Abstract
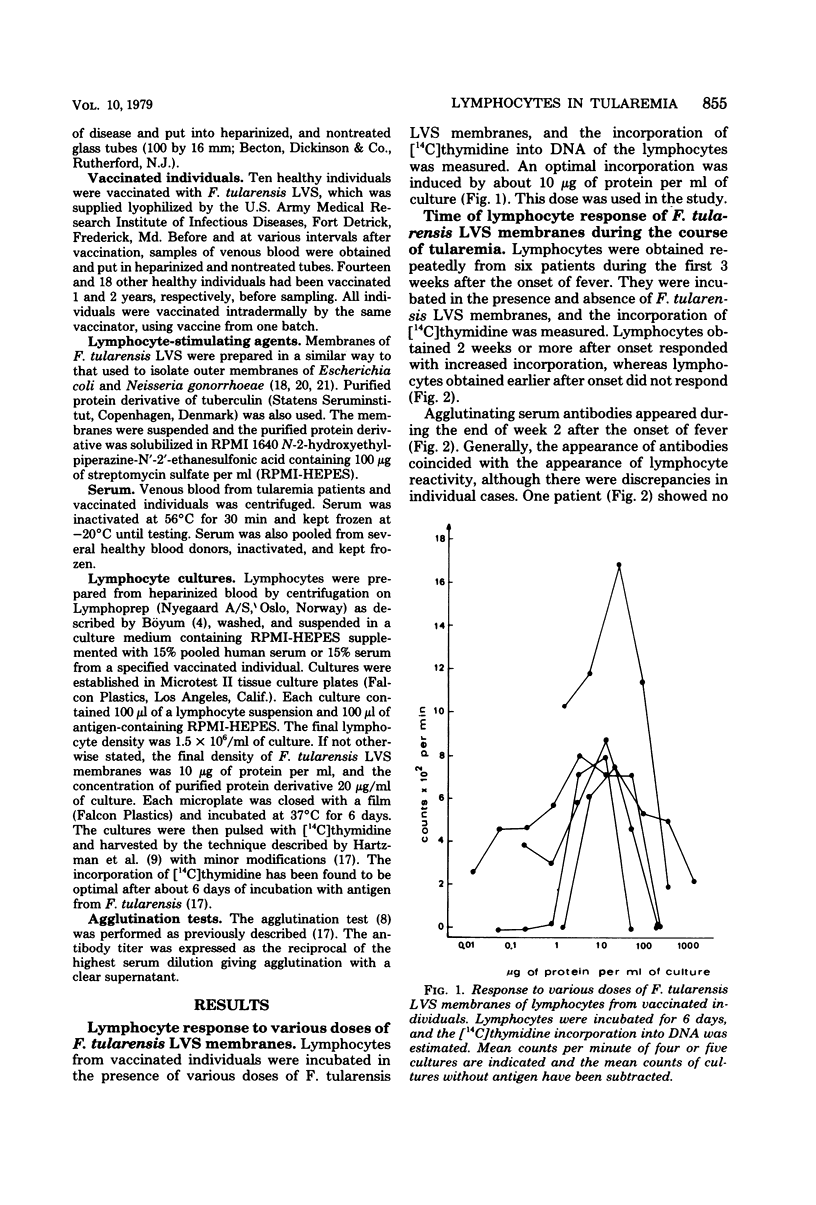
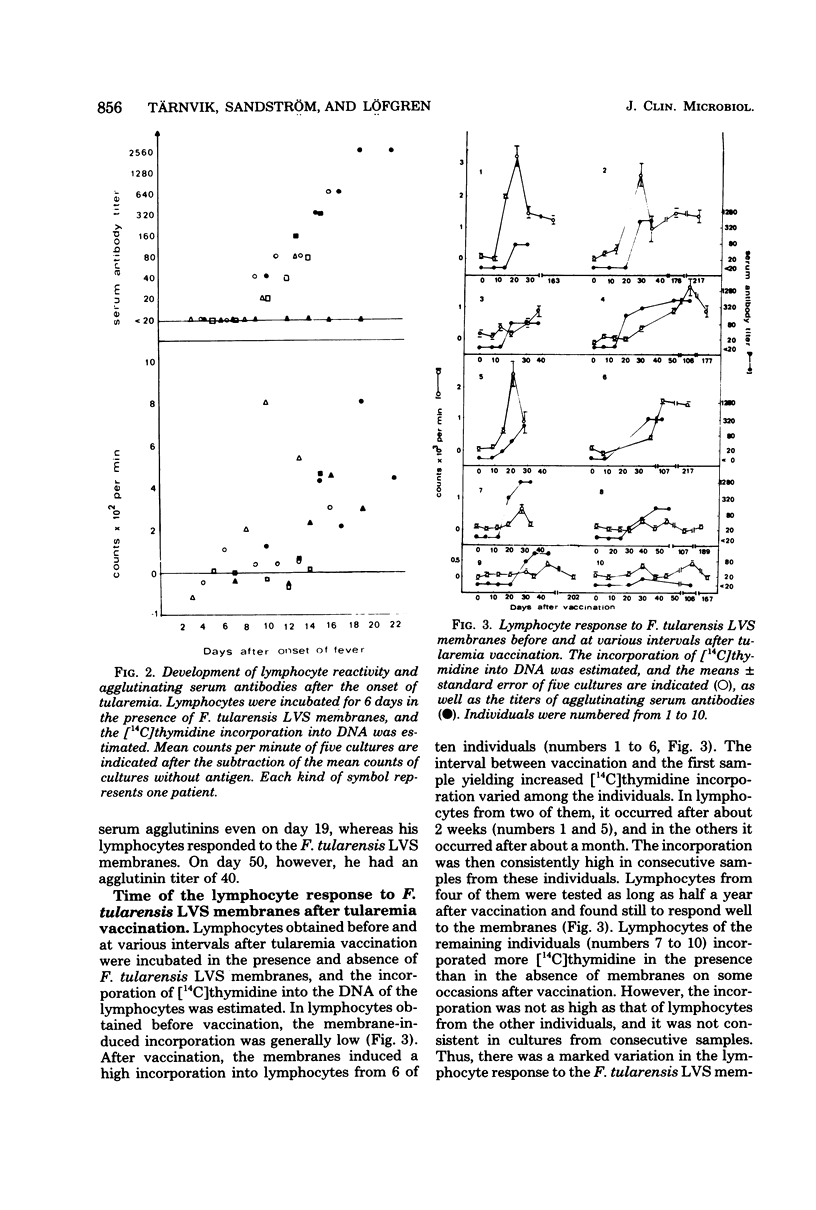
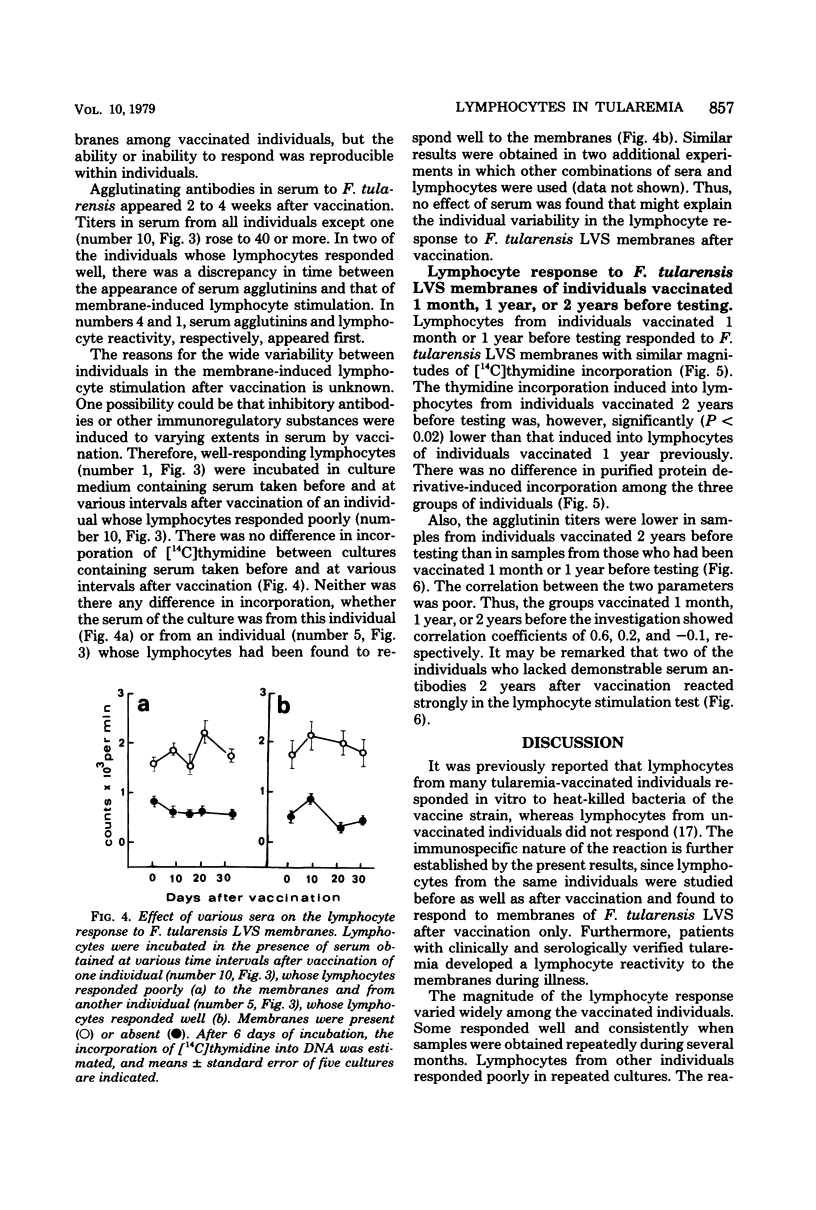
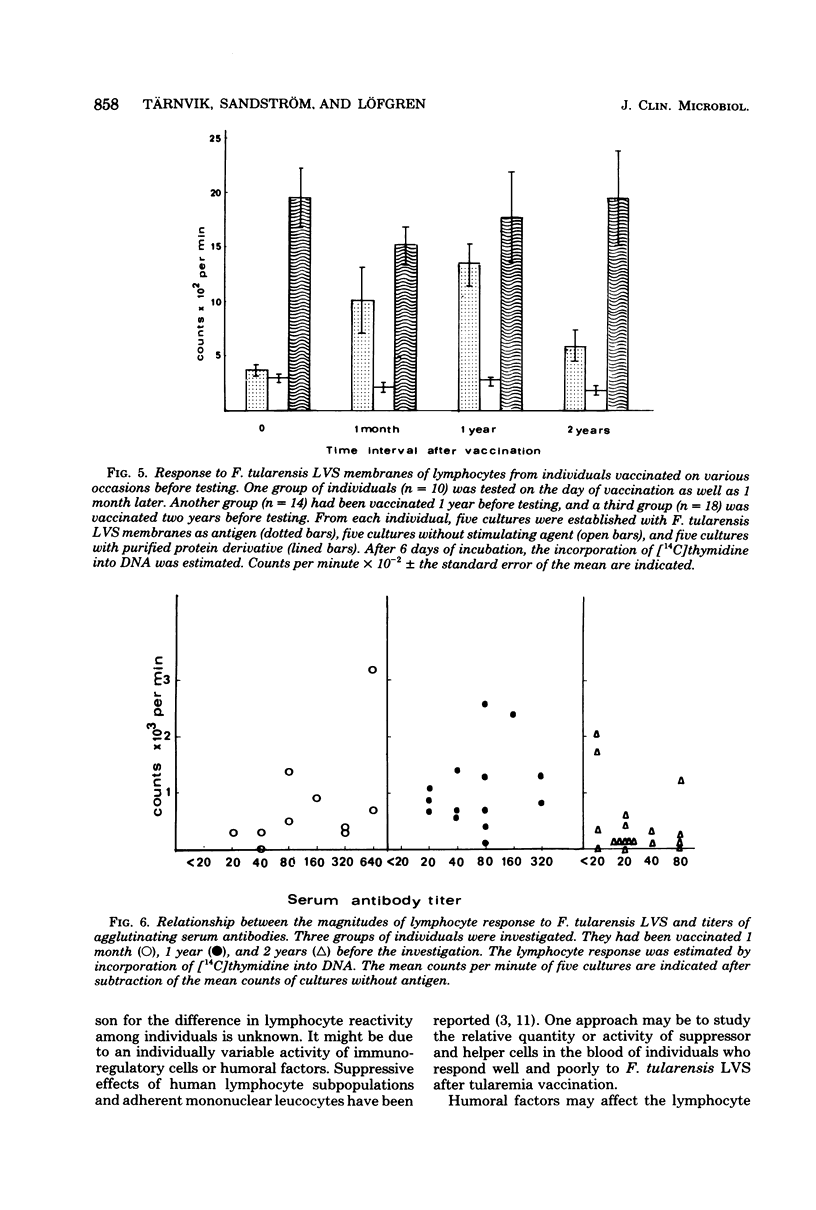
Blood lymphocytes were prepared from 6 patients at various time intervals after the onset of tularemia and from 10 subjects after vaccination against this disease. Lymphocytes were also prepared from subjects who had been vaccinated 1 and 2 years previously. The lymphocytes were incubated in the presence of membranes of the vaccine strain. Lymphocytes obtained 2 weeks or later after onset of the disease responded to the membranes with increased deoxyribonucleic acid synthesis, whereas lymphocytes obtained earlier than 2 weeks after onset did not respond. Lymphocytes of the vaccinated subjects did not respond to the membranes of the vaccine strain before vaccination. Two to 4 weeks after vaccination lymphocytes from six of the vaccinees yielded a high response, and this response was consistently high for several months. Lymphocytes from four of the vaccinated individuals responded to a low extent only, and this was consistently low for several months. Lymphocytes from individuals vaccinated 1 year before testing responded to a similar extent to the membranes, as did lymphocytes from those who had been vaccinated 1 month previously. Lymphocytes from individuals vaccinated 2 years previously, however, showed a diminished response to the membranes. There was no correlation between titer of agglutinating antibodies and magnitude of lymphocyte reactivity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN W. P. Immunity against tularemia: passive protection of mice by transfer of immune tissues. J Exp Med. 1962 Feb 1;115:411–420. doi: 10.1084/jem.115.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen V., Soborg M., Sorensen S. F. Antigen-induced lymphocyte transformation in vitro during primary immunization in man. 1. Development and course. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(4):489–494. doi: 10.1111/j.1699-0463.1971.tb03799.x. [DOI] [PubMed] [Google Scholar]
- Bona C., Chedid L. Stimulation of lymphocytes by purified protein derivative: suppression by cells from human vaccinated with Bacille Calmette-Guérin. J Infect Dis. 1976 Apr;133(4):465–468. doi: 10.1093/infdis/133.4.465. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Brooks G. F., Brachman P. S. The tularemia skin test. 325 skin tests in 210 persons: serologic correlation and review of the literature. Ann Intern Med. 1971 Mar;74(3):336–343. doi: 10.7326/0003-4819-74-3-336. [DOI] [PubMed] [Google Scholar]
- Burke D. S. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977 Jan;135(1):55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- Claflin J. L., Larson C. L. Infection-immunity in tularemia: specificity of cellular immunity. Infect Immun. 1972 Mar;5(3):311–318. doi: 10.1128/iai.5.3.311-318.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzman R. J., Bach M. L., Bach F. H., Thurman G. B., Sell K. W. Precipitation of radioactively labeled samples: a semi-automatic multiple-sample processor. Cell Immunol. 1972 Jun;4(2):182–186. doi: 10.1016/0008-8749(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Kostiala A. A., McGregor D. D., Logie P. S. Tularaemia in the rat. I. The cellular basis on host resistance to infection. Immunology. 1975 May;28(5):855–869. [PMC free article] [PubMed] [Google Scholar]
- Kurnick J. T., Bell C., Grey H. M. PHA-induced activation of suppressor cells in normal human peripheral blood lymphocytes. Scand J Immunol. 1976;5(6-7):771–778. doi: 10.1111/j.1365-3083.1976.tb03026.x. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Sigel M. M. A differential effect of IgM and IgG antibodies on the blastogenic response of lymphocytes to rubella virus. Cell Immunol. 1974 Jul;13(1):22–31. doi: 10.1016/0008-8749(74)90223-8. [DOI] [PubMed] [Google Scholar]
- Nelson D. S., Gatti R. A. Humoral factors influencing lymphocyte transformation. Prog Allergy. 1976;21:261–341. [PubMed] [Google Scholar]
- Nutter J. E., Myrvik Q. N. In vitro interactions between rabbit alveolar macrophages and Pasteurella tularensis. J Bacteriol. 1966 Sep;92(3):645–651. doi: 10.1128/jb.92.3.645-651.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkany I., Hales H. Lymphocyte transformation following B.C.G. vaccination. Br J Dermatol. 1968 Jan;80(1):29–34. doi: 10.1111/j.1365-2133.1968.tb11902.x. [DOI] [PubMed] [Google Scholar]
- THORPE B. D., MARCUS S. PHAGOCYTOSIS AND INTRACELLULAR FATE OF PASTEURELLA TULARENSIS. 3. IN VIVO STUDIES WITH PASSIVELY TRANSFERRED CELLS AND SERA. J Immunol. 1965 Apr;94:578–585. [PubMed] [Google Scholar]
- Tärnvik A., Holm S. E. Stimulation of subpopulations of human lymphocytes by a vaccine strain of Francisella tularensis. Infect Immun. 1978 Jun;20(3):698–704. doi: 10.1128/iai.20.3.698-704.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tärnvik A., Löfgren S. Stimulation of human lymphocytes by a vaccine strain of Francisella tularensis. Infect Immun. 1975 Nov;12(5):951–957. doi: 10.1128/iai.12.5.951-957.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Elmros T., Normark S., Bloom G. D. Cell envelope of Neisseria gonorrhoeae: outer membrane and peptidoglycan composition of penicillin-sensitive and-resistant strains. Infect Immun. 1975 Jun;11(6):1332–1341. doi: 10.1128/iai.11.6.1332-1341.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Normark S., Bloom G. D. Rapid method for isolation of large quantities of outer membrane from Escherichia coli K-12 and its application to the study of envelope mutants. J Bacteriol. 1973 Sep;115(3):1191–1197. doi: 10.1128/jb.115.3.1191-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



