Abstract
Serotonin (5-HT) plays a critical role in modulating synaptic plasticity in the marine mollusc Aplysia and in the mammalian nervous system. In Aplysia sensory neurons, 5-HT can activate several signal cascades, including PKA and PKC, presumably via distinct types of G protein-coupled receptors. However, the molecular identities of these receptors have not yet been identified. We here report the cloning and functional characterization of a 5-HT receptor that is positively coupled to adenylyl cyclase in Aplysia neurons. The cloned receptor, 5-HTapAC1, stimulates the production of cAMP in HEK293T cells and in Xenopus oocytes. Moreover, the knockdown of 5-HTapAC1 expression by RNA interference blocked 5-HT-induced cAMP production in Aplysia sensory neurons and blocked synaptic facilitation in nondepressed or partially depressed sensory-to-motor neuron synapses. These data implicate 5-HTapAC1 as a major modulator of learning related synaptic facilitation in the direct sensory to motor neuron pathway of the gill withdrawal reflex.
Keywords: 5-HT receptor, memory, cAMP, protein kinase A
5-Hydroxytryptamine (5-HT), or serotonin, is a key neurotransmitter that modulates a variety of behaviors in both invertebrate and vertebrate animals and is involved in the regulation of mood and mood disorders in humans (1). Serotonin also modulates synaptic plasticity in the marine mollusc Aplysia (2, 3). Synaptic facilitation of the connections between sensory and motor neurons of the gill-withdrawal reflex is mediated by 5-HT, and this form of synaptic plasticity has been found to be a critical cellular mechanism of behavioral sensitization (4–6). A number of pharmacological studies have found that, depending on the behavioral history and pattern of sensory stimulation, 5-HT stimulates several downstream signaling pathways, including protein kinase A (PKA), protein kinase C (PKC), and mitogen-activated protein kinase (MAPK), suggesting that serotonin acts on more than one receptor type (2, 3, 7, 8). Of these signaling cascades, the adenylyl cyclase-cAMP-PKA cascade has been most extensively investigated because of its important roles in both behavioral sensitization and synaptic facilitation (3, 4, 9, 10). Historically, this was the initially identified second-messenger system involved in the regulation of synaptic plasticity, behavior, and memory storage (4).
A single pulse of 5-HT activates PKA, which phosphorylates and inactivates potassium channels (11) and subsequently increases synaptic strength at nondepressed synapses. At depressed synapses, however, PKC becomes the major downstream kinase to be activated by a single pulse of 5-HT (8). In addition, repetitive exposures to 5-HT that induce long-term facilitation result in the activation of additional kinases, including MAPK (12), that translocate to the nucleus to induce gene expression. However, the molecular mechanism for this dynamic coupling specificity of downstream signaling pathways is not known.
In vertebrates, seven families of 5-HT receptors have been characterized; six of these include G protein-coupled receptors, and only the 5-HT3 family is composed of ionotropic receptors (13). The G protein-coupled 5-HT receptors are classified on the basis of the second messenger systems to which they are coupled (14). The 5-HT1 and 5-HT5 receptors inhibit adenylyl cyclase, whereas 5-HT4, 5-HT6, and 5-HT7 activate adenylyl cyclase, and the 5-HT2 receptor stimulates phospholipase C (PLC). Molecular evolutionary analyses indicate that primordial 5-HT receptors differentiated into 5-HT1, 5-HT2, and 5-HT6 approximately 800 million years ago (mya) (15). Since vertebrates differentiated from invertebrates 600 mya, one might predict that invertebrates may have 5-HT receptor families that are homologous to at least three subtypes of vertebrate 5-HT receptors: 5-HT1, 5-HT2 and 5-HT6. However, considering that a hundred million years have passed since they have diverged, it is by no means certain that the invertebrate receptors have similar pharmacological characteristics to the vertebrate receptors within each family (16).
Aplysia 5-HT receptors have been characterized mainly by pharmacological means. For example, Abrams et al. characterized the 5-HT receptor(s) that are positively coupled to adenylyl cyclase (5-HTapAC) pharmacologically (17, 18). So far, four full-length 5-HT receptors have been cloned in Aplysia (19, 20), two of which—5-HTap1 and 5-HTap2—inhibit adenylyl cyclase activity (19, 20). However, no receptor positively coupled to adenylyl cyclase and involved in synaptic facilitation has yet been cloned, leaving questions about the receptors that initiate synaptic facilitation unanswered. Here, we report the molecular cloning and functional characterization of a 5-HT receptor that is positively coupled to adenylyl cyclase in the Aplysia nervous system.
Results
The Molecular Cloning of 5-HTapAC1 from the Aplysia Nervous System.
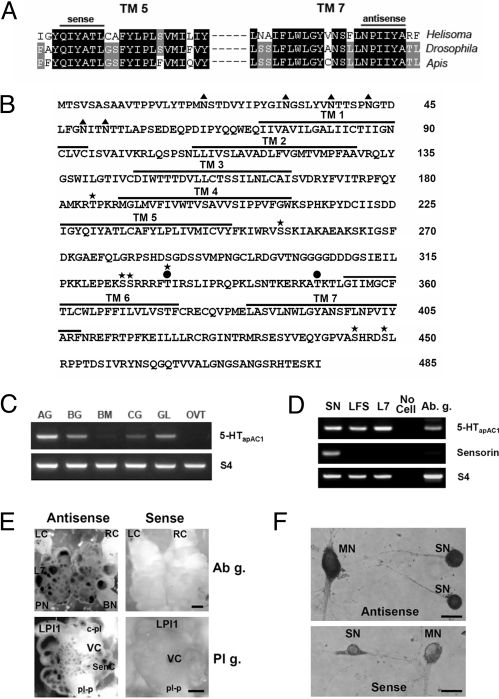
To isolate genes encoding 5-HT receptors that are positively coupled to adenylyl cyclase in Aplysia, we designed two degenerative PCR primers based on the peptide sequences of the fifth and seventh transmembrane domains of invertebrate 5-HT7 receptors (Fig. 1A). In addition to the highly conserved amino acids sequences, NPXXY, in the seventh transmembrane domains of G protein-coupled receptors, we found that another motif (QIYATL) is strikingly conserved only in invertebrate 5-HT7 receptors. We obtained a 519-bp PCR product showing ≈80% sequence homology with the 5-HT7 receptor of Helisoma trivolvis. Using this fragment as a probe, we screened the Aplysia kurodai cDNA library and isolated a full-length cDNA clone, 5-HTapAC1. The putative 1,458 bp ORF encodes a protein of 485 amino acid with a predicted molecular weight of 54 kDa (Fig. 1B). Phylogenetic analysis with other invertebrate 5-HT receptors indicates that 5-HTapAC1 clusters with 5-HT receptor type 7 from the pond snail, honey bee, and fruit fly (Fig. S1A). Our 5-HTapAC1 clone also shows a great homology to a partial EST clone, PEG003-C-228120–501 (GenBank accession, EB245546) from A. californica, suggesting that this clone is highly conserved between two closely related species of Aplysia (21). Comparisons between 5-HTapAC1 and mammalian receptors also reveal that the cloned 5-HTapAC1 belongs within the 5-HT7 family of receptors, which is positively coupled to adenylyl cyclase (Fig. S1B).
Fig. 1.
Molecular cloning and expression pattern of 5-HTapAC1. (A) Multiple sequence alignments of TM5 and TM7 of invertebrate 5-HT7 receptors. (B) Deduced amino acid sequence of 5-HTapAC1. Seven transmembrane (TM) domains are indicated and numbered. Predicted phosphorylation sites for PKA (●) and PKC (*) are marked. Triangles (▴) indicate N-glycosylation sites. (C) Multiple tissue RT-PCR analysis. AG, abdominal ganglia; BG, buccal ganglia; BM, buccal mass; CG, central ganglia (cerebral, pleural and pedal); GL, gill; OVT, ovotestis. (D) Single cell RT-PCR analysis of 5-HTapAC1. 5-HTapAC1 was expressed in sensory neurons and LFS, L7 motor neurons. The sensory neuron specific gene sensorin was expressed only in sensory cells. The housekeeping gene S4 was used as a control. Total RNA isolated from the whole abdominal ganglion was used as another control. SN, sensory neuron; LSF, LSF motor neuron; L7, L7 motor neuron; Ab. g., total RNA from the abdominal ganglion. (E) In situ hybridization of 5-HTapAC1 mRNA in sensory clusters. Dorsal abdominal ganglia and left pleural ganglia were shown. 5-HTapAC1 was expressed in sensory cells in the sensory cluster in pleural ganglia, as well as in L7 motor neurons. LC, left pleuroabdominal connective; RC, right pleuroabdominal connective; PN, pericardial nerve; BN, branchial nerve; L7, L7 motor neuron; LPI1, left pleural giant neuron; SenC, sensory cluster; c-pl, cerebro-pleural connective; pl-p, pleuropedal connective. (Scale bars, 500 μm for Upper; 200 μm Lower.) (F) In situ hybridization of 5-HTapAC1 mRNA in sensory-to-motor coculture. (Right) Shown are the hybridization with sense nucleotide probe. (Scale bar, 50 μm.)
The deduced amino acid sequences of 5-HTapAC1 show the hallmarks of known G protein-coupled receptors, including seven transmembrane domains, the tripeptide DRY (Asp-169–Arg-170–Tyr-171) required for G protein coupling (22), and the NPXXY motif (Asn-401–Pro-402–Tyr-405) for receptor desensitization and internalization (23) (Fig. 1B). Four potential sites for PKC phosphorylation and two sites for PKA are found within the third intracellular loop. Three additional PKC sites are also found in the second intracellular loop and C-terminal intracellular tail. In addition, six consensus asparagine residues for N-glycosylation are found in the extracellular N terminus. 5-HTapAC1 also contains, at its C terminus, a putative PDZ-binding motif (Ser-483–Lys-484– Ile-485), suggesting that this receptor may be clustered by scaffolding proteins to form a signaling complex.
We next examined the tissue distribution of the cloned receptor by RT-PCR analysis. For this purpose we used total RNA isolated from the abdominal ganglia, the buccal ganglia, the buccal mass, the central ganglia containing the pleural-pedal ganglia, and the gill and ovotestis of Aplysia (Fig. 1C). A specific fragment was detected in the CNS tissue, with the strongest signal coming from the abdominal ganglia. The mRNA was also detected in muscle and gill tissue, which is consistent with an earlier study that used an [3H]LSD binding assay (24). To further validate the expression of 5-HTapAC1 in specific neurons involved in synaptic facilitation and behavioral sensitization, we performed single cell RT-PCR analysis (Fig. 1D). Our results clearly showed that 5-HTapAC1 is expressed both in the sensory neurons and in the L7 and LFS motor neurons.
The expression pattern of 5-HTapAC1 was further examined by in situ hybridization in the abdominal and pleural ganglia of A. kurodai. In situ staining revealed that the cloned receptor is widely expressed in the nervous system, including sensory neurons in the sensory clusters of both abdominal and pleural ganglia, as well as L7 motor neurons in abdominal ganglia (Fig. 1E). Expression of 5-HTapAC1 in pleural sensory neurons and in LFS motor neurons was also confirmed by in situ hybridization in sensory-to-motor coculture (Fig. 1F).
Subcellular Localization of 5-HTapAC1.
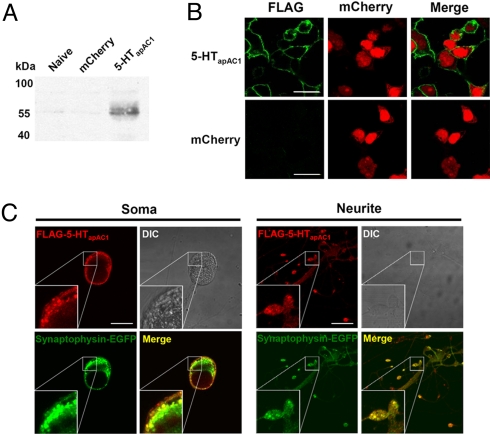
To investigate the subcellular localization of 5-HTapAC1, we transiently expressed 5-HTapAC1 fused with a FLAG tag at its N terminus in HEK293T cells. We confirmed the expression by western blot and immunocytochemistry with an anti-FLAG antibody (Fig. 2 A and B). Immunocytochemistry analysis revealed the membrane expression of 5-HTapAC1 in HEK293T cells (Fig. 2B). Overexpressed 5-HTapAC1 was detected at the plasma membrane in Aplysia sensory neurons (Fig. 2C). Importantly, 5-HTapAC1 was co-localized with synaptophysin-EGFP at neurites and varicosities in sensory neurons, indicating that 5-HTapAC1 is expressed at synapses (Fig. 2C).
Fig. 2.
Subcellular localization of 5-HTapAC1. (A and B) Expression of FLAG-5-HTapAC1 in HEK293T cell. Expression was confirmed by western blot (A) and immunocytochemistry (B). In immunocytochemistry, FLAG-5-HTapAC1 (green) localized in the cytoplasmic membrane. mCherry-N1 (red) was co-transfected as a expression marker and diffusely distributed in the cytosol. (C) Co-localization of overexpressed FLAG-5-HTapAC1 (red) and synaptophysin-EGFP (green) in Aplysia sensory cells co-cultured with LFS motor neurons. Insets show three fold magnification images. FLAG-5-HTapAC1 and synaptophysin-EGFP are highly co-localized at synaptic varicosities, and partially co-localized at neurites and the plasma membrane, but not co-localized at the cytosol. (Scale bars, 30 μm.)
Stimulation of Adenylyl Cyclase by Heterologously Expressed 5-HTapAC1.
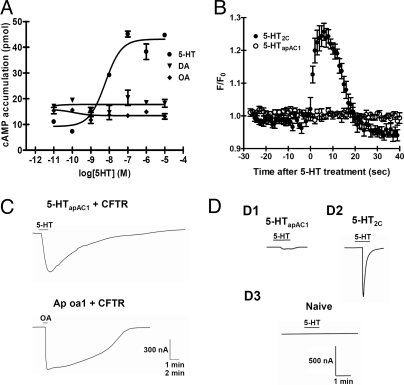
To determine whether the heterologously expressed 5-HTapAC1 can stimulate cAMP production in response to 5-HT in HEK293T cells, we treated cells with either vehicle or varying concentrations of 5-HT and then measured the amount of cellular cAMP. The addition of 5-HT stimulated the production of cAMP in a dose-dependent manner with an EC50 of 6.0 nM (logEC50 = −8.22 ± 0.21), which is comparable to that of other invertebrate 5-HT type 7 receptors, consistent with 5-HTapAC1 stimulating cAMP signaling (25, 26) (Fig. 3A). This stimulation by 5-HT was specific. Neither dopamine nor octopamine stimulated cAMP accumulation. To further investigate whether 5-HTapAC1 can also activate other second messenger cascades such as intracellular Ca2+ signaling, we performed Ca2+ imaging. As a positive control, we used mouse 5-HT2c, the Gq-coupled 5-HT receptor that stimulates intracellular Ca2+ elevation. Exposure to 5-HT (1 μM) triggered a Ca2+ signal only in 5-HT2c-expressing cells, not in 5-HTapAC1-expressing cells (F/F0: 5-HT2c, 1.257 ± 0.026, n = 6; 5-HTapAC1, 1.003 ± 0.019, n = 6) (Fig. 3B).
Fig. 3.
Coupling specificity of 5-HTapAC1. (A) Dose-response curve of 5-HT on cAMP production in HEK293T cells transiently expressing 5-HTapAC1. Various concentrations of 5-HT were applied in the presence of 500 μM 3-isobutyl-1-methylxanthine (IBMX), an inhibitor of the cAMP dependent phosphodiesterase. Neither octopamine (OA) nor dopamine (DA) was able to stimulate cAMP production. (B) Ca2+ imaging in HEK293T cell overexpressing mouse 5-HT2C or 5-HTapAC1. After loading the calcium dye, Calcium Green-1, the cells were exposed to 1 μM 5-HT and imaged under the confocal microscope. Increase of cellular Ca2+ was detected only in cells expressing 5-HT2C, which is known to be coupled to the Gq protein. (C) Functional expression and Gs coupling in Xenopus oocytes. CFTR cRNA (2.5 ng) was co-injected with either 5-HTapAC1 (2.5 ng) or Ap oa1 cRNA (2.5 ng). CFTR current is known to be activated by cAMP/PKA. Ap oa1, which is specifically coupled to Gs, was used as a positive control. 5-HT (1 μM) and OA (1 μM) were used to stimulate 5-HTapAC1 or Ap oa1. Scale bars, 300 nA and 1 min for Upper, and 300 nA and 2 min for Lower. CFTR current was observed in both 5-HTapAC1- and Ap oa1-injected oocytes. (D) 5-HTapAC1 does not activate Gq in oocytes. Oocytes were injected with the following cRNAs: 5-HTapAC1 (2.5 ng) (D1), 5-HT2c cRNA (100 pg) (D2), and distilled water (D3). (Scale bars, 500 nA and 1 min.) A transient Cl− current activated by Gq-mediated signaling was observed in 5-HT2c-injected oocytes, not in 5-HTapAC1-injected oocytes.
To examine electrophysiologically the functional expression and G protein coupling specificity of 5-HTapAC1, we expressed 5-HTapAC1 in Xenopus oocytes with or without the cystic fibrosis transmembrane conductance regulator (CFTR). The CFTR, a Cl− channel, is activated by PKA and has been used to determine whether a receptor is coupled to the Gs protein (27, 28). Treatment of 1 μM 5-HT induced significant CFTR current (320.6 ± 123.8 nA, n = 8) in oocytes injected with 5-HT apAC1 and CFTR cRNA (Fig. 3C). As a positive control, we coinjected the CFTR with an Aplysia octopamine receptor Ap oa1, which activates adenylyl cyclase (28). The activation of Ap oa1 with 1 μM octopamine generated a similar CFTR current (565.0 ± 104.1 nA, n = 8) (Fig. 3C). Xenopus oocytes have an endogenous Cl− channel that is quickly activated by Ca2+ after activation of Gq (29). The typical agonist-activated transient inward current was observed in the 5-HT2c-injected oocytes in response to 5-HT treatment (835.0 ± 35.4 nA, n = 2). However, 5-HT treatment did not induce such a large and transient Cl− current in 5-HTapAC1 cRNA-injected oocytes (Fig. 3D). Taken together, these heterologous expression analyses strongly suggest that the cloned 5-HTapAC1 is positively linked specifically to Gs, and not to Gq.
Pharmacological Properties of 5-HTapAC1.
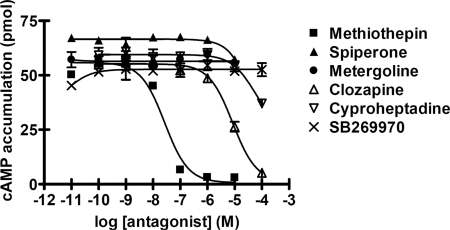
To characterize the pharmacological properties of 5-HTapAC1, we examined the effects of known 5-HT receptor antagonists in HEK293T cells in which we had transiently expressed 5-HTapAC1 in the presence of 10 nM 5-HT. Of the antagonists tested, the nonselective 5-HT receptor antagonist methiothepin was most effective. Clozapine also blocked 5-HT-induced cAMP production, but with less potency than methiothepin (Fig. 4). Cyproheptadine, spiperone and metergoline, however, were much less effective than methiothepin and clozapine, or not effective at all. These data are consistent with a previous report by Abrams and his colleagues (17) using Aplysia CNS membranes, except that in the previous study cyproheptadine and metergoline also blocked 5-HT-induced cAMP production. We also found that SB269970, a specific 5-HT7 antagonist in mammals (30), was not effective for 5-HTapAC1. SB269970 was also found to be ineffective for Am5-HT7, a recently cloned invertebrate 5-HTR, whose sequence is more closely related to 5-HTapAC1 than mammalian 5-HT7 (26).
Fig. 4.
Pharmacological characterization of 5-HTapAC1. Effects of antagonists on 5-HT stimulated cAMP production were examined in HEK293T cells transiently transfected with 5-HTapAC1. Various concentrations of antagonists were treated in the presence of 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) 20 min before 5-HT (10 nM) treatment. Methiothepin and clozapine blocked 5-HT-induced cAMP production with pKi values of 7.53 and 5.5, respectively. pKi values were determined by the equation, Ki = IC50/(1+C/Kd), where C is the 5-HT concentration (10 nM) and Kd is the EC50 value for 5-HT (6.0 nM). The presented data are representative of at least two independent experiments with each point measured in duplicate (mean ± SEM).
5-HTapAC1 Modulates Membrane Excitability and Spike Duration.
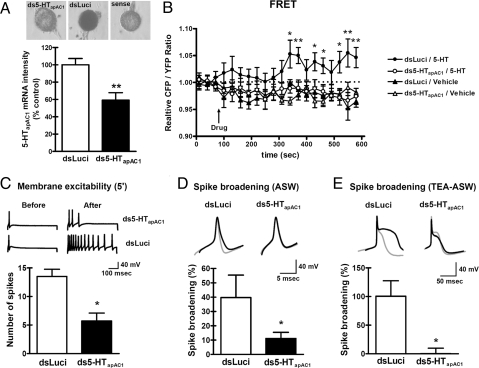
To investigate the role of endogenous 5-HTapAC1 in sensory neurons, we blocked 5-HTapAC1 gene expression by injecting double stranded (ds) RNA (ds5-HTapAC1) (31). The effectiveness of dsRNA was confirmed by performing in situ hybridization (Fig. 5A). As a control, dsLuci was injected into the sensory neuron using EGFP as an expression marker. An antisense RNA against partial 5-HTapAC1 was used as a probe (see Materials and Methods for the details). In situ hybridization showed that ds5-HTapAC1 significantly reduced the expression of 5-HTapAC1 in sensory neurons by almost 50% (relative percent of 5-HTapAC1 mRNA intensity: dsLuci, 100.0 ± 7.2, n = 10; ds5-HTapAC1, 59.2 ± 8.7, n = 18; P < 0.01, Student's t test). To determine whether 5-HTapAC1 is critically involved in cAMP production in response to 5-HT in Aplysia sensory neurons, we monitored changes in the cAMP level using the Epac-based fluorescence resonance energy transfer (FRET) sensor, CFP-Epac(δDEP-CD)-YFP (Fig. 5B). Epac is a well-known guanine nucleotide exchange factor (GEF) that is activated by direct binding of cAMP (32). Binding of cAMP to Epac induces conformational changes in Epac, resulting in changes in the CFP/YFP ratio, which serves as a measure of the cAMP level (33). 5-HT (10 μM) increased cAMP level in dsLuci-injected sensory cells. However, there was no significant change in the CFP/YFP ratio in ds5-HTapAC1-injected sensory neurons, suggesting that 5-HTapAC1 plays a critical role in 5-HT-induced cAMP production in Aplysia sensory neurons (Fig. 5B).
Fig. 5.
Blockage of 5-HTapAC1 expression impaired the increases in intracellular cAMP, membrane excitability and spike duration by 5-HT. (A) Effectiveness of 5-HTapAC1 dsRNA was measured by in situ hybridization. Sensory neurons were injected with dsRNA for 5-HT apAC1 or with dsLuci as a control. (B) ds5-HTapAC1 blocked the increase in the intracellular cAMP level stimulated by 5-HT treatment (10 μM). Relative CFP/YFP ratios are measured as an index of the cellular cAMP level. One-way ANOVA followed by a Newman-Keuls post hoc test was performed for each time point. Asterisks indicate significant differences in the post hoc tests between dsLuci/5-HT and ds5-HTapAC1/5-HT. *, P < 0.05; **, P < 0.01. (C) ds5-HTapAC1 blocked the increase in membrane excitability normally induced by 5-HT treatment (10 μM for 5 min). Representative traces of action potentials during a 500 ms depolarizing pulse are indicated. Unpaired, two-tailed t test; *, P < 0.05. (D and E) Spike broadening induced by 5-HT treatment (10 μM for 7 min) was blocked only by ds5-HTapAC1 microinjection, not by dsLuci microinjection under normal or TEA/nifedipine-ASW conditions. Representative traces of single action potentials before (gray line), and after 10 μM 5-HT treatment (black line) are indicated. Summary bar graphs are represented as mean ± SEM. Unpaired, two-tailed t test; *, P < 0.05.
The activation of adenylyl cyclase by 5-HT is known to modulate K+ currents to produce an increase in membrane excitability and in spike duration in sensory neurons (8, 11, 28, 34). ds5-HTapAC1 significantly suppressed the increase in membrane excitability produced by a 5-min exposure to 10 μM 5-HT (number of spikes: dsLuci, 13.5 ± 1.3, n = 4 vs. ds5-HTapAC1, 5.7 ± 1.4, n = 14; P < 0.05, Student's t test) (Fig. 5C). Similarly, spike broadening was also reduced in ds5-HTapAC1-injected sensory neurons as compared with dsLuci-injected control neurons (% increase of spike duration in normal ASW: dsLuci, 39.7 ± 15.7, n = 5 vs. ds5-HTapAC1, 11.1 ± 4.3, n = 14; P < 0.05, Student's t test) (Fig. 5D). To isolate spike broadening, which is specifically mediated by the inhibition of the cAMP dependent-S-type K+ current, we recorded spike broadening in the presence of 100 mM TEA and 20 μM nifedipine. In the presence of these two drugs, all K+ currents other than the 5-HT-modulated S-type K+ currents are blocked (11, 35). Blockage of 5-HTapAC1 under these conditions also dramatically impaired the spike broadening (in TEA/nifedipine-ASW: % increase of spike duration: dsLuci, 100.5 ± 27.0, n = 6 vs. ds5-HTapAC1, 0.2 ± 9.5, n = 5; P < 0.05, Student's t test) (Fig. 5E). These results indicate that 5-HTapAC1 is required for short-term activation of the cAMP-PKA pathway and for its actions on spike duration and excitability.
Critical Role of 5-HTapAC1 in Synaptic Facilitation.
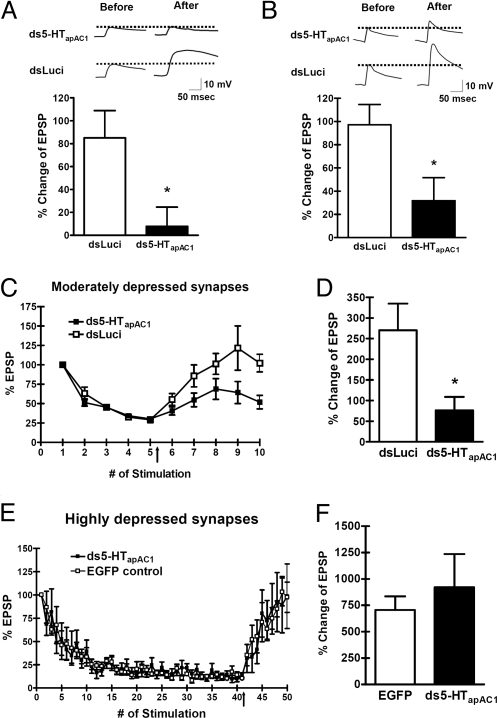
Synaptic facilitation produced by 5-HT is state-dependent. PKA plays a major role in synaptic facilitation of a nondepressed synapse that is recruited by behavioral sensitization, whereas PKC activity becomes dominant in facilitation of a depressed synapse involved in dishabituation (8, 28, 36–38). To study the role of 5-HTapAC1 in synaptic facilitation in a nondepressed synapse, we first injected ds5-HTapAC1 into the presynaptic sensory neuron of a sensory-to-motor neuron synapse. Injection of ds5-HTapAC1 did not affect basal synaptic transmission (% change of EPSP: dsLuci, 105.6 ± 53.0, n = 3 vs. ds5-HTapAC1, 109.8 ± 31.1, n = 3; P = 0.9477, Student's t test). In nondepressed synapses, we examined the effect of knock-down of 5-HTapAC1 by dsRNA microinjection in two ways. First, we applied 5-HT (10 μM) for 1 min and then washed out (36, 39). In this condition, knock-down of 5-HTapAC1 completely blocked short-term facilitation (% change of EPSP: dsLuci, 85.0 ± 23.9, n = 7; vs. ds5-HTapAC1, 7.7 ± 16.8, n = 9; P < 0.05, Student's t test) (Fig. 6A). Second, we examined a longer application—5 min—of 5-HT exposure. Knock-down of 5-HTapAC1 again significantly impaired short-term synaptic facilitation although with their longer exposure it did not fully block synaptic facilitaion (% change of EPSP: dsLuci, 97.1 ± 17.5, n = 10 vs. ds5-HTapAC1, 31.8 ± 19.7, n = 12; P < 0.05, Student's t test) (Fig. 6B).
Fig. 6.
Blockage of 5-HTapAC1 expression impaired short-term facilitation in nondepressed and moderately depressed synapses. (A and B) Short-term facilitation induced by 5-HT treatment (10 μM, 1 min) followed by 4 min of wash out (A) or 5-HT treatment (10 μM, 5 min) (B) was significantly reduced both cases in ds5-HTapAC1-injected synapses compared to control synapses. EPSP amplitudes were measured both before and 5 min after 5-HT treatment, and the percent of amplitude change was calculated. (C and D) 5-HT treatment (10 μM, 5 min) reversed a moderate degree of synaptic depression only in control synapses, indicating that cAMP is required for this degree of depression. % Change of EPSP = (mean EPSP at the 9th and 10th stimuli) − (mean EPSP at the 4th and 5th stimuli)/(mean EPSP at the 4th and 5th stimuli) × 100 (%). (E and F) By contrast, 5-HT treatment reversed a high degree of synaptic depression comparably in both dsRNA-injected and control synapses. Percent change of EPSP = (mean EPSP between the 36th and 40th stimuli) − (mean EPSP between the 46th and 50th stimuli)/(mean EPSP between the 46th and 50th stimuli) × 100 (%). Unpaired, two-tailed t test; *, P < 0.05.
Next, we stimulated the sensory neuron of the sensory-to-motor neuron synapse repeatedly to achieve either a moderate (Fig. 6 C and D) or high degree of synaptic depression (Fig. 6 E and F). In moderately depressed synapses, reversal of synaptic depression by exposure to one pulse of 5-HT was significantly lower in ds5-HTapAC1-injected cells than that of dsLuci-injected cells (% change of EPSP: dsLuci, 270.3 ± 64.2, n = 7 vs. ds5-HTapAC1, 76.2 ± 32.7, n = 7; P < 0.05, Student's t test) (Fig. 6D). In highly depressed synapses, one pulse of 5-HT reversed the synaptic depression of ds5-HTapAC1-injected synapses as well as EGFP-injected synapses (% change of EPSP: EGFP, 705.4 ± 129.9, n = 5 vs. ds5-HTapAC1, 920.5 ± 315.8, n = 4; P > 0.05, Student's t test) (Fig. 6F). These results demonstrate that 5-HTapAC1 is critically involved in short-term facilitation of nondepressed synapses as well as the reversal of moderately depressed synapses, both of which are mediated by the cAMP–PKA pathway. By contrast, 5-HTapAC1 may not be involved in the reversal of highly depressed synapses where the reversal depends on PKC.
Discussion
In the present study, we report the cloning and functional characterization of a 5-HT receptor that is positively coupled to adenylyl cyclase in the Aplysia nervous system. 5-HT plays a central role in synaptic plasticity in Aplysia. Our molecular, pharmacological, and electrophysiological data are consistent with earlier pharmacological studies and reveal that cloned 5-HTapAC1 is critically involved in synaptic facilitation via stimulating the production of cAMP (3).
We used degenerative PCR as a cloning strategy. Extensive sequence comparisons with previously cloned invertebrate 5-HT7 receptors enabled us to find a unique motif, QIYATL, in the fifth transmembrane domain (Fig. 1). To our knowledge, this motif has no known function and is not found in other closely related biogenic amine receptors, such as dopamine receptors, or even in other 5-HT receptor subtypes in Aplysia. We named the cloned receptor 5-HTapAC1, considering that it is a cloned Aplysia 5-HT receptor positively coupled to adenylyl cyclase; however, we do not exclude the possibility that other similar receptor subtypes exist in the Aplysia nervous system. For example, we found that several 5-HT receptor antagonists that are known to block both cAMP production and synaptic facilitation in other studies are ineffective against 5-HTapAC1 expressed in HEK293 cells (7, 17). While this discrepancy may arise from the different experimental conditions, it may imply that more than one subtype of 5-HT receptors can stimulate adenylyl cyclase in the Aplysia nervous system. It is also possible, however, that in the previous biochemical and electrophysiological analyses treatments with the antagonists might have influenced crosstalk between different types of 5-HT receptors.
5-HTapAC1 also differs pharmacologically from the mammalian 5-HT7 family (Fig. 4). A mammalian 5-HT7 antagonist—SB269970—was ineffective at inhibiting the AC activity of 5-HTapAC1. This antagonist is known to also be ineffective in Am5-TH7, which was recently cloned in the invertebrate Apis mellifera (26). Furthermore, of several 5-HT receptor antagonists that we tested, only two, methiothepin and clozapine, proved to be effective. The ineffectiveness of mammalian receptor antagonists might be due to the evolutionary divergence of 5-HT receptors between vertebrates and invertebrates over 600 million years. Completion of the ongoing Aplysia genome project or extensive analysis of expressed sequence tags will clarify this issue (21).
The endogenous function of 5-HTapAC1 was investigated using RNA interference. Our electrophysiological analyses with this technique revealed the critical role of this receptor in learning related heterosynaptic facilitation (Figs. 5 and 6). Due to the lack of an antibody against ds5-HTapAC1, we assessed the efficiency of dsRNA by performing in situ hybridization. Interestingly, we found that an ≈40% reduction in mRNA levels was enough to significantly block the cellular and synaptic response to 5-HT treatment. The efficacy of dsRNA on protein expression remains to be examined. If the protein expression is also partially reduced, it may suggest that there is a certain threshold of expression which is necessary for the receptor to be fully functional. We also cannot exclude the possibility of the off-target effects of dsRNA, such as inhibiting other unknown Gs-coupled 5-HT receptors in sensory neurons. So far, however, the only match (PEG003-C-228120–501) for the ds5-HTapAC1 sequence in the Aplysia EST database (http://www.seahare.org) is thought to be an A. californica homolog of 5-HTapAC1, suggesting that ds5-HTapAC1 specifically knocks down 5-HTapAC1.
We also found that 5-HTapAC1 dsRNA impaired the 5-HT-induced increases in membrane excitability, spike duration, and synaptic facilitation in nondepressed and moderately depressed synapses. Consistent with earlier pharmacological analysis, we also found that the knock-down of 5-HTapAC1 does not reverse the synaptic depression in highly depressed synapses where PKC is critically involved in this reversal (8). Along with calcium imaging data, these results indicate that PKA and PKC are activated by independent G protein-coupled receptors in Aplysia sensory neurons in a state-dependent manner.
Cloning of 5-HTapAC1 allows us to revisit several still unsolved questions about the mechanism of synaptic facilitation in Aplysia: What happens to the adenylyl cyclase coupled receptor in a highly depressed synapse? Is the receptor desensitized or internalized as the synapse becomes depressed? If so, what are the mechanisms for this functional down-regulation? What are the receptor's binding proteins? Is the same receptor involved in long-term synaptic facilitation? What is the role of the receptor in the postsynaptic neurons? Further molecular, biochemical, and electrophysiological studies on 5-HTapAC1 should answer these questions.
Materials and Methods
Molecular Cloning of 5-HTapAC1 cDNA.
To clone 5-HTapAC1 cDNA from Aplysia kurodai, we performed degenerative PCR. Two degenerative PCR primers were designed based on the peptide sequences of the highly conserved amino acid sequence, NPXXY in the fifth transmembrane domains, and the QIYATL motif in the seventh transmembrane domains of invertebrate 5-HT7 receptors (sense, 5′-CARATITAYGCMACICTA-3′; antisense, 5′-TGYRTADATIAYIGGRTT-3′). Amplification was carried out for 3 min at 94°C (one cycle), followed by 35 cycles of 15 s at 94°C, 15 s at 60°C, 1.5 min at 68°C, and a final extension of 5 min at 68°C. The PCR yielded a DNA fragment of 519 bp, showing approximately 80% sequence homology with the Helisoma trivolvis 5-HT7 receptor. Using this fragment as a probe, we screened approximately 2.0 × 105 clones from the Aplysia kurodai cDNA library (40) to obtain the entire ORF, and one positive signal was found. This clone was analyzed by DNA sequencing to reveal that it, in fact, contained the entire ORF (5-HTapAC1). The sequence of 5-HTapAC1 was subcloned into HindIII/KpnI-digested pNEXδ (41) to create pNEXδ-5-HTapAC1.
Single Cell RT-PCR.
Single cell RT-PCR protocol was modified from the previously described methods (42, 43). Please see SI Text for the details.
Intracellular cAMP Level Measurement Using FRET.
The construct for monitoring the intracellular cAMP level was a gift from Changjoon Justin Lee (Korea Institute of Science and Technology, Korea), and the analysis was performed as described previously (44). Please see SI Text for the details.
Aplysia Cell Culture and Electrophysiology.
Sensory cell culture and sensory-to-motor coculture were performed as described previously (45, 46). Please see SI Text for the details.
Statistical Analysis.
The results are expressed as mean ± SEM. The unpaired Student's t test was used for comparison between two groups. One-way ANOVA followed by Newman-Keuls post-hoc test was used for comparison between three- or more groups.
Supplementary Material
Acknowledgments.
This work was supported by the National Creative Research Initiative Program of the Korean Ministry of Science and Technology and the Marine and Extreme Genome Research Center Program, Ministry of Marine Affairs and Fisheries, Republic of Korea. Y.-S.L., S.-L.C., and D.J-.J. are supported by Brain Korea 21 fellowships. S.-L.C. is supported by Seoul Science Fellowship. E.R.K. is supported by the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
The sequence reported in this paper has been deposited in the GenBank database (accession no. FJ477896).
This article contains supporting information online at www.pnas.org/cgi/content/full/0907502106/DCSupplemental.
References
- 1.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological, and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 2.Barbas D, DesGroseillers L, Castellucci VF, Carew TJ, Marinesco S. Multiple serotonergic mechanisms contributing to sensitization in Aplysia: Evidence of diverse serotonin receptor subtypes. Learn Mem. 2003;10:373–386. doi: 10.1101/lm.66103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 4.Brunelli M, Castellucci V, Kandel ER. Synaptic facilitation and behavioral sensitization in Aplysia: Possible role of serotonin and cyclic AMP. Science. 1976;194:1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- 5.Montarolo PG, et al. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- 6.Lee YS, Bailey CH, Kandel ER, Kaang BK. Transcriptional regulation of long-term memory in the marine snail Aplysia. Molecular brain. 2008;1:3. doi: 10.1186/1756-6606-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercer AR, Emptage NJ, Carew TJ. Pharmacological dissociation of modulatory effects of serotonin in Aplysia sensory neurons. Science. 1991;254:1811–1813. doi: 10.1126/science.1662413. [DOI] [PubMed] [Google Scholar]
- 8.Byrne JH, Kandel ER. Presynaptic facilitation revisited: State and time dependence. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrams TW, Castellucci VF, Camardo JS, Kandel ER, Lloyd PE. Two endogenous neuropeptides modulate the gill and siphon withdrawal reflex in Aplysia by presynaptic facilitation involving cAMP-dependent closure of a serotonin-sensitive potassium channel. Proc Natl Acad Sci USA. 1984;81:7956–7960. doi: 10.1073/pnas.81.24.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glanzman DL, et al. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J Neurosci. 1989;9:4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldsmith BA, Abrams TW. cAMP modulates multiple K+ currents, increasing spike duration and excitability in Aplysia sensory neurons. Proc Natl Acad Sci USA. 1992;89:11481–11485. doi: 10.1073/pnas.89.23.11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin KC, et al. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- 13.Hoyer D, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 14.Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 15.Peroutka SJ, Howell TA. The molecular evolution of G protein-coupled receptors: Focus on 5-hydroxytryptamine receptors. Neuropharmacology. 1994;33:319–324. doi: 10.1016/0028-3908(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 16.Tierney AJ. Structure and function of invertebrate 5-HT receptors: A review. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:791–804. doi: 10.1016/s1095-6433(00)00320-2. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JE, Onyike CU, McElroy VL, Lin AH, Abrams TW. Pharmacological characterization of an adenylyl cyclase-coupled 5-HT receptor in Aplysia: Comparison with mammalian 5-HT receptors. J Neurophysiol. 2003;89:1440–1455. doi: 10.1152/jn.01004.2002. [DOI] [PubMed] [Google Scholar]
- 18.Dumitriu B, Cohen JE, Wan Q, Negroiu AM, Abrams TW. Serotonin receptor antagonists discriminate between PKA- and PKC-mediated plasticity in Aplysia sensory neurons. J Neurophysiol. 2006;95:2713–2720. doi: 10.1152/jn.00642.2005. [DOI] [PubMed] [Google Scholar]
- 19.Angers A, Storozhuk MV, Duchaine T, Castellucci VF, DesGroseillers L. Cloning and functional expression of an Aplysia 5-HT receptor negatively coupled to adenylate cyclase. J Neurosci. 1998;18:5586–5593. doi: 10.1523/JNEUROSCI.18-15-05586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbas D, et al. Functional characterization of a novel serotonin receptor (5-HTap2) expressed in the CNS of Aplysia californica. J Neurochem. 2002;80:335–345. doi: 10.1046/j.0022-3042.2001.00703.x. [DOI] [PubMed] [Google Scholar]
- 21.Moroz LL, et al. Neuronal transcriptome of Aplysia: Neuronal compartments and circuitry. Cell. 2006;127:1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moro O, Lameh J, Hogger P, Sadee W. Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J Biol Chem. 1993;268:22273–22276. [PubMed] [Google Scholar]
- 23.Barak LS, et al. A highly conserved tyrosine residue in G protein-coupled receptors is required for agonist-mediated beta 2-adrenergic receptor sequestration. J Biol Chem. 1994;269:2790–2795. [PubMed] [Google Scholar]
- 24.Drummond AH, Bucher F, Levitan IB. Distribution of serotonin and dopamine receptors in Aplysia tissues: Analysis by [3H]LSD binding and adenylate cyclase stimulation. Brain Res. 1980;184:163–177. doi: 10.1016/0006-8993(80)90595-8. [DOI] [PubMed] [Google Scholar]
- 25.Obosi LA, et al. Functional characterisation of the Drosophila 5-HTdro1 and 5-HTdro2B serotonin receptors in insect cells: Activation of a G(alpha) s-like protein by 5-HTdro1 but lack of coupling to inhibitory G-proteins by 5-HTdro2B. FEBS Lett. 1996;381:233–236. doi: 10.1016/0014-5793(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 26.Schlenstedt J, Balfanz S, Baumann A, Blenau W. Am5-HT7: Molecular and pharmacological characterization of the first serotonin receptor of the honeybee (Apis mellifera) J Neurochem. 2006;98:1985–1998. doi: 10.1111/j.1471-4159.2006.04012.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee YS, et al. Cloning and expression of a G protein-linked acetylcholine receptor from Caenorhabditis elegans. J Neurochem. 1999;72:58–65. doi: 10.1046/j.1471-4159.1999.0720058.x. [DOI] [PubMed] [Google Scholar]
- 28.Chang DJ, et al. Activation of a heterologously expressed octopamine receptor coupled only to adenylyl cyclase produces all the features of presynaptic facilitation in Aplysia sensory neurons. Proc Natl Acad Sci USA. 2000;97:1829–1834. doi: 10.1073/pnas.97.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubbert H, et al. cDNA cloning of a serotonin 5-HT1C receptor by electrophysiological assays of mRNA-injected Xenopus oocytes. Proc Natl Acad Sci USA. 1987;84:4332–4336. doi: 10.1073/pnas.84.12.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovell PJ, et al. A novel, potent, and selective 5-HT(7) antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl) phenol (SB-269970) J Med Chem. 2000;43:342–345. doi: 10.1021/jm991151j. [DOI] [PubMed] [Google Scholar]
- 31.Lee JA, et al. Overexpression of and RNA interference with the CCAAT enhancer-binding protein on long-term facilitation of Aplysia sensory to motor synapses. Learn Mem. 2001;8:220–226. doi: 10.1101/lm.40201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci USA. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponsioen B, et al. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 2004;5:1176–1180. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baxter DA, Byrne JH. Differential effects of cAMP and serotonin on membrane current, action-potential duration, and excitability in somata of pleural sensory neurons of Aplysia. J Neurophysiol. 1990;64:978–990. doi: 10.1152/jn.1990.64.3.978. [DOI] [PubMed] [Google Scholar]
- 35.Baxter DA, Byrne JH. Serotonergic modulation of two potassium currents in the pleural sensory neurons of Aplysia. J Neurophysiol. 1989;62:665–679. doi: 10.1152/jn.1989.62.3.665. [DOI] [PubMed] [Google Scholar]
- 36.Ghirardi M, et al. Roles of PKA and PKC in facilitation of evoked and spontaneous transmitter release at depressed and nondepressed synapses in Aplysia sensory neurons. Neuron. 1992;9:479–489. doi: 10.1016/0896-6273(92)90185-g. [DOI] [PubMed] [Google Scholar]
- 37.Braha O, et al. Second messengers involved in the two processes of presynaptic facilitation that contribute to sensitization and dishabituation in Aplysia sensory neurons. Proc Natl Acad Sci USA. 1990;87:2040–2044. doi: 10.1073/pnas.87.5.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang DJ, Lim CS, Lee JA, Kaang BK. Synaptic facilitation by ectopic octopamine and 5-HT receptors in Aplysia. Brain Res Bull. 2003;60:73–79. doi: 10.1016/s0361-9230(03)00016-9. [DOI] [PubMed] [Google Scholar]
- 39.Hawkins RD, Kandel ER, Bailey CH. Molecular mechanisms of memory storage in Aplysia. Biol Bull. 2006;210:174–191. doi: 10.2307/4134556. [DOI] [PubMed] [Google Scholar]
- 40.Lee YS, et al. Transcriptome analysis and identification of regulators for long-term plasticity in Aplysia kurodai. Proc Natl Acad Sci USA. 2008;105:18602–18607. doi: 10.1073/pnas.0808893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaang BK, Kandel ER, Grant SG. Activation of cAMP-responsive genes by stimuli that produce long-term facilitation in Aplysia sensory neurons. Neuron. 1993;10:427–435. doi: 10.1016/0896-6273(93)90331-k. [DOI] [PubMed] [Google Scholar]
- 42.Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 43.Li HL, et al. Dscam mediates remodeling of glutamate receptors in Aplysia during de novo and learning-related synapse formation. Neuron. 2009;61:527–540. doi: 10.1016/j.neuron.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park H, et al. An Aplysia type 4 phosphodiesterase homolog localizes at the presynaptic terminals of Aplysia neuron and regulates synaptic facilitation. J Neurosci. 2005;25:9037–9045. doi: 10.1523/JNEUROSCI.1989-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schacher S, Proshansky E. Neurite regeneration by Aplysia neurons in dissociated cell culture: Modulation by Aplysia hemolymph and the presence of the initial axonal segment. J Neurosci. 1983;3:2403–2413. doi: 10.1523/JNEUROSCI.03-12-02403.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H, et al. A nucleolar protein ApLLP induces ApC/EBP expression required for long-term synaptic facilitation in Aplysia neurons. Neuron. 2006;49:707–718. doi: 10.1016/j.neuron.2006.01.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.