C, Kinzler KW, Vogelstein B. Facile methods for generating human somatic cell gene knockouts using recombinant adeno-associated viruses. Nucleic Acids Res. 2004;32:e3. doi: 10.1093/nar/gnh009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topaloglu O, Hurley PJ, Yildirim O, Civin CI, Bunz F. Improved methods for the generation of human gene knockout and knockin cell lines. Nucleic Acids Res. 2005;33:e158. doi: 10.1093/nar/gni160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rago C, Vogelstein B, Bunz F. Genetic knockouts and knockins in human somatic cells. Nat Protoc. 2007;2:2734–2746. doi: 10.1038/nprot.2007.408. [DOI] [PubMed] [Google Scholar]
- 14.Matoba S, et al. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 15.Matthew EM, et al. Replication stress, defective S-phase checkpoint and increased death in Plk2-deficient human cancer cells. Cell Cycle. 2007;6:2571–2578. doi: 10.4161/cc.6.20.5079. [DOI] [PubMed] [Google Scholar]
- 16.Pak DT, Sheng M. Targeted protein degradation and synapse remodeling by an inducible protein kinase. Science. 2003;302:1368–1373. doi: 10.1126/science.1082475. [DOI] [PubMed] [Google Scholar]
- 17.Butow RA, Avadhani NG. Mitochondrial signaling: The retrograde response. Mol Cell. 2004;14:1–15. doi: 10.1016/s1097-2765(04)00179-0. [DOI] [PubMed] [Google Scholar]
- 18.Walsh C, Barrow S, Voronina S, Chvanov M, Petersen OH, Tepikin A. Modulation of calcium signalling by mitochondria. Biochim Biophys Acta. 2009 Jan 21; doi: 10.1016/j.bbabio.2009.01.007. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Jang YJ, Ma S, Terada Y, Erikson RL. Phosphorylation of threonine 210 and the role of serine 137 in the regulation of mammalian polo-like kinase. J Biol Chem. 2002;277:44115–44120. doi: 10.1074/jbc.M202172200. [DOI] [PubMed] [Google Scholar]
- 20.van de Weerdt BC, et al. Uncoupling anaphase-promoting complex/cyclosome activity from spindle assembly checkpoint control by deregulating polo-like kinase 1. Mol Cell Biol. 2005;25:2031–2044. doi: 10.1128/MCB.25.5.2031-2044.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma S, Charron J, Erikson RL. Role of Plk2 (Snk) in mouse development and cell proliferation. Mol Cell Biol. 2003;23:6936–6943. doi: 10.1128/MCB.23.19.6936-6943.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burns TF, Fei P, Scata KA, Dicker DT, El-Deiry WS. Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (taxol)-exposed cells. Mol Cell Biol. 2003;23:5556–5571. doi: 10.1128/MCB.23.16.5556-5571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith P, Syed N, Crook T. Epigenetic inactivation implies a tumor suppressor function in hematologic malignancies for Polo-like kinase 2 but not Polo-like kinase 3. Cell Cycle. 2006;5:1262–1264. doi: 10.4161/cc.5.12.2813. [DOI] [PubMed] [Google Scholar]
- 24.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 25.Toyoshima-Morimoto F, Taniguchi E, Nishida E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 2002;3:341–348. doi: 10.1093/embo-reports/kvf069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–1658. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macurek L, et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455:119–123. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 28.Park JE, et al. Direct quantification of polo-like kinase 1 activity in cells and tissues using a highly sensitive and specific ELISA assay. Proc Natl Acad Sci USA. 2009;106:1725–1730. doi: 10.1073/pnas.0812135106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu-Yoshida Y, et al. Radiation-inducible hSNK gene is transcriptionally regulated by p53 binding homology element in human thyroid cells. Biochem Biophys Res Commun. 2001;289:491–498. doi: 10.1006/bbrc.2001.5993. [DOI] [PubMed] [Google Scholar]
- 30.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 31.Syed N, et al. Transcriptional silencing of Polo-like kinase 2 (SNK/PLK2) is a frequent event in B-cell malignancies. Blood. 2006;107:250–256. doi: 10.1182/blood-2005-03-1194. [DOI] [PubMed] [Google Scholar]
- 32.Tan LB, Chen KT, Yuan YC, Liao PC, Guo HR. Identification of urine PLK2 as a marker of bladder tumors by proteomic analysis. World J Urol. 2009 Jan 9; doi: 10.1007/s00345-009-0432-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Sur S, et al. A panel of isogenic human cancer cells suggests a therapeutic approach for cancers with inactivated p53. Proc Natl Acad Sci USA. 2009;106:3964–3969. doi: 10.1073/pnas.0813333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inglis KJ, et al. Polo-like kinase 2 (PLK2) phosphorylates alpha-synuclein at serine 129 in central nervous system. J Biol Chem. 2009;284:2598–2602. doi: 10.1074/jbc.C800206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patino WD, et al. Circulating transcriptome reveals markers of atherosclerosis. Proc Natl Acad Sci USA. 2005;102:3423–3428. doi: 10.1073/pnas.0408032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Ejeh F, Darby JM, Brown MP. Chemotherapy synergizes with radioimmunotherapy targeting La autoantigen in tumors. PLoS ONE. 2009;4:e4630. doi: 10.1371/journal.pone.0004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.