Abstract
In daily life, memories are intertwined events. Little is known about the mechanisms involved in their interactions. Using two hippocampus-dependent (spatial object recognition and contextual fear conditioning) and one hippocampus-independent (conditioned taste aversion) learning tasks, we show that in rats subjected to weak training protocols that induce solely short term memory (STM), long term memory (LTM) is promoted and formed only if training sessions took place in contingence with a novel, but not familiar, experience occurring during a critical time window around training. This process requires newly synthesized proteins induced by novelty and reveals a general mechanism of LTM formation that begins with the setting of a “learning tag” established by a weak training. These findings represent the first comprehensive set of evidences indicating the existence of a behavioral tagging process that in analogy to the synaptic tagging and capture process, need the creation of a transient, protein synthesis-independent, and input specific tag.
Keywords: hippocampus, insular cortex, memory consolidation, protein synthesis, novelty
Acquisition of new information can be stored into at least two temporally and mechanistically different memory types: a short-term (STM) and a long-term memory (LTM). It is well known that in order for LTM to be established, synaptic changes must be stabilized by the action of newly synthesized proteins (1). This process of memory trace consolidation takes place in the brain areas where such forms of learning and memory are likely to reside. Surprisingly, recent evidence demonstrated that the supply of newly synthesized proteins may also derived from another behavioral event occurring in a relatively long-lasting associative time window, helping to promote LTM for a weak learning task that otherwise would only induce STM (2).
Current models, based on seminal ideas, propose that memories are stored by stable changes in synaptic weight modifying the activity of specific neuronal circuits (3–5). Therefore, those specific synapses activated by a given learning will require the supply of new plasticity-related proteins (PRPs) for LTM to be formed. As a consequence, there should be a mechanism that restricts the action of PRPs to recently activated synapses but not to others. To address this biological problem, it was suggested that a transient local synaptic tag is established at those recently activated synapses where PRPs are specifically captured. This idea was originally postulated by Frey and Morris (6) and it is now known as the synaptic tagging and capture (STC) hypothesis (7–9). In their seminal work they showed that early-LTP, a transient form of LTP that is induced by a weak stimulus, could be extended to late-LTP, a more persistent form of LTP, if the weak and the strong stimuli were applied in a relatively long-lasting associative time window on different synapses of the same neuron. Frey and colleagues also found that an hippocampal LTP can be reinforced by exposing rats to a novelty or to a holeboard training and this phenomenon is protein synthesis-dependent (10, 11).
Considering that LTP and some forms of LTM share a number of properties, such as associativity, durability and protein synthesis dependence (12), it is feasible that a mechanism of STC, also operates in the formation of LTM.
The main goal of the present work is to study the process of behavioral tagging using several combinations of behavioral tasks, to investigate whether a behavioral experience can extend the duration of the memory for an independent task by providing the PRPs needed for its consolidation. We found that the behavioral tagging process operates in different hippocampus- and cerebral cortex-dependent learning tasks, suggesting that it represents a general mechanism of LTM formation.
Results
To study behavioral tagging processes we subjected rats to different hippocampus-dependent or independent learning tasks, and tested whether a weak training gave rise to a LTM by the relatively long-lasting temporal association with a novel experience in a protein synthesis-dependent way.
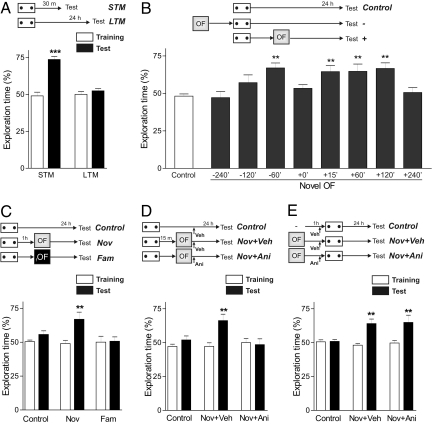
First, rats were trained in a hippocampus-dependent spatial object recognition (SOR) task, consisting of 4 min exploration of two identical objects located in a familiar arena. Fig. 1A shows that time spent exploring both objects in the training session was similar, resulting in an exploration time near 50%. In the test session, performed 30 min after this weak training protocol, animals showed STM expressed by a preferential exploration of the object switched to a new location (P < 0.001, Fig. 1A). This weak training was ineffective to yield LTM, because an independent group of rats tested 24 h later did not display a preferential exploration of the objects (P > 0.05, Fig. 1A). Next, we asked whether a LTM lasting at least 24 h could be obtained by pairing the SOR with the exploration of a novel environment. Therefore, we exposed rats to a 5 min open field (OF) session at different times before or after the weak SOR training task. OF exploration performed 1 h before the SOR training or between 15 min to 2 h after it, promoted the formation of a SOR-LTM (P < 0.01, Fig. 1B). Control animals, that were not exposed to the OF, and those groups that were exposed to the novel OF 4 or 2 h before SOR training did not express SOR-LTM (P > 0.05, Fig. 1B). The same applied to the groups that explored the novel OF immediately or 4 h after SOR training (P > 0.05, Fig. 1B). Thus, this permissive action of a spatial novelty on memory formation is restricted to a critical time window. To directly address whether the promotion of LTM was due to the novel nature of the OF environment, a group of animals was exposed to a 30 min OF session the previous day to familiarize them with the arena. In contrast to the promoting effect induced by exploration of a novel OF, no SOR-LTM formation was observed when rats explored the familiar OF 1 h after a weak SOR training (P > 0.05, Fig. 1C).
Fig. 1.
Spatial novelty promotes SOR-LTM in a protein synthesis-dependent manner. A–E show the percentage of exploration time as mean ± SEM. (A) Animals explored the arena for 4 min. Independent groups of rats were tested at 30 min (STM, n = 13) or 24 h (LTM, n = 15) after training. ***, P < 0.001 vs. training, Student's t test. (B) Control animals (n = 20) received 4 min SOR training. Animals in the OF group were exposed to a novel OF 240, 120, or 60 min before (n = 15, 17, and 17, respectively) or 0, 15, 60, 120, or 240 min after (n = 15, 20, 15, 18, and 15, respectively) SOR training. In the group + 0′, animals explore the OF immediately after the SOR training. **, P < 0.01 vs. control, Dunnet test after one-way ANOVA. (C) Animals were subjected, or not (Control, n = 20), to a novel (n = 18) or familiar (n = 15) OF 1 h after a 4 min SOR training and LTM was tested 24 h later. **, P < 0.01 vs. Control and Fam test sessions, Newman-Keuls after one-way ANOVA. (D) Animals were subjected, or not (Control, n = 20), to a novel OF 15 min after a 4 min SOR training. Experimental groups received intra-CA1 infusions of vehicle (Nov + Veh, n = 17) or anisomycin (Nov + Ani, n = 15) immediately after OF. **, P < 0.01 vs. all groups in test sessions, Newman-Keuls after one-way ANOVA. (E) Rats were tested 24 h after a weak SOR training in the absence (Control, n = 8) or in the presence of OF 1 h before training. Experimental groups received intra-CA1 infusions of vehicle (Nov + Veh, n = 8) or anisomycin (Nov + Ani, n = 7) 15 min before training. **, P < 0.01 vs. Control test session, Newman-Keuls after one-way ANOVA.
By analogy to the STC hypothesis, protein synthesis products derived from one behavioral task and used to stabilize a transient STM into LTM of another learning task reveal the existence of a behavioral tagging process. Thus, it is imperative to demonstrate that OF exploration provides the proteins necessary to consolidate SOR-LTM. For this reason, we infused the translation inhibitor anisomycin in the CA1 region of the dorsal hippocampus, immediately after the OF exploration performed 15 min after a weak SOR training. We found that the infusion of anisomycin blocked the promoting effect of OF on SOR-LTM (P < 0.01, Fig. 1D). Consistent with previous results obtained in an inhibitory avoidance task (2), de novo protein synthesis elicited by spatial novelty is necessary to promote SOR-LTM. However, the intra-CA1 infusion of anisomycin 15 min before a weak SOR training did not impair the promoting effect of novel OF exposure when given 1 h before training, suggesting that the setting of a “learning tag” by weak SOR training does not require protein synthesis (Fig. 1E).
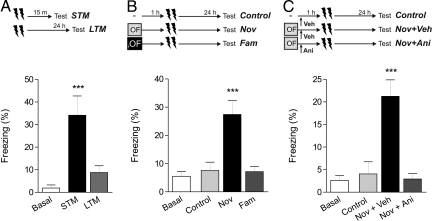
Next, we tested if LTM formation of another hippocampus-dependent memory task, the contextual fear conditioning (CFC) paradigm, could be promoted by a novel exposure to an OF. Thus, we trained rats in CFC paradigm employing a weak protocol that only induced STM. An increase in the percentage of freezing was observed when animals were tested in the same context 15 min after the training session (P < 0.001, Fig. 2A), but not observed 24 h later (P > 0.05, Fig. 2A). To test whether CFC-LTM was promoted by an exploration of a novel arena, we subjected rats to a 5 min OF session 1 h before a weak CFC training. As expected, in test session performed 24 h after training, CFC-LTM was observed only if the OF experience was novel to the animal (P < 0.001, Fig. 2B). When rats explored a familiar OF, the promoting effect on CFC-LTM was prevented (P > 0.05, Fig. 2B). We next determined whether proteins synthesized after a novel OF exposure were necessary to induce the consolidation of CFC-LTM after a weak training. We infused vehicle or anisomycin into the CA1 immediately after the OF session. One hour later, rats were trained with a weak CFC protocol and LTM tested the following day. Fig. 2C shows the promoting effect of OF exposure on CFC-LTM in animals infused with vehicle solution (P < 0.001); again, as happened with the SOR task, this effect was totally abolished by the protein synthesis inhibitor.
Fig. 2.
Spatial novelty promotes CFC-LTM in a protein synthesis-dependent manner. All data is expressed as mean ± SEM. (A) Animals received a weak training and were tested 15 min (STM, n = 7) or 24 h (LTM, n = 7) after the training session. ***, P < 0.001 vs. all groups, Newman-Keuls after one-way ANOVA (Basal, n = 7). (B) Animals were subjected, or not (Control, n = 13), to a novel (n = 13) or familiar (n = 13) OF 1 h before a weak CFC training. ***, P < 0.001 vs. all groups, Newman-Keuls after one-way ANOVA (Basal, n = 15). (C) Animals were subjected, or not (Control, n = 13), to a novel OF 1 h before a weak CFC training. Experimental groups received intra-CA1 infusions of vehicle (Nov + Veh, n = 11) or anisomycin (Nov + Ani, n = 11) immediately post-OF. ***, P < 0.001 vs. all groups, Newman-Keuls after one-way ANOVA (Basal, n = 11).
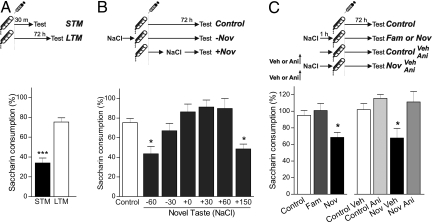
These experiments, together with others reported recently (2), strongly suggest that a mechanism of behavioral tagging operates in three different hippocampus-dependent memory tasks in rats. Several important questions arise from these results. Is this phenomenon selective for hippocampal memories? Could a cerebral cortex-dependent memory be promoted by an appropriate stimulus? Is the exploration of a novel OF effective in the promotion of a cortical-dependent memory? To answer these questions, we subjected rats to a conditioned taste aversion (CTA) paradigm, largely known to be dependent on the activation of the insular cortex (13, 14). In this task, rats associate the consumption of saccharin with the i.p. injection of a lithium chloride (LiCl) solution, which induces digestive malaise. As a consequence, there is a decrease in saccharin consumption during the test session with respect to the acquisition session intake, which is used as a measure of the aversion strength. Classically, LTM for this learning task is measured 3 days after the acquisition session. However, the injection of a low LiCl dose induced a negligible CTA-LTM, but expressed a strong STM 30 min after the acquisition session (P < 0.001, Fig. 3A). Considering that the insular cortex is involved in the acquisition and storage of taste memory traces (14), we reasoned that another gustative stimulus that activates the same cortical region may improve the consolidation of the CTA-LTM. Accordingly, at different times before or after the intake of saccharin in CTA training, rats drank a solution of sodium chloride (NaCl) (Fig. 3B). Although rats were trained using a low dose of LiCl, a strong CTA-LTM was observed when NaCl intake took place 1 h before or 2.5 h after the CTA training (P < 0.05, Fig. 3B). No effects were observed when NaCl was consumed between 30 min before to 1 h after the CTA training session (P > 0.05, Fig. 3B).
Fig. 3.
Novel taste promotes CTA-LTM in a protein synthesis-dependent manner. Saccharin consumption in the test session is expressed as mean percentage ± SEM relative to acquisition session (A) Animals were subjected to a weak CTA training session and memory was tested 30 min (STM, n = 15) or 72 h (LTM, n = 24) after acquisition. ***, P < 0.001 Student's t test. (B) Animals were subjected to a weak CTA training and LTM was tested. Rats in the novel taste groups drank 10 mL of NaCl 0.3% at 60 or 30 min before (n = 10 and 8) or 0, 30, 60, or 150 min after (n = 10, 12, 8, and 18, respectively) saccharin intake. In the group + 0′, animals drank NaCl immediately after the saccharin intake. *, P < 0.05 vs. Control, Dunnet test after one-way ANOVA. (C) Animals were subjected to a weak CTA training and LTM was tested. (Left) Animals drank, or not (Control, n = 13), a novel (n = 9) or familiar (n = 10) NaCl (0.3%) taste 1 h before saccharin intake. *, P < 0.05 vs. all groups, Newman-Keuls after one-way ANOVA. (Right) All groups received intra-insular cortex infusion of vehicle (n = 12) or anisomycin (n = 12) 75 min before saccharin intake. Half of them also drank a novel taste 60 min before drinking the saccharin solution. *, P < 0.05 vs. all groups, Newman-Keuls after one-way ANOVA.
Similarly to what is observed for SOR and CFC, stimuli familiarization has not a promoting effect on CTA-LTM. When rats drank from a NaCl solution that become a familiar taste, by consecutive administration for 3 days before training, the promoting effect of salt intake on CTA-LTM was not observed (P > 0.05, Fig. 3C Left). Next, we asked if the effect on CTA-LTM caused by pairing the novel taste consumption with a weak CTA training session was dependent on insular cortex protein synthesis. We bilaterally infused vehicle or anisomycin in this region 15 min before rats drank a novel NaCl solution and 60 min later they were trained in a CTA task. In control animals that did not drink the NaCl solution, the infusion of either vehicle or anisomycin did not affect the weak CTA-LTM (Fig. 3C Right). However, the infusion of anisomycin in the group of rats drinking NaCl blocked the promoting effect of novel salt consumption on the CTA-LTM (P < 0.05, Fig. 3C Right).
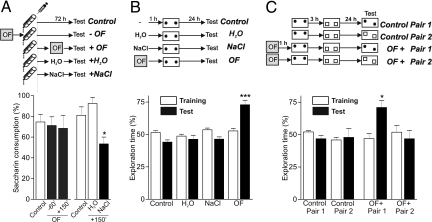
Our results suggest that several common features exist between the STC processes and the behavioral tagging. In sum, the tag setting by a weak training does not require protein synthesis and has a transient lifetime. Then, tags capture proteins synthesized by novel associated stimuli. However, the behavioral tagging hypothesis requires the integration of separate experienced stimuli arriving at a common population of neurons within a period of few hours. Based on the fact that a taste induces the activation of insular cortex and spatial information of hippocampus, we tested if a novel environment promotes CTA-LTM and if a novel taste promotes SOR-LTM. When rats were exposed to a novel 5 min OF session 1 h before or 2.5 h after a weak CTA training, the promoting effect on CTA-LTM did not occur (P > 0.05, Fig. 4A Left). Confirming our previous results (Fig. 3B), consumption of a novel taste 2.5 h after a weak CTA training, promoted CTA-LTM compared with control animals and a group of rats that drank water (P < 0.05, Fig. 4A Right). Consistent with these findings, we demonstrated that the hippocampus is not necessary for the acquisition and/or storage of CTA memory: the induction of pharmacological inactivation of the dorsal hippocampus, by local infusion of GABAA receptor agonist muscimol, 10 min before a CTA training (0.4 M LiCl) did not prevent the expression of a strong CTA-LTM [percentage of saccharin consumption for vehicle group: 30.41 ± 5.01; muscimol group: 30.62 ± 3.49, P > 0.05 Student's t test (n = 8–10)].
Fig. 4.
Novelty promotes LTM in specific inputs: regional and temporal requirements. (A) Saccharin consumption in the test session is expressed as mean percentage ± SEM relative to acquisition session. (Left) Animals were subjected to a weak CTA training session and exposed to a novel OF 60 min before or 150 min after saccharin intake (n = 8 and 12). Control animals (n = 13) did not explore the arena. P > 0.05, one-way ANOVA. (Right) Animals were subjected to a weak CTA training and drank, or not (Control, n = 13), NaCl (0.3%) (n = 11) or water (H2O, n = 7) 150 min after saccharin intake. *, P < 0.05 vs. all groups, Newman-Keuls after one-way ANOVA. (B) Rats were deprived of water for 24 h and then habituated to drink water from a graduated tube twice a day for 2 days. The next day animals received a 4 min SOR training 1 h after the consumption of water (H2O, n = 9), a novel taste (NaCl, n = 8) or the exposure to a novel open field (OF, n = 8). Control rats did not drink water (n = 8). SOR-LTM was tested 24 h after training. Exploration time is expressed as mean ± SEM. ***, P < 0.001 vs. all test sessions, Newman-Keuls after one-way ANOVA. (C) Rats were trained with two consecutive (3-h spaced) weak SOR tasks, using two different pair of identical objects at different position. 24-h later half of the animals were tested for SOR-LTM, changing the position of one object of the pair 1 (Control Pair 1, n = 8) and the rest changing one of the pair 2 (Control Pair 2, n = 7). Exploration time is expressed in percentage of mean ± SEM. P > 0.05, one-way ANOVA. Separate groups were trained as above but 1-h before they were exposed to a novel OF. Again, one group of rats was tested for pair 1 SOR- LTM (OF + Pair 1, n = 7) and the other for pair 2 (OF + Pair 2, n = 7). *, P < 0.05, vs. all groups; Newman-Keuls after one-way ANOVA.
In addition, at the time that a novel OF exploration promoted SOR-LTM (P < 0.001, Fig. 4B), neither the groups of rats that drank water or a novel taste expressed SOR-LTM (P > 0.05, Fig. 4B).
Finally, we determined whether behavioral tagging, resembling STC processes, exhibits input specificity. For that purpose, rats were trained in SOR task for 4 min using two identical objects (Pair 1) and 3 h later they were trained in the same cage to explore a different pair of identical objects (Pair 2), also for 4 min. The next day, half of the animals were tested for SOR-LTM changing the position of one object of Pair 1 and the other half of rats were tested with one of the objects of Pair 2 switched to a new position. It was not observed SOR-LTM neither for Pair 1 nor Pair 2 of objects, confirming that a weak 4 min training is not sufficient to form SOR-LTM (P > 0.05, Fig. 4C). A separate group of animals was subjected to the same procedure but in the presence of a novel OF 1 h before the first SOR training. Fig. 4C shows that SOR-LTM was observed specifically for Pair 1 (P < 0.05); the absence of LTM for Pair 2 confirms that the promoting effect of novelty on SOR-LTM lasts <4 h (see Fig. 1B). Thus, these findings indicate that behavioral tagging displays input specificity allowing LTM formation for the first but not the second learning tag.
Discussion
We have recently found that spatial novelty promotes the formation of a long lasting form of a hippocampus-dependent aversive memory in the inhibitory avoidance (IA) task (2). This effect is dependent on protein synthesis induced by the novel stimulus, suggesting that a behavioral analogue of the STC hypothesis occurs in vivo. The hypothesis was formulated to explain the phenomenon of late associativity of LTP (6, 15). This consists in the stabilization of an early-LTP, induced by a weak stimulus, into a late-LTP due to the delayed temporal association with a strong stimulus, delivered in a convergent pathway to a common neuronal population. To explain these observations, it was proposed that the activation of synapses by a weak stimulus sets a tag that propitiates the capture of proteins synthesized by a strong stimulus (6, 16, 17). Similarly, a behavioral tag phenomenon predicts that a weak learning sets a tag in those activated synapses that will eventually capture the proteins synthesized by another behavioral experience, enabling the stabilization of the mnemonic trace into a LTM.
The main contribution of the present study is the demonstration that behavioral tagging is a general process taking place in the formation of hippocampus- and cerebral cortex-dependent memories. It is based on results obtained from training rats in SOR, CFC, and CTA paradigms. SOR, CFC, and IA are hippocampus-dependent tasks (12, 18, 19) but they are different in many ways. For example, SOR involves a natural tendency toward free spatial exploration without punishment and CFC is based on the association of a foot-shock within a context, not engaging instrumental conditioning as in the IA task. In contrast, CTA depends on a different sensorial modality (taste) and on cortical areas activity (14, 20). Altogether, we observed that the exploration to a novel environment promoted the formation of both SOR and CFC-LTM for learning sessions that only induce STM. These effects depend on the novel nature of the stimulus, because a familiar environment did not exert any action on the promotion of the LTM. Similarly, in the case of CTA task, a novel but not a familiar taste transformed a weak memory for saccharin into a strong one. Importantly, in the three tasks, the effect of novel stimuli on LTM was dependent on protein synthesis because it was blocked by local infusion of anisomycin into the dorsal hippocampus for SOR and CFC, and into the insular cortex for CTA. These results support the hypothesis that novel stimuli provide PRPs that can be captured by the learning tag induced by weak trainings to allow memories to last. The results resemble the pioneering work described by Frey and Morris (6) in electrophysiological model of plasticity.
As predicted by the STC hypothesis, the action of novelty was symmetric, exerting a positive effect on LTM formation either when it was experienced before or after training sessions (17). The biphasic windows of novelty sensitivity observed for memory consolidation rely on two main features: (i) the availability of PRPs synthesized by novel stimuli that depends on the speed of their synthesis and half lives and (ii) their spatial and temporal coincidence with the transient learning tag, set by the weak training. Regarding the absence of LTM promotion by novelty occurring near training sessions and in accordance to LTP protocols (21–23), we propose that novelty given close to the acquisition session, may prevent the formation of, or eliminate the learning tag. In the SOR task a short gap was observed, where novelty disrupts the learning tag set by the SOR training. In the CTA paradigm, given the delayed association between the saccharin consumption and LiCl injection, it is reasonable to find a long gap where the novel stimulus fails to promote CTA-LTM. This fact was probably due to a late setting of the CTA learning tag and a prolonged sensitivity to the disruption by novel stimuli. Alternatively, we cannot rule out the possibility that a direct sensory interference occurs when two sets of dissimilar information are present within the temporal window of STM.
Another important requisite of the behavioral tagging hypothesis is that both events must activate a common neuronal population, where the learning tag and the PRPs can interact. It is predicted that a behavioral event has no chance to influence the LTM formation of another event, if they activate different brain structures. In this context, we assessed the prediction about the absence of a behavioral tagging process when the animal was subjected to two tasks that induce activation of different brain areas. As expected, the exploration of a novel environment (a hippocampus-dependent task) did not affect the formation of CTA-LTM (an insular cortex-dependent learning) nor did the consumption of a novel taste promoted SOR-LTM. In addition, our results demonstrated that novelty acts on specific inputs, promoting LTM for those learning tag set inside its critical temporal window of efficacy.
Further studies will be needed to elucidate the identity of the PRPs and the underlying mechanisms involved in the promoting effect of novelty on LTM formation. In this regard, 1 h after a novel exploration (Nov) the ratio of phosphorylated/total cAMP response element-binding protein (pCREB/CREB) increased in the hippocampal nuclear fraction; this effect was not observed in rats that also explored the arena the previous day (Fam) (Naive: 1,00 ± 0.16; Nov: 2,76 ± 0.39; Fam: 0.86 ± 0.17; P < 0.01 Nov vs. other groups, Newman Keuls after ANOVA, n = 7–9). CREB phosphorylation in Ser 133 serves to recruit CREB-binding protein facilitating gene transcription (24), which is probably involved in the expression of PRPs needed for the promotion of LTM by novelty. So, which are the PRPs involved in formation and persistence of LTM? Recently, it was showed that GluR1-contaning AMPARs are likely candidate molecules selectively delivered to tagged synapses by CFC training, suggesting that at the time of learning changes occur in some dendrite that allow the capture of newly synthesized AMPARs at later time points (25). Another molecule with a well established role in synaptic plasticity and memory processing is the protein kinase Mζ (PKMζ). Sajikumar et al. (26) found that persistent PKMζ activity maintains potentiated responses in hippocampal slices, not only of the strongly tetanized pathway, but also of the weakly tetanized costimulated pathway and propose PKMζ as the first LTP-specific PRP during synaptic tagging and cross-tagging processes.
In addition, several lines of evidence point to a crucial role for activity-induced brain-derived neurotrophic factor (BDNF) expression in generating sustained structural and functional changes at hippocampal synapses thought to underlie some forms of LTM (27–29). In particular, BDNF is sufficient to induce the transformation of early into late-phase LTP in the presence of protein synthesis inhibitors, and the inhibition of BDNF signaling impairs LTM formation. An interesting alternative is that BDNF-TrkB signaling is involved in synaptic tagging (30). This proposal is reasonable considering the great body of information pointing the requirement of BDNF in formation, storage and persistence of several types of memories (28, 31–33).
An implication of the present findings is that LTM formation and the stability of a given experience not only depends on the characteristics and strength of the experience but also on the prior and the future activation of the neural circuits involved in its cellular consolidation. Here, we show that in rats weakly trained in different tasks, LTMs are formed only if training sessions take place in contingence with a novel event appearing in a critical time window. This phenomenon was blocked by local injection of a protein synthesis inhibitor delivered around the exposure to the novel stimulus. Last, the inability to promote LTM in rats subjected to tasks involving the activation of different brain areas supports the notion that convergent neuronal activation is necessary for behavioral tagging to occur.
It is widely known that LTM consolidation requires the synthesis of PRPs. Our results show that the setting of a learning tag is also needed. Since they were triggered by independent behavioral events, the experimental designs used in this work clearly allow separating the tagging process from the PRP synthesis process. If there is a spatiotemporal overlapping between learning tag and PRPs, the consolidation of LTM occurs. The fact that different hippocampus and cortical-dependent tasks are capable of using PRPs synthesized by a novel experience occurring in a relatively long-lasting associative time window supports the idea that the behavioral tagging and capture process operates as a general mechanism of LTM formation. We propose that the formation of LTMs depends on two stages: an initial stage consisting on the tagging of the specific sites where the information is codified, and a later one in which PRPs are captured at those tagged sites. Our results strongly support that memories go through these processes for their consolidation, where PRPs are provided by the learning experience (if it is strong enough) or by another associated event. This new perspective in the analysis of LTM formation opens new avenues to study and understand how different experiences interact within neural circuits.
Materials and Methods
Animals.
Male adult Wistar rats (weight 180–220 g) from our own breeding colony were used. Animals were housed in groups of 6 per cage, with food and water available ad libitum, under a 12 h light/dark cycle (lights on at 7 AM) at a room temperature of 23 °C. Animals were handled for 3 min for 3 consecutive days before the experiment to avoid emotional stress. All procedures complied with the National Institutes of Health guide for care and use of laboratory animals and were approved by the Animal Care and Use Committee of Universidad de Buenos Aires.
Open Field (OF).
The apparatus is a 50 cm wide × 50 cm long × 39 cm high arena with black plywood walls and wooden floor, divided into 9 squares by black lines. A novel environment exploration consists of a 5 min OF session. To familiarize the animals with the arena, a 30 min OF session was performed one day before the experiment (34, 35).
Spatial Object Recognition (SOR).
The SOR apparatus is a 60 cm wide × 40 cm long × 50 cm high acrylic box. The floor is white as well as two of its walls, which have different visual clues. The frontal wall is transparent and the back wall is hatched. For habituation to the context, animals explored the arena without objects for 20 min once a day for 2 days. On the training day, two identical objects were included in the arena in two adjacent corners, 10 cm from lateral walls. In the training session, animals were left to explore the arena for 4 min and exploration time for each of the objects was measured. In test session, one of the objects was switched to a new position and animals were allowed to explore for to 2 min. Animals expressed SOR memory when they spent more time exploring the object in the novel position. Exploration was defined as sniffing or touching the object with the nose or forepaws. The time of exploration to each object was recorded and expressed as a percentage of the total exploration time to both objects (19).
Contextual Fear Conditioning (CFC).
Animals were placed in a Plexiglas box (30 cm wide × 25 cm long × 25 cm high with a metallic grid floor) for 2 min, then 3 consecutive shocks (0.3 mA 2 sec) were applied with a 30 sec interval. Basal freezing behavior was registered previous to the administration of the shocks. Rats were left in the conditioning chamber for another 15 sec and then placed back into their home cages. To assess CFC learning, animals were returned to the training context 15 min or 24 h posttraining, and freezing behavior was scored for 5 min. Freezing behavior was assessed with a time sampling procedure where the animal was observed during 2 sec every 5 sec, and expressed as percentage of freezing (36).
Conditioned Taste Aversion (CTA).
Animals were deprived of water for 24 h and then habituated to drink water from a graduated tube for 20 min each day for 3 days, until stable water consumption was reached. In training session, water was substituted with a 0.1% saccharin solution and 30 min later the animals were injected i.p. with 0.075 M LiCl solution (7.5 mL/kg) that did not produce a clear CAT-LTM. After 2 more days of water baseline consumption, water was once replaced by a 0.1% saccharin solution to test CTA-LTM. CTA-STM was measured 30 min after training. Saccharin consumption (in percentage) was calculated as consumption test × 100/consumption acquisition. For the induction of a strong CTA-LTM, the LiCl solution used was 0.4 M (7.5 mL/kg) (37).
As a novel taste, 10 mL of a NaCl 0.3% solution was administered at different times before or after the saccharin solution intake. To familiarize the rats to the NaCl 0.3% solution taste, they drank 10 mL of it for 3 d before the CTA acquisition session.
Surgery.
Animal canulation and drug infusion were performed as described elsewhere (2). Cannulae were implanted bilaterally by stereotaxic surgery 1.0 mm above the dorsal hippocampus (AP − 4.0, L ± 3.0 mm, V 3.0 mm relative to Bregma) or the insular neocortex (AP + 1.4 mm, L ± 5.5 mm, V 6 mm relative to Bregma).
Cannula localization was checked as described elsewhere (2). Only data from animals with correct cannula implants (95%) were included in statistical analyses.
Biochemical Procedures.
Samples of hippocampal nuclear fraction were subjected to SDS/PAGE and immunoblots for CREB and pCREB (pSer 133) were performed as described elsewhere (34).
Drugs.
Anisomycin, muscimol, and saccharin were purchased from Sigma. Eighty micrograms of Anisomycin were infused in a volume of 0.8 μL per side and dissolved in HCl, diluted in saline, and adjusted to pH 7.4 with NaOH. Muscimol was dissolved in saline up to a 10 μg/μL concentration, and 1 μg was administered per side per animal.
Acknowledgments.
We thank Jorge H. Medina and Pedro Bekinschtein for their helpful comments and discussion of the manuscript. This work was supported by grants from the Universidad de Buenos Aires para Ciencia y Tecnica and Agencia Nacional de Promoción Científica y Tecnológica (Argentina).
Footnotes
The authors declare no conflict of interest.
References
- 1.Costa-Mattioli M, Sonenberg N. Translational control of gene expression: A molecular switch for memory storage. Prog Brain Res. 2008;169:81–95. doi: 10.1016/S0079-6123(07)00005-2. [DOI] [PubMed] [Google Scholar]
- 2.Moncada D, Viola H. Induction of long-term memory by exposure to novelty requires protein synthesis: Evidence for a behavioral tagging. J Neurosci. 2007;27:7476–7481. doi: 10.1523/JNEUROSCI.1083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebb D, editor. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- 4.Konorski J, editor. Conditioned Reflexes and Neuron Organization. Cambridge, UK: Cambridge Univ Press; 1948. [Google Scholar]
- 5.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 6.Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 7.Barco A, Lopez de Armentia M, Alarcon JM. Synapse-specific stabilization of plasticity processes: The synaptic tagging and capture hypothesis revisited 10 years later. Neurosci Biobehav Rev. 2008;32:831–851. doi: 10.1016/j.neubiorev.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Frey S, Frey JU. “Synaptic tagging” and “cross-tagging” and related associative reinforcement processes of functional plasticity as the cellular basis for memory formation. Prog Brain Res. 2008;169:117–143. doi: 10.1016/S0079-6123(07)00007-6. [DOI] [PubMed] [Google Scholar]
- 9.Govindarajan A, Kelleher RJ, Tonegawa S. A clustered plasticity model of long-term memory engrams. Nat Rev Neurosci. 2006;7:575–583. doi: 10.1038/nrn1937. [DOI] [PubMed] [Google Scholar]
- 10.Straube T, Korz V, Balschun D, Frey JU. Requirement of beta-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J Physiol. 2003;552(Pt 3):953–960. doi: 10.1113/jphysiol.2003.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uzakov S, Frey JU, Korz V. Reinforcement of rat hippocampal LTP by holeboard training. Learn Mem. 2005;12:165–171. doi: 10.1101/lm.89305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izquierdo I, et al. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: Dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- 14.Bermudez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci. 2004;5:209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- 15.Reymann KG, Frey JU. The late maintenance of hippocampal LTP: Requirements, phases, ‘synaptic tagging’, ‘late-associativity’ and implications. Neuropharmacology. 2007;52:24–40. doi: 10.1016/j.neuropharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Frey U, Morris RG. Synaptic tagging: Implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- 17.Frey U, Morris RG. Weak before strong: Dissociating synaptic tagging and plasticity-factor accounts of late-LTP. Neuropharmacology. 1998;37:545–552. doi: 10.1016/s0028-3908(98)00040-9. [DOI] [PubMed] [Google Scholar]
- 18.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 19.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bermudez-Rattoni F, McGaugh JL. Insular cortex and amygdala lesions differentially affect acquisition on inhibitory avoidance and conditioned taste aversion. Brain Res. 1991;549:165–170. doi: 10.1016/0006-8993(91)90616-4. [DOI] [PubMed] [Google Scholar]
- 21.Sajikumar S, Frey JU. Resetting of “synaptic tags” is time- and activity-dependent in rat hippocampal CA1 in vitro. Neuroscience. 2004;129:503–507. doi: 10.1016/j.neuroscience.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Young JZ, Isiegas C, Abel T, Nguyen PV. Metaplasticity of the late-phase of long-term potentiation: A critical role for protein kinase A in synaptic tagging. Eur J Neurosci. 2006;23:1784–1794. doi: 10.1111/j.1460-9568.2006.04707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young JZ, Nguyen PV. Homosynaptic and heterosynaptic inhibition of synaptic tagging and capture of long-term potentiation by previous synaptic activity. J Neurosci. 2005;25:7221–7231. doi: 10.1523/JNEUROSCI.0909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chrivia JC, et al. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 25.Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–1107. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sajikumar S, Navakkode S, Sacktor TC, Frey JU. Synaptic tagging and cross-tagging: The role of protein kinase Mzeta in maintaining long-term potentiation but not long-term depression. J Neurosci. 2005;25:5750–5756. doi: 10.1523/JNEUROSCI.1104-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem. 2004;11:172–178. doi: 10.1101/lm.67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alonso M, et al. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002;12:551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka J, et al. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Christian K, Lu B. BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bekinschtein P, et al. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 32.Bekinschtein P, et al. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci USA. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moguel-Gonzalez M, Gomez-Palacio-Schjetnan A, Escobar ML. BDNF reverses the CTA memory deficits produced by inhibition of protein synthesis. Neurobiol Learn Mem. 2008;90:584–587. doi: 10.1016/j.nlm.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Moncada D, Viola H. Phosphorylation state of CREB in the rat hippocampus: A molecular switch between spatial novelty and spatial familiarity? Neurobiol Learn Mem. 2006;86:9–18. doi: 10.1016/j.nlm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Moncada D, Viola H. PKMzeta inactivation induces spatial familiarity. Learn Mem. 2008;15:810–814. doi: 10.1101/lm.1139508. [DOI] [PubMed] [Google Scholar]
- 36.Sweatt JD. Toward a molecular explanation for long-term potentiation. Learn Mem. 1999;6:399–416. doi: 10.1101/lm.6.5.399. [DOI] [PubMed] [Google Scholar]
- 37.Miranda MI, Ferreira G, Ramirez-Lugo L, Bermudez-Rattoni F. Glutamatergic activity in the amygdala signals visceral input during taste memory formation. Proc Natl Acad Sci USA. 2002;99:11417–11422. doi: 10.1073/pnas.182200499. [DOI] [PMC free article] [PubMed] [Google Scholar]