Abstract
Worldwide a number of fish stocks have collapsed because of overfishing and climate-induced ecosystem changes. Developing ecosystem-based fisheries management (EBFM) to prevent these catastrophic events in the future requires ecological models incorporating both internal food-web dynamics and external drivers such as fishing and climate. Using a stochastic food-web model for a large marine ecosystem (i.e., the Baltic Sea) hosting a commercially important cod stock, we were able to reconstruct the history of the stock. Moreover we demonstrate that in hindsight the collapse could only have been avoidable by adapting fishing pressure to environmental conditions and food-web interactions. The modeling approach presented here represents a significant advance for EBFM, the application of which is important for sustainable resource management in the future.
Keywords: ecosystem-based fisheries management, sustainability
Atlantic cod (Gadus morhua) is among the commercially most important fish species of the European waters. Many of the stocks have declined dramatically and still remain at historically low levels (1, 2). These collapses have largely resulted from overfishing (3, 4) and climate-driven declines in productivity (5, 6). The climate effect generally works through changes in the physical environment (e.g., temperature and salinity), but also through altered food supply for early life-history stages, eventually affecting recruitment (5, 6). In accordance with this effect, recruitment failure of Eastern Baltic cod was caused mainly by high egg and larval mortalities as a result of climate-induced hydrographic change (7, 8). In several areas the collapses of cod stocks were part or major drivers of large-scale reorganizations of ecosystems (9). These so-called regime shifts are frequently caused by climatic changes (9, 10) and/or overexploitation resulting in cascading trophic interactions (11, 12). Similarly to other areas, the Baltic Sea underwent both regime shifts and trophic cascades (8, 13). Such alterations in ecosystem structure typically affect species interactions, eventually influencing food-web dynamics through both positive and negative feedback loops (14).
The recognition of the ecosystem context in the collapse of fish stocks has led to the development of more holistic ecosystem-based fisheries management (EBFM) approaches (15, 16). To be of value in decision making and management, ecosystem-based models require a manageable degree of complexity, to incorporate ecological detail, represent environmental and fishing effects, and provide estimates of uncertainty (17, 18). Statistical models have proven useful in incorporating key ecological and environmental drivers and evaluating multiple sources of uncertainty (19–21), an advantage over existing multispecies models for the Baltic Sea (22, 23). Multivariate autoregressive models [MAR(1)] provide a statistical framework for modeling food-web interactions at multiple trophic levels (14). Within this modeling framework, stochastic events such as environmental variability can be included to account for external forcing on the system's dynamics. By integrating multiple drivers and uncertainties the MAR(1) framework thus provides an important tool for EBFM. Surprisingly, it has rarely been extended beyond theory, and its application to real food webs (24, 25) has, to our knowledge, never been used in fisheries management.
We applied the MAR(1) approach to the food web of the Baltic Sea, host to one of the formerly most productive cod stocks (7). The Baltic Sea upper trophic food web is dominated by cod and two competing planktivorous fish species, herring (Clupea harengus) and sprat (Sprattus sprattus). Additionally, the species are forced top-down by fishing and bottom-up through zooplankton and environmental effects (Fig. 1). We modeled this simple food web by fitting a MAR(1) state-space model to a time series of population biomasses, fishing mortalities (F), and a number of abiotic and biotic variables, selected based on prior knowledge of their effects on fish stocks (see Table S1 and SI Text). Finally, the most parsimonious model in terms of the number of parameters and the explained variance was selected (Table S2 and Fig. S1).
Fig. 1.
A schematic view of the Baltic Sea upper-trophic food web. Black arrows and parameters represent species interactions between cod (Top), sprat (Left), and herring (Right). Gray arrows and parameters demonstrate the effects of fishing, climate, and zooplankton on the three species. Interactions with the key zooplankton species Acartia spp. (Left) and P. acuspes (Right) are illustrated by dotted arrows. Negative parameter values indicate negative effects on the biomass of the species and vice versa. Intraspecific parameters <1 indicate an increasing degree of density dependence in the population. Zero parameter values indicate interactions excluded during model selection. Note that even though it is statistically uncertain we decided to include the fishing effect on sprat and herring because they are heavily exploited by commercial fishing. Climate image is from www.ldeo.columbia.edu/res/pi/NAO.
Model Results and Validation
Model parameters of the selected model (Tables S3–S6) accurately captured the known mechanisms of species interactions within the food web and the effects of fishing, zooplankton, and environmental variability on the three species (Fig. 1). Density dependence was detected for all species. The strong effect on cod probably is caused by cannibalism (26), whereas food competition (27) and egg cannibalism may explain the effect on herring and sprat (28). Herring is additionally affected by sprat competition (27), although the opposite is not seen. Cod predation influences both prey species negatively, whereas only herring shows a significant net positive foraging effect on cod. Sprat predation on cod eggs may underlie the lack of positive effect on cod (29).
Climate-induced hydrographic change has markedly altered the Baltic Sea ecosystem (8, 13). Changes in salinity and oxygen conditions act both directly on cod recruitment (i.e., through egg and larval survival) or indirectly on the availability of Pseudocalanus acuspes, the main food source for larval cod (7). In our model, a 3-year lagged positive effect of salinity indicates a stronger direct environmental effect (Fig. 2B). The time lag corresponds to the recruitment phase of Baltic cod reaching maturity at the age of 4. Sea surface temperature (SST) lagged by 1 year strongly affects sprat (Fig. 2D), demonstrating its positive effect on recruitment (30). Although increased prey availability may indirectly add to this effect (30), the interaction with Acartia spp. did not significantly explain the variance in sprat spawning stock biomass (SSB). On the contrary, our model displays a significant effect of P. acuspes on herring (Fig. 2F). With P. acuspes being a key prey species for herring (27), this interaction indicates a stronger indirect environmental effect on herring, i.e., because P. acuspes critically depends on high salinities for reproduction (31). We found a negative effect of commercial fishing on all three species, with the largest effect on cod.
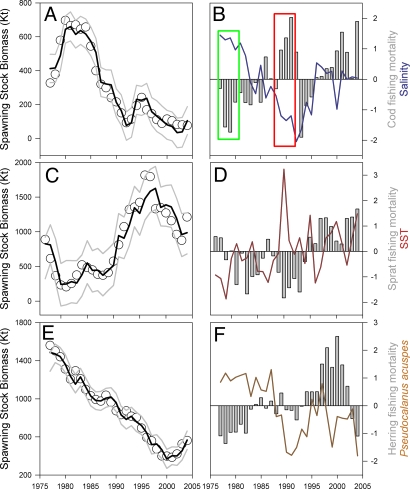
Fig. 2.
Historical development of the Baltic Sea fish populations. (A, C, and E) The fitted biomass values (black lines) represent well the observed biomasses (circles) of cod (r2 = 0.95) (A), sprat (r2 = 0.89) (C), and herring (ir2 = 0.98) (E) from 1977 to 2004. Upper and lower 95% prediction intervals are displayed by gray lines. (B, D, and F) The main external drivers, i.e., fishing mortalities and environmental variables are shown as anomalies (i.e., standardized to zero mean and unit variance) for each species. In B a combination of low fishing mortalities and record high salinities explains the sharp increase in cod during the late 1970s, i.e., the gadoid outburst (green box). On the contrary, decreasing salinities combined with high fishing pressure explain the dramatic decline and collapse of the cod stock in the early 1990s (red box).
By combining internal food-web dynamics with external forcing through fishing, zooplankton and the physical environment our fitted model clearly reproduces the observed fish stock dynamics in the Baltic Sea. These are characterized by the decrease of cod from high levels during the early 1980s (Fig. 2A), the drastic increase of sprat since the late 1980s (Fig. 2C), and a constant decline of herring (Fig. 2E). We validated our model by sequentially refitting the model for varying time periods and hindcasting the observed dynamics based on only the starting biomass values (Fig. S2). Forced by the observed time series of fishing mortality and salinity (Fig. 2B) the simulations recreate accurately both the sharp increase in cod biomass of the late 1970s, the so-called “gadoid outburst,” and the dramatic decline and collapse of the stock in the early 1990s (Fig. S2b).
The gadoid outburst in the North Sea has been explained mainly by indirect climate forcing caused by “match–mismatch” in the timing and abundance of key copepod prey for larval cod (i.e., Calanus finmarchicus and Calanus helgolandicus; ref. 5). In our model, the gadoid outburst of Baltic cod is explained mainly by a period of anomalously low fishing mortalities in combination with record high salinities (Fig. 2B), enabling successful spawning and strong recruitment caused by high egg and larvae survival in the entire Baltic Sea (7, 32). Although the ecological mechanisms differ between areas, the largely synchronous outburst of cod in the North and Baltic Seas suggests a common underlying driver. Because both variability in C. finmarchicus and C. helgolandicus and hydrographic conditions suitable for recruitment of Baltic cod (i.e., salinity and oxygen) have been strongly linked to oceanic inflow events from the North Atlantic (33, 34), climate-driven changes in large-scale circulation patterns (35, 36) seem to be the most likely driver.
Management Scenarios and Simulations
The validated model was then used to explore whether given the present knowledge on environmental forcing and species interactions, we in hindsight could have managed the cod stock in ways to avoid a collapse. To this end we applied an adaptive management approach (37), taking into account both environmental conditions and food-web interactions in deciding precautionary exploitation levels for Baltic cod. First, to derive precautionary exploitation levels (38), we performed multiple stochastic simulations over a range of fishing mortalities (F from 0 to 1.2) and salinity levels (i.e., corresponding to the observed salinity range from 1974 to 2004). The response of the cod stock was calculated as the percentage of simulations where SSB recovers above the precautionary stock level (Bpa) and the limiting stock level (Blim) in 1992 and 2004, respectively, providing a measure of the probability of stock recovery above ecologically safe levels.
In both scenarios the probability of recovery increase rapidly with decreasing F and increasing salinity levels (Fig. 3 A and C). At F levels close to 0.3 and 0.5 the probability of recovery above Bpa and Blim, respectively, approach 100% regardless of salinity level, whereas at exploitation >1.0 and 1.2 a collapse below ecologically safe levels is inevitable. It is noteworthy that the actual F before the collapse and in several recent years were well above this critical threshold (e.g., 1.41 in 1991 and 1.29 in 2004). During a period of highly unfavorable salinity conditions for spawning and recruitment (i.e., because of a lack of inflows from the North Sea) these extreme exploitation levels (Fig. 2B) no doubt caused the decline and collapse of Baltic cod (7). Furthermore, when fished according to the previously recommended reference levels, the precautionary fishing mortality (Fpa) may promote a stock recovery above Bpa only during highly favorable salinity conditions, whereas at the limiting fishing mortality (Flim) not even maximum observed salinity levels can guarantee a recovery above Blim. Hence, fixed reference levels not taking into consideration environmental forcing and indirect effects of food-web interactions seem far from precautionary and would not have promoted a recovery of the stock.
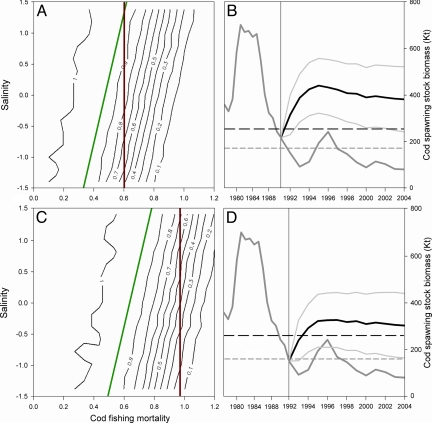
Fig. 3.
An adaptive management strategy for Baltic cod. (A and C) The probability of cod stock recovery above Bpa (A) and Blim (C) in 1992–2004 is shown over a range of fishing mortalities (F from 0 to 1.2) and salinity levels (i.e., corresponding to the observed standardized salinity range from 1974 to 2004). The red vertical lines show the recommended fixed reference levels, Fpa and Flim, and the green diagonal lines show the adaptive management approach deciding F levels based on salinity conditions. (B and D) The dark gray line represents the observed biomass and the black line represents the simulated biomass following the adaptive management strategy outlined in A and C. The simulations are initialized in 1991 (B) and 1992 (D), the years after the collapse below Bpa and Blim, respectively. Light-gray lines indicate the upper and lower 95% confidence interval of the simulations. Horizontal lines define the ecologically safe levels Bpa (long dash) and Blim (short dash) above which the stock is considered recovered.
Based on the above analysis of recovery potential at different environmental conditions we formulated our adaptive management strategy by assigning exploitation levels relative to the observed salinity conditions during 1992–2004. As such, our adaptive strategy allow F levels to increase during favorable salinity conditions, whereas during unfavorable conditions lower F levels may compensate for recruitment reductions. To ensure precautionary management (38) we applied F levels corresponding to a <5% risk of population decline below ecologically safe levels. By adopting this strategy, we initialized the simulations in 1991 and 1992, the years after the collapse below Bpa and Blim, and managed the stock forward until 2004. By running 1,000 stochastic simulations, we show that our precautionary management strategy, by adapting fishing pressure to environmental conditions and food-web interactions, could significantly have prevented the cod stock from collapsing and promoted a stock recovery above ecologically safe levels (Fig. 3 B and D).
Additionally, to demonstrate that our adaptive management strategy is essential in preventing future stock collapses we simulated a set of long-term dynamics of Baltic cod under three different management scenarios: (i) fishing mortalities fluctuate at mean historical levels (1974–2004); (ii) fishing mortalities remain fixed at Fpa = 0.6; and (iii) fishing levels are adapted to salinity conditions (i.e., F varies according to 0.6 ± 0.3). Forced under the same environmental scenario (i.e., resembling the historical range of fluctuations in SST and salinity conditions) only the adaptive management approach may prevent future stock collapses and maintain the stock stably above ecologically sustainable levels, Bpa and Blim (Fig. 4C). Although the fixed reference approach may reduce large-scale variability in SSB (Fig. 4B) compared with a more “random” harvesting scenario (Fig. 4A), it does not sufficiently buffer against climate-driven recruitment failure nor prevent recurrent stock collapses.
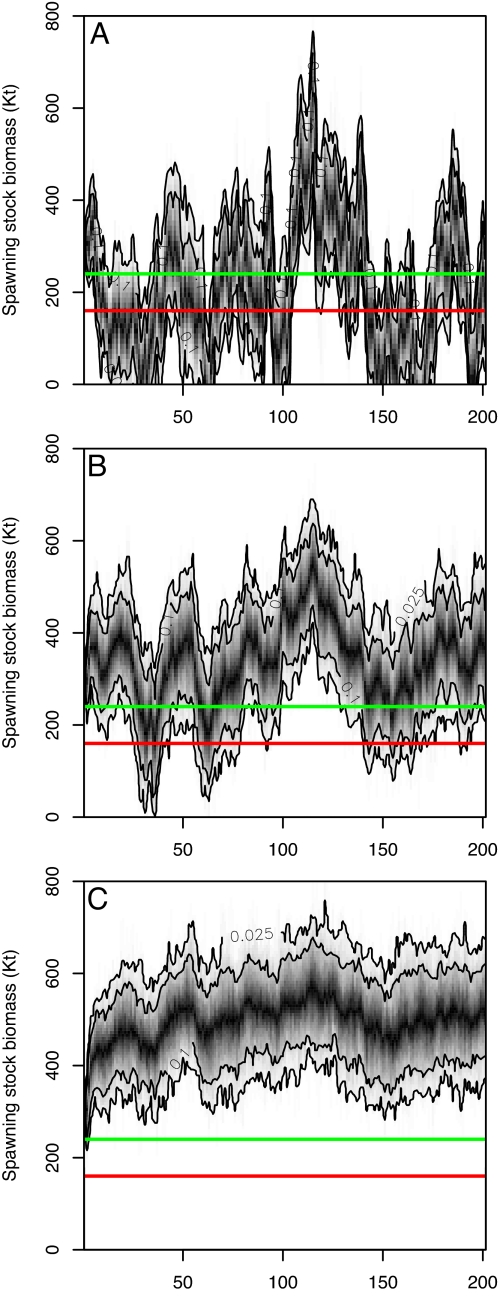
Fig. 4.
Simulated long-term dynamics of Baltic cod under different management scenarios. (A) Fishing mortalities fluctuate at mean historical levels. (B) Fishing mortalities are fixed at Fpa (0.6). (C) Fishing levels are adapted to salinity conditions (i.e., F = 0.6 ± 0.3). To resemble the historical range of fluctuations in SST and salinity, environmental conditions were simulated based on the mean, variance, and autocorrelation structure of the observed time series (1974–2004) using an approach for modeling “red-shifted,” i.e., positively autocorrelated marine environments (39, 40). Simulations were initialized at the mean historical SSB levels for each species and replicated 1,000 times including stochastic process noise. Solid horizontal lines mark the recommended ecologically safe levels of Baltic cod, the precautionary stock level, Bpa (green), and limiting stock level, Blim (red). Black contour lines show the 90% and 95% prediction intervals within which the cod stock dynamics of each replicated run fluctuates.
Bio-Economic Scenarios
In an effort to adopt a holistic management perspective we finally coupled the food-web model to a simple bio-economic model (41), aiming to compare revenues of the Baltic cod fishery across management strategies. To that end, we investigated the net present value (NPV) of the cod fishery under a fixed and adaptive management strategy. NPVs were calculated over a range of fishing mortalities (F from 0 to 1.2) and discount rates (0–15%) by simulating the yield and stock dynamics of Baltic cod over a 20-year period. Simulations were initiated at mean SSB levels for all species and run with environmental conditions (i.e., salinity and SST) fluctuating at mean historical levels.
In all simulations the adaptive management strategy (Fig. 5B) yields average NPVs above the fixed management strategy (Fig. 5A). At F levels < 0.4 the percentage difference corresponds to a net gain of ≈25–50 m€ summed over the simulated period, and as F levels rise, the net gain increase even further (Fig. 5C). As such, we can show that our adaptive approach would not only be ecologically (Fig. 4) but economically profitable because of increased landings and reduced fishing costs as the stock and hence the catchability is allowed to increase (Fig. S3). Our findings thus support the need to invest in “natural capital” (i.e., in future stock size) as a long-term management strategy for Baltic cod (41).
Fig. 5.
Economic revenue (m€) of the Baltic cod fishery. (A and B) Revenue is shown under a fixed (A) and adaptive management (B) strategy. NPVs are shown over a range of fishermen discount rates (0–15%) and fishing mortalities (F from 0 to 1.2). Fishing mortalities for sprat and herring are maintained at mean historical levels. (C) The net revenues (i.e., the percentage difference between the adaptive and fixed management scenario) are shown. The economically optimal exploitation levels (Fopt) is shown in green, and the horizontal red line marks the precautionary fishing mortality, Fpa.
Fleet overcapitalization is argued to be a serious threat to marine resources worldwide (42). One of the main reasons is conventional discounting favoring aggressive short-term harvest policies (43). In our simulations, increasing discount rates cause optimal exploitation levels (Fopt) to rise (i.e., even above recommended precautionary fishing levels, Fpa) as fishermen seek to optimize short-term profits (Fig. 5 A and C). Because increasing discount rates are largely caused by the great uncertainty perceived about future landings (44), Döring and Egelkraut (41) stress the need to reduce fishermen's long-term uncertainty by guaranteeing specific shares of total future landings and profits, e.g., by restricting the number of fishing licenses ad/or introducing a system of individual transferable quotas in the Baltic Sea cod fishery. We show here that not only traditional management advice but conventional discounting may have fueled overfishing and caused the collapse of Baltic cod. We thus reinforce the importance of establishing political and economical incentives to rebuild rather than deplete fish stocks (43).
Conclusions
The modeling approach presented here represents an important step away from traditional fisheries management practices and provides the necessary hindsight needed for developing successful management alternatives in the future. Using the Baltic cod as an example, we have demonstrated that only by adopting a holistic management approach taking into account both ecological and economic effects could we in hindsight have prevented this important fish stock from collapsing. Hence, for a future recovery of depleted fish stocks, such as Baltic cod, and an ecologically and economically sustainable fishery exploitation levels must be adapted to the full ecosystem context of the targeted species.
Materials and Methods
The Food-Web Model.
We modeled the food-web dynamics of Baltic cod, sprat, and herring by using a linear state-space approach based on the MAR(1) framework of Ives et al. (14). A MAR(1) model can be viewed as a linear approximation to a nonlinear stochastic process (14) and essentially functions as a set of lagged linear equations (one for each species) solved simultaneously to arrive at the most parsimonious model overall1 (25). Written in state-space form the MAR(1) model we used is given by:
where X is SSB of cod, sprat, and herring at time t and t − 1, and B is a 3 × 3 matrix of species interactions, hence an analogue of the “community matrix” used in food-web theory (45, 46). The covariate vector U encompass the effects of fishing, climate, and zooplankton through values of annual fishing mortalities (F) and a number of selected climate and zooplankton variables known to affect recruitment of cod, sprat, and herring (Table S1). Consequently, C is a 3 × 9 matrix whose diagonal elements specify the effect of covariates on each species. The process error E(t) is assumed to be multivariate normal and temporally uncorrelated. Likewise, the observation error of the covariance matrix of the normal random variable V(t) is assumed to be independent. Regression parameters were found by maximum-likelihood estimation using a Kalman filter (47). The Kalman filter sequentially calculates the unobserved SSB values X(t) from the previous time step by using the model formula in Eq. 1. The predictions are then updated by using the actual observed SSB values, Y(t) of the “true” observed state (Eq. 2). Model fitting was performed on time series from 1974 to 2004. Finally, the most parsimonious model in terms of the number of parameters and the explained variance was selected (Table S2) and validated (Fig. S2).
The Bio-Economic Model.
Coupled to the food-web model is a brief and simplified bio-economic model aiming to quantify the economic value of applying different exploitation levels to the Baltic cod fishery from 1992 to 2004. Based on a model by Döring and Egelkraut (41), the NPV is computed as:
 |
where δ denotes the discount rate of the fishermen, L(t) is the simulated landings, and N(t) is the simulated stock size. Because market price depends on supply (i.e., the price decrease with landings), we consider the market price, p (€/tonne) a linear function of landings. Regression parameters were calculated from the observed market price (adjusted for inflation rate for each year) and landings from 1974 to 2004. The fishing costs, c, were calculated on a fixed number of fishing licenses and include investments and running costs for 2004 (41). Fishing costs are assumed to decrease as stock biomass increase because of increased catchability and reduced fishing effort.
Supplementary Material
Acknowledgments.
We thank our colleagues at the Technical University of Denmark–National Institute of Aquatic Resources and Institute for Hydrobiology and Fisheries Science and J. Ripa for providing input and criticisms. This work was supported by the European Union Marie Curie Early Stage Research Training Project METAOCEANS (Grant MEST-CT-2005-019678) and is a contribution to the European Union 6th Framework Projects ″Understanding the mechanisms of stock recovery″ (UNCOVER, 022717) and ″Indicators for fisheries Management in Europe″ (IMAGE, 044227).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906620106/DCSupplemental.
References
- 1.Murawski SA, et al. In: Northest Atlantic Groundfish: Perspectives on a Fishery Collapse. Boreman J, et al., editors. Bethesda: American Fisheries Society; 1997. pp. 27–70. [Google Scholar]
- 2.Hutchings JA. Perspectives on a fishery collapse. Nature. 2000;406:882–885. doi: 10.1038/35022565. [DOI] [PubMed] [Google Scholar]
- 3.Cook RM, Sinclair A, Stefansson G. Potential collapse of North Sea cod stocks. Nature. 1997;385:521–522. [Google Scholar]
- 4.Myers RA, Hutchings JA, Barrowman NJ. Why do fish stocks collapse? The example of cod in Atlantic Canada. Ecol Appl. 1997;7:91–106. [Google Scholar]
- 5.Beaugrand G, Brander KM, Lindley JA, Souissi S, Reid PC. Plankton effect on cod recruitment in the North Sea. Nature. 2003;426:661–664. doi: 10.1038/nature02164. [DOI] [PubMed] [Google Scholar]
- 6.Stige LC, Ottersen G, Brander K, Chan KS, Stenseth NC. Cod and climate: Effect of the North Atlantic Oscillation on recruitment in the North Atlantic. Mar Ecol Prog Ser. 2006;325:227–241. [Google Scholar]
- 7.Köster FW, et al. Baltic cod recruitment: The impact of climate variability on key processes. ICES J Mar Sci. 2005;62:1408–1425. [Google Scholar]
- 8.Möllmann C, Muller-Karulis B, Kornilovs G, St John MA. Effects of climate and overfishing on zooplankton dynamics and ecosystem structure: Regime shifts, trophic cascade, and feedback coops in a simple ecosystem. ICES J Mar Sci. 2008;65:302–310. [Google Scholar]
- 9.Frank KT, Petrie B, Choi JS, Leggett WC. Trophic cascades in a formerly cod-dominated ecosystem. Science. 2005;308:1621–1623. doi: 10.1126/science.1113075. [DOI] [PubMed] [Google Scholar]
- 10.Hare SR, Mantua NJ. Empirical evidence for North Pacific regime shifts in 1977 and 1989. Prog Oceanogr. 2000;47:103–145. [Google Scholar]
- 11.Scheffer M, Carpenter S, de Young B. Cascading effects of overfishing marine systems. Trends Ecol Evol. 2005;20:579–581. doi: 10.1016/j.tree.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Daskalov GM, Grishin AN, Rodionov S, Mihneva V. Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proc Natl Acad Sci USA. 2007;104:10518–10523. doi: 10.1073/pnas.0701100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casini M, et al. Multilevel trophic cascades in a heavily exploited open marine ecosystem. Proc R Soc London Ser B. 2008;275:1793–1801. doi: 10.1098/rspb.2007.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ives AR, Dennis B, Cottingham KL, Carpenter SR. Community interaction webs and zooplankton responses to planktivory manipulations. Ecol Monogr. 2003;73:301–330. [Google Scholar]
- 15.Pikitch EK, et al. Ecosystem-based fishery management. Science. 2004;305:346–347. doi: 10.1126/science.1098222. [DOI] [PubMed] [Google Scholar]
- 16.Marasco RJ, et al. Ecosystem-based fishery management: Some practical suggestions. Can J Fish Aquat Sci. 2007;64:928–939. [Google Scholar]
- 17.Clark JS, et al. Ecological forecasts: An emerging imperative. Science. 2001;293:657–660. doi: 10.1126/science.293.5530.657. [DOI] [PubMed] [Google Scholar]
- 18.deYoung B, et al. Challenges of modeling ocean basin ecosystems. Science. 2004;304:1463–1466. doi: 10.1126/science.1094858. [DOI] [PubMed] [Google Scholar]
- 19.Walters CJ, Stocker M, Tyler AV, Westrheim SJ. Interaction between Pacific cod (Gadus macrocephalus) and herring (Clupea harengus pallasi) in the Hecate Strait, British Columbia. Can J Fish Aquat Sci. 1986;43:830–837. [Google Scholar]
- 20.Harwood J, Stokes K. Coping with uncertainty in ecological advice: Lessons from fisheries. Trends Ecol Evol. 2003;18:617–622. [Google Scholar]
- 21.Hjermann DO, Ottersen G, Stenseth NC. Competition among fishermen and fish causes the collapse of Barents Sea capelin. Proc Natl Acad Sci USA. 2004;101:11679–11684. doi: 10.1073/pnas.0402904101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The International Council for the Exploration of the Sea. Report of the Working Group on Multispecies Assessments of Baltic Fish. Copenhagen: International Council for the Exploration of the Sea; 1996. [Google Scholar]
- 23.Harvey CJ, Cox SP, Essington TE, Hansson S, Kitchell JF. An ecosystem model of food web and fisheries interactions in the Baltic Sea. ICES J Mar Sci. 2003;60:939–950. [Google Scholar]
- 24.Ives AR, Carpenter SR, Dennis B. Community interaction webs and zooplankton responses to planktivory manipulations. Ecology. 1999;80:1405–1421. [Google Scholar]
- 25.Hampton SE, Schindler DE. Empirical evaluation of observation scale effects in community time series. Oikos. 2006;113:424–439. [Google Scholar]
- 26.Neuenfeldt S, Köster FW. Trophodynamic control on recruitment success in Baltic cod: The influence of cannibalism. ICES J Mar Sci. 2000;57:300–309. [Google Scholar]
- 27.Möllmann C, Kornilovs G, Fetter M, Köster FW. Climate, zooplankton, and pelagic fish growth in the central Baltic Sea. ICES J Mar Sci. 2005;62:1270–1280. [Google Scholar]
- 28.Köster FW, Möllmann C. Egg cannibalism in Baltic sprat Sprattus sprattus. Mar Ecol Prog Ser. 2000;196:269–277. [Google Scholar]
- 29.Köster FW, Möllmann C. Trophodynamic control by clupeid predators on recruitment success in Baltic cod? ICES J Mar Sci. 2000;57:310–323. [Google Scholar]
- 30.MacKenzie BR, Köster FW. Fish production and climate: Sprat in the Baltic Sea. Ecology. 2004;85:784–794. [Google Scholar]
- 31.Möllmann C, Kornilovs G, Fetter M, Köster FW, Hinrichsen HH. The marine copepod, Pseudocalanus elongatus, as a mediator between climate variability and fisheries in the Central Baltic Sea. Fish Oceanogr. 2003;12:360–368. [Google Scholar]
- 32.Bagge O, Thurow F, Steffensen E, Bay J. The Baltic cod. Dana. 1994;10:1–24. [Google Scholar]
- 33.Matthaus W, Franck H. Characteristics of major Baltic inflows: A statistical analysis. Cont Shelf Res. 1992;12:1375–1400. [Google Scholar]
- 34.Reid PC, Edwards M, Beaugrand G, Skogen M, Stevens D. Periodic changes in the zooplankton of the North Sea during the 20th century linked to oceanic inflow. Fish Oceanogr. 2003;12:260–269. [Google Scholar]
- 35.Parsons LS, Lear WH. Climate variability and marine ecosystem impacts: A North Atlantic perspective. Prog Oceanogr. 2001;49:167–188. [Google Scholar]
- 36.Ottersen G, et al. Ecological effects of the North Atlantic oscillation. Oecologia. 2001;128:1–14. doi: 10.1007/s004420100655. [DOI] [PubMed] [Google Scholar]
- 37.Herrick SF, Norton JG, Mason JE, Bessey C. Management application of an empirical model of sardine-climate regime shifts. Mar Policy. 2007;31:71–80. [Google Scholar]
- 38.Garcia SM. The precautionary principle: Its implications in capture fisheries management. Ocean Coast Manag. 1994;22:99–125. [Google Scholar]
- 39.Ripa J, Lundberg P. Noise Colour and the Risk of Population Extinctions. Proc R Soc London Ser B. 1996;263:1751–1753. [Google Scholar]
- 40.Steele JH, Henderson EW. Modeling Long-Term Fluctuations in Fish Stocks. Science. 1984;224:985–987. doi: 10.1126/science.224.4652.985. [DOI] [PubMed] [Google Scholar]
- 41.Döring R, Egelkraut TM. Investing in natural capital as management strategy in fisheries: The case of the Baltic Sea cod fishery. Ecol Econ. 2008;64:634–642. [Google Scholar]
- 42.Pauly D, et al. Toward sustainability in world fisheries. Nature. 2002;418:689–695. doi: 10.1038/nature01017. [DOI] [PubMed] [Google Scholar]
- 43.Sumaila UR, Walters C. Intergenerational discounting: A new intuitive approach. Ecol Econ. 2005;52:135–142. [Google Scholar]
- 44.Hillis JP, Wheelan BJ. In: Antona M, Catanzano J, Sutinen JG, editors. Proceedings of the Sixth Conference of the International Institute of Fisheries Economics and Trade; Paris: International Institute of Fisheries Economics and Trade; 1994. pp. 657–670. [Google Scholar]
- 45.Pimm SL. Food Webs. London: Chapman and Hall; 1982. [Google Scholar]
- 46.May RM. Will a large complex system be stable? Nature. 1972;238:413. doi: 10.1038/238413a0. [DOI] [PubMed] [Google Scholar]
- 47.Harvey AC. Forecasting, Structural Time Series Models, and the Kalman Filter. Cambridge, UK: Cambridge Univ Press; 1989. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.