Foraminifers are single-celled, testate protists with a rich geologic record spanning at least 500 million years of Earth's most recent history. Their abundance, diversity, and diagnostic distribution patterns make them very useful to Earth scientists for purposes of relative age determination, correlation from one locality to another, and paleoenvironmental reconstructions. Benthic foraminifers occupy nearly every conceivable marine habitat ranging from coastal salt marshes and estuaries to marginal silled basins and deep-sea trenches. They range from pole to pole and are particularly diverse and abundant in shallow waters of the tropics. Planktic foraminifers have a shorter geologic history that begins ≈180 Ma in the late Early Jurassic. Traditional classification has assigned all of the planktic forms to a single suborder Globigerinina (1). Such a classification scheme implies a monophyletic origin for the planktic foraminifers, although many specialists have long suspected a polyphyletic origin from benthic ancestors.
In a recent issue of PNAS, Darling et al. (2) provide compelling evidence to further support the polyphyletic hypothesis, while also demonstrating a very peculiar dual mode of life for one particular species of foraminifer. Here, for the first time, Darling et al. conclusively demonstrate that the benthic Bolivina variabilis and planktic Streptochilus globigerus are one and the same species. This taxon can actively grow in seafloor sediments of a continental shelf or in surface waters of the open ocean. The existence of such a tychopelagic mode of life in foraminifers provides a glimpse at a possible mechanism by which planktic lineages may have evolved multiple times from benthic ancestors since Jurassic time.
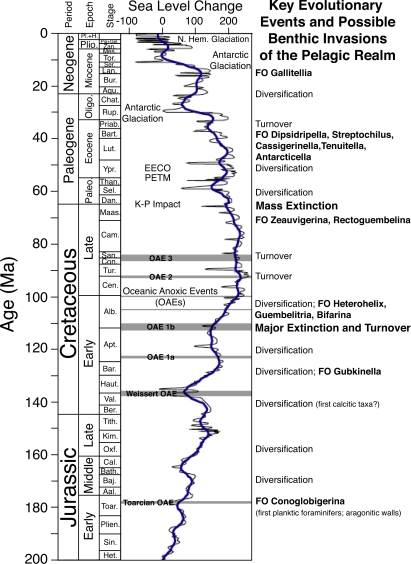
The Earth has experienced a dynamic history of global change during the Mesozoic and Cenozoic eras (past 200 million years). As the Supercontinent Pangaea broke apart, new ocean basins were created, oceanic gateways opened and others closed, new mountain belts formed, and the redistribution of land and sea altered global climate and heat dispersal around the planet. Plate tectonic activity, chemical weathering, ocean circulation, and metabolic processes of the biosphere have modulated atmospheric greenhouse gas concentrations, while orbital forcing has modulated incoming solar radiation. Importantly, global climate change has been both gradual and abrupt. During this time, foraminiferal evolution occurred in the context of global warming and cooling, growth and decay of continental ice sheets, fluctuating global sea level including the creation of vast epicontinental seas, and major events such as the Oceanic Anoxic Events (OAEs) of the Mesozoic, an era-defining bolide impact and mass extinction at the Cretaceous–Paleogene boundary (65.5 Ma), and the Paleocene–Eocene Thermal Maximum (PETM; 55 Ma). The well-known iterative pattern of planktic foraminiferal evolution (3) points to multiple turnovers in planktic foraminiferal communities with subsequent opportunities to invade and repopulate the pelagic realm (Fig. 1).
Fig. 1.
Key diversification and turnover events in the evolution of planktic foraminifers during the Mesozoic and Cenozoic eras and planktic genera (bold) suspected to be closely related to benthic ancestors. These trends are compared with changes in global sea level (7) and other events affecting the ocean–climate system. FO, first occurrence; EECO, Early Eocene Climatic Optimum. Based in part on refs. 8, 11, 12, 15, 16, 19, and 22–24.
Phytoplankton evolution accelerated during the Mesozoic as dinoflagellates, and coccolithophorids rose to dominate marine phytoplankton communities (4–6). Sea level rose during this time, peaking in the Late Cretaceous with the creation of vast epicontinental seas (7). At times, low oxygen waters of the oxygen minimum zone (OMZ) shoaled into the neritic zone and spread into epicontinental seas during global OAEs (8). This was also a time of diversification of deep-sea benthic foraminifers and the development of diagnostic “depth” assemblages along the continental margins, probably in response to changing oxygen levels and trophic resources (9, 10).
The outer continental shelf and upper slope lie at the transition from the blue water conditions of the open ocean to the brown or green water of the coasts and shelf seas (10). Here, an ample food supply from riverine sources and a rain of organic matter from the sunlit surface waters provide an optimum environment for the benthos, including the biserial benthic foraminifer B. variabilis. This transition zone lies in an area of seasonally high biological productivity and shifting water masses. In addition, the thermocline, chlorophyll maximum zone, and OMZ may intersect outer neritic–upper bathyal benthic habitats. This part of the seafloor is particularly sensitive to sea-level change and OMZ intensification. Therefore, the dynamic interface near the shelf-slope break makes this environment a likely “jumping off” platform for benthics to spring into the planktic realm, as is apparently the case for B. variabilis/S. globigerus (2).
The first planktic foraminiferal genus, Conoglobigerina, possesses a microperforate, aragonitic wall and was likely derived from an aragonitic benthic of the Family Duostominidae (11, 12). Planktic foraminifers of the Jurassic occupied shallow seas and hugged the margins of Tethys (12–14). They expanded their range in the Early Cretaceous (15), but then experienced an explosion of diversity and morphologic form during the mid-Cretaceous that continued through the Late Cretaceous (e.g., refs. 8, 13, and 16). Planktic foraminiferal diversity generally parallels global sea-level fluctuations of the Mesozoic–Cenozoic. Expanded shelf areas and shallow epicontinental seas during times of high global sea level provided increased opportunities for neritic and upper bathyal benthic foraminifers to gain access to the pelagic realm. OAEs of the Jurassic and Cretaceous may also have been important contributing factors in some benthic foraminifera evolving a planktic mode of life. Hart et al. (12) suggested that the evolution of the earliest planktic foraminifers during the late Early Jurassic may have been related to the development of an OAE in the Toarcian.
The foray into the pelagic realm by benthic foraminifers is likely to have occurred a number of times over the past 180 Myr (Fig. 1). The genus Heterohelix is a good example. The late Albian–Cenomanian Heterohelix flourished along productive continental margins and in the expanding shallow waters of epicontinental seas (17–19). This genus and its descendents diversified rapidly in the later Cretaceous to become dominant and widespread in open ocean planktic foraminiferal assemblages as evidenced by the high diversity of morphologic form and sizes, and great abundances in deep-sea pelagic sections (20, 21).
The ancestry of a number of planktic foraminiferal lineages remains a mystery. For example, the iterative origin of Mesozoic–Cenozoic planktic taxa with a microperforate wall structure is poorly known, including the mid to late Cretaceous Guembelitria, Maastrichtian–Paleocene Zeauvigerina, Eocene Cassigerinella, Tenuitella, and Antarcticella lineages, and the modern triserial Gallitellia vivans (19, 22–24); all may have had benthic ancestors. Guembelitria survived the end-Cretaceous mass extinction and gave rise to several microperforate genera during the Paleocene (25). One group that may be similar to Bolivina/Streptochilus, described by Darling et al. (2), is Zeauvigerina, another survivor of the Cretaceous–Paleogene boundary. These taxa also resemble benthic taxa, but they have stable isotopic values that are equivocal regarding a benthic or planktic mode of life (23).
A repetitive pattern is emerging: multiple invasions of the plankton by a number of different benthics, typically during times of high global sea level and perhaps initiated by a dynamic food supply or oxygen stress in the benthos or extinction in the plankton. Many of these unusual planktics existed in epicontinental seas or had restricted geographic distributions along continental margins, such as Bifarina, Tenuitella insolita, Rectoguembelina, Zeauvigerina, and Antarcticella. Others like Heterohelix (and their descendents), Guembelitria, Tenuitella, Cassigerinella chipolensis, Streptochilus, and Gallitellia became very successful in the pelagic realm, with some more common and distributed more widely than others. Fluctuating sea level and changing conditions of the OMZ are two of many factors that may have provided opportunities for benthic foraminifers to make the leap into the plankton. Changing phytoplankton community structure of the upper water column, including the diversification and rise in dominance of dinoflagellates and coccolithophorids during the Mesozoic Era, and diatoms during the Cenozoic (4–6), may have also facilitated planktic foraminiferal evolution by stimulating experimental forays into the photic zone of the upper water column.
Footnotes
The author declares no conflict of interest.
See companion article on page 12629 in issue 31 of volume 106.
References
- 1.Loeblich AR, Jr, Tappan H. Suprageneric classification of the Foraminiferida (Protozoa) Micropaleontology. 1984;30:1–70. [Google Scholar]
- 2.Darling KF, et al. Surviving mass extinction by bridging the benthic/planktic divide. Proc Natl Acad Sci USA. 2009;106:12629–12633. doi: 10.1073/pnas.0902827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frerichs WE. Evolution of planktonic foraminifera and paleotemperatures. J Paleontol. 1971;45:963–968. [Google Scholar]
- 4.Falkowski PG, et al. The evolution of modern eukaryotic phytoplankton. Science. 2004;305:354–360. doi: 10.1126/science.1095964. [DOI] [PubMed] [Google Scholar]
- 5.Katz ME, Finkel ZV, Grzebyk D, Knoll AH, Falkowski PG. Evolutionary trajectories and biogeochemical impacts of marine eukaryotic phytoplankton. Annu Rev Ecol Evol Syst. 2004;35:523–556. [Google Scholar]
- 6.Erba E. The first 150 million years history of calcareous nannoplankton: Biosphere–geosphere interactions. Palaeogeogr Palaeoclimatol Palaeoecol. 2006;232:237–250. [Google Scholar]
- 7.Haq BU, Hardenbol J, Vail PR. Chronology of fluctuating sea levels since the Triassic (250 million years ago to present) Science. 1987;235:1156–1167. doi: 10.1126/science.235.4793.1156. [DOI] [PubMed] [Google Scholar]
- 8.Leckie RM, Bralower TJ, Cashman R. Oceanic anoxic events and plankton evolution: Biotic response to tectonic forcing during the mid-Cretaceous. Paleoceanography. 2002;17:PA000623. [Google Scholar]
- 9.Sliter WV, Baker BA. Cretaceous bathymetric distribution of benthic foraminifers. J Foram Res. 1972;2:167–183. [Google Scholar]
- 10.Leckie RM, Olson HC. Foraminifera as proxies for sea-level change on siliciclastic margins. In: Olson HC, Leckie RM, editors. Micropaleontologic Proxies for Sea-Level Change and Stratigraphic Discontinuities. Tulsa, OK: Society for Sedimentary Geology; 2003. pp. 5–19. Society for Sedimentary Geology Special Publication 75. [Google Scholar]
- 11.BouDagher-Fadel MK, Banner FT, Whittaker JE, Simmons MD. The Early Evolutionary History of Planktonic Foraminifera. London: Chapman & Hall; 1997. [Google Scholar]
- 12.Hart MB, et al. The search for the origin of the planktic Foraminifera. Geol Soc London. 2003;160:341–343. [Google Scholar]
- 13.Caron M, Homewood P. Evolution of early planktic foraminifers. Mar Micropaleontol. 1983;7:453–462. [Google Scholar]
- 14.Hudson W, Hart MB, Smart CW. Palaeogeography of early planktonic foraminifera. Bull Soc Géol Fr. 2009;180:27–38. [Google Scholar]
- 15.Coccioni R, Premoli Silva I. Planktonic foraminifera from the Lower Cretaceous of Rio Argos sections (southern Spain) and biostratigraphic implications. Cretaceous Res. 1994;15:645–687. [Google Scholar]
- 16.Premoli Silva I, Sliter WV. Cretaceous paleoceanography: Evidence from planktonic foraminiferal evolution. In: Barrera E, Johnson CC, editors. The Evolution of the Cretaceous Ocean-Climate System. Boulder, CO: Geological Society of America; 1999. pp. 301–328. Geological Society of America Special Paper 332. [Google Scholar]
- 17.Eicher DL, Worstell P. Cenomanian and Turonian foraminifera from the Great Plains, United States. Micropaleontology. 1970;16:269–324. [Google Scholar]
- 18.Leckie RM. Paleoecology of mid-Cretaceous planktonic foraminifera: A comparison of open ocean and epicontinental sea assemblages. Micropaleontology. 1987;33:164–176. [Google Scholar]
- 19.Georgescu MD. On the origins of superfamily Heterohelicacea Cushman, 1927 and the polyphyletic nature of planktic foraminifera. Rev Españ Micropaleontol. 2009;41:1–38. [Google Scholar]
- 20.Nederbragt AJ. Late Cretaceous biostratigraphy and development of Heterohelicidae (planktic foraminifera) Micropaleontology. 1991;37:329–372. [Google Scholar]
- 21.Abramovich S, Keller G, Stüben D, Berner Z. Characterization of late Campanian and Maastrichtian planktonic foraminiferal depth habitats and vital activities based on stable isotopes. Palaeogeogr Palaeoclimatol Palaeoecol. 2003;202:1–29. [Google Scholar]
- 22.Liu C, Olsson RK, Huber BT. A benthic paleohabitat for Praepararotalia gen. nov. and Antarcticella Loeblich and Tappan. J Foram Res. 1998;28:3–18. [Google Scholar]
- 23.Huber BT, Olsson RK, Pearson PN. Taxonomy, biostratigraphy, and phylogeny of Eocene microperforate Planktonic foraminifera (Jenkinsina, Cassigerinelloita, Chiloguembelina, Streptochilus, Zeaurvigerina, Tenuitella, and Cassigerinella) and Problematica (Dipsidripella) In: Pearson PN, Olsson RK, Huber BT, Hemleben C, Berggren WA, editors. Atlas of Eocene Planktonic Foraminifera. Washington, DC: Cushman Foundation; 2006. pp. 461–508. Cushman Foundation Special Publication 41. [Google Scholar]
- 24.Ujiié Y, Kimoto K, Pawlowski J. Molecular evidence for an independent origin of modern triserial planktonic foraminifera from benthic ancestors. Mar Micropaleontol. 2008;69:334–340. [Google Scholar]
- 25.Olsson RK, Hemleben C, Berggren WA, Huber BT, editors. Atlas of Paleocene Planktonic Foraminifera. Washington, DC: Smithsonian Institution Press; 1999. [Google Scholar]