Abstract
Activation of the NF-κB pathway in T cells is required for induction of an adaptive immune response. Hematopoietic progenitor kinase (HPK1) is an important proximal mediator of T-cell receptor (TCR)-induced NF-κB activation. Knock-down of HPK1 abrogates TCR-induced IKKβ and NF-κB activation, whereas active HPK1 leads to increased IKKβ activity in T cells. Yet, the precise molecular mechanism of this process remains elusive. Here, we show that HPK1-mediated NF-κB activation is dependent on the adaptor protein CARMA1. HPK1 interacts with CARMA1 in a TCR stimulation-dependent manner and phosphorylates the linker region of CARMA1. Interestingly, the putative HPK1 phosphorylation sites in CARMA1 are different from known PKCθ consensus sites. Mutations of residues S549, S551, and S552 in CARMA1 abrogated phosphorylation of a CARMA1-linker construct by HPK1 in vitro. In addition, CARMA1 S551A or S5549A/S551A point mutants failed to restore HPK1-mediated and TCR-mediated NF-κB activation and IL-2 expression in CARMA1-deficient T cells. Thus, we identify HPK1 as a kinase specific for CARMA1 and suggest HPK1-mediated phosphorylation of CARMA1 as an additional regulatory mechanism tuning the NF-κB response upon TCR stimulation.
Keywords: CBM complex, IKK, TCR, PKC
Induction of an adaptive immune response depends on activation of the NF-κB pathway in lymphocytes (1–3). NF-κB activation in response to antigen receptor ligation is mediated by the IκB kinase (IKK) complex (4, 5), which comprises two enzymatic subunits, IKKα and IKKβ, and IKKγ (NEMO) (6). Activation of the IKK complex upon T-cell receptor (TCR) stimulation depends on the caspase-recruitment domain (CARD)-containing signaling adaptor protein CARMA1 (CARD11) (7). TCR stimulation leads to protein kinase C (PKC) θ activation which in turn phosphorylates CARMA1 within its linker region between the coiled-coil and the PDZ domains (8–10). Subsequently, CARMA1 interacts with BCL-10 and MALT1 (11). The MALT1-interacting E3 ubiquitin ligase TRAF6 triggers Lys-63-linked polyubiquitinylation of IKKγ and causes activation of the IKK complex (12). Loss of CARMA1 causes profound defects in NF-κB activation (13–18). Thus, the CARMA1-BCL-10-MALT1 (CBM) complex is central for activation of the IKK complex in lymphocytes (6).
A molecular link between TCR stimulation and NF-κB activation in T cells is the hematopoietic progenitor kinase 1 (HPK1). HPK1 comprises an N-terminal kinase domain and a C-terminal citron homology (CNH) domain (19). Antigen receptor cross-linking leads to activation of HPK1 in T and B cells (20, 21). HPK1 activation involves relocation to the plasma membrane, autophosphorylation and transphosphorylation by protein kinase D1 (22). HPK1 activities are implicated in monocytic differentiation (23) and in negative regulation of the immune response involving phosphorylation of SLP-76 and binding of 14–3-3 proteins (24, 25). HPK1 has also been linked to T-cell apoptosis (26). Upon activation and expansion of T cells cleavage of HPK1 separates the kinase domain, HPK1-N, from the C terminus, HPK1-C, which leads to sensitization toward activation-induced cell death (27, 28). Full length HPK1 selectively activates the JNK and the NF-κB pathways (29, 30). HPK1 interacts with IKKβ in T cells and mediates phosphorylation of IKKβ and activation of NF-κB upon TCR stimulation (27). By controlling the NF-κB pathway HPK1 plays a central role in life and death decisions in T cells (31, 32). However, the molecular details of HPK1-mediated IKK activation are still elusive.
Here, we show that HPK1 is a regulator of TCR-mediated NF-κB activation and that NF-κB activation via HPK1 is dependent on CARMA1. HPK1 interacts with CARMA1 in a TCR stimulation-dependent manner. Upon TCR stimulation HPK1 phosphorylates CARMA1 within its linker region. HPK1-mediated CARMA1 phosphorylation involves the non-PKCθ consensus residues S549 and S551. A mutant of CARMA1 lacking the putative phosphorylation site S551 failed to trigger HPK1- and TCR-mediated NF-κB activation and IL-2 expression. Our results indicate that CARMA1 serves as a physiological substrate for HPK1 upon TCR stimulation.
Results
HPK1 Is Specific for TCR-Mediated NF-κB Activation and Depends on CARMA1.
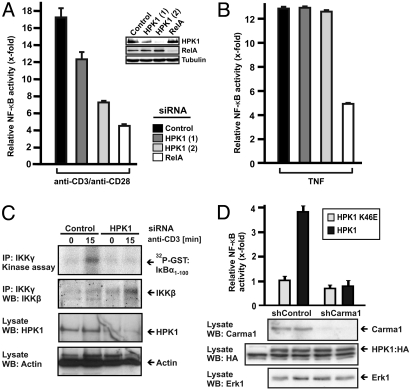
HPK1 is contributing to activation of the IKK complex upon TCR stimulation (27). To investigate the mechanism through which HPK1 mediates NF-κB activation, we compared TCR-mediated and TNF-mediated NF-κB activation in Jurkat T cells. Clearly, siRNA-mediated knock-down of HPK1 by different siRNAs prevented TCR-specific NF-κB activation in a dose dependent manner (Fig. 1A). However, knock-down of HPK1 did not affect TNF-mediated NF-κB activation (Fig. 1B). This result indicates that the presence of HPK1 specifically mediates NF-κB activation upon TCR stimulation. To substantiate this finding, we performed siRNA-mediated knock-down in primary human T cells and analyzed activation of the IKK complex by an in vitro kinase assay using GST: IκBα as a substrate (Fig. 1C). Again, knock-down of HPK1 led to a loss of TCR-mediated IKK activation. This confirms HPK1 to be a critical mediator of NF-κB activation upstream of the IKK complex.
Fig. 1.
HPK1 is a critical regulator of TCR-mediated NF-κB activation and depends on CARMA1. (A and B) Jurkat T cells harboring a stably integrated NF-κB-specific luciferase reporter gene were transfected with HPK1-specific, RelA-specific, or non-targeting siRNA oligonucleotides. Seventy-two hours later cells were stimulated with 10 μg/mL plate bound anti-CD3/anti-CD28 or 0.8 nM TNF for 8 h and NF-κB activation was measured in triplicates to calculate mean values and standard deviations. (C) Primary human T cells were transfected with HPK1-specific or non-targeting siRNA oligonucleotides. Forty-eight hours later, cells were stimulated or not with anti-CD3. Endogenous IKKγ proteins were immunoprecipitated and the activity of the coprecipitated IKK complex was measured by in vitro transphosphorylation of recombinant GST:IκBα using 32P-γ-ATP. Endogenous IKKβ, HPK1 and actin was controlled by western blot analysis (WB). The experiment was done three times with similar outcome. (D) Jurkat T cells with stable expression of control shRNA (shControl) or CARMA1-specific shRNA (shCARMA1) were transfected with a NF-κB-specific reporter plasmid and expression plasmids encoding for HPK1:HA or kinase-inactive HPK1(K46E):HA. Forty-eight h later cells were lysed and NF-κB activity was measured. Standard deviation refers to triplicate measurements. Expression levels of CARMA1, HPK1, and Erk1 were visualized by WB. The experiments were repeated at least three times with similar outcome.
Activation of the IKK complex upon TCR stimulation requires the adaptor protein CARMA1. To delineate the requirement for CARMA1 in HPK1-mediated NF-κB activation we used Jurkat T cells in which we silenced CARMA1 expression by shRNA. While expression of kinase active HPK1 induced NF-κB in control Jurkat T cells, HPK1 failed to induce NF-κB in CARMA1-silenced Jurkat T cells (Fig. 1D). This result implies that signaling from HPK1 to NF-κB depends on CARMA1 and that HPK1 and CARMA1 are crucial for NF-κB activation.
HPK1 Interacts with CARMA1 in a TCR Stimulation-Dependent Manner.
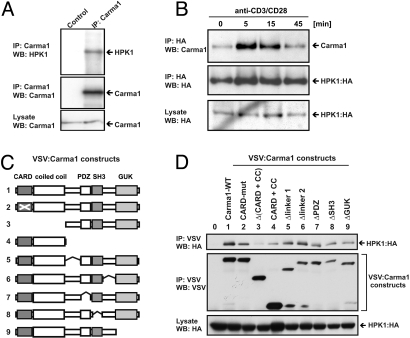
The previous result has shown a functional dependency of HPK1 on the presence of CARMA1. Therefore, we investigated a potential interaction of both proteins. Interestingly, endogenous CARMA1 coprecipitated with endogenous HPK1 (Fig. 2A). This demonstrates a constitutive association of endogenous HPK1 and CARMA1 in T cells.
Fig. 2.
HPK1 interacts with CARMA1 in a TCR stimulation-dependent manner. (A) Jurkat T cells were used to immunoprecipitate (IP) endogenous CARMA1 using anti-CARMA1 abs and tested for the presence of coimmunoprecipitated endogenous HPK1 by anti-HPK1 WB. A non-precipitating rabbit serum was used as control. (B) Jurkat T cells expressing HA-tagged HPK1 were stimulated with anti-CD3/anti-CD28, lysed and used to coimmunoprecipitate endogenous CARMA1 using anti-HA. Presence of CARMA1 and HPK1 is shown by WB. (C) Overview of wild type (WT) and truncated VSV-tagged CARMA1 proteins. Numeration of constructs corresponds to D. (D) 293T cells were used to express HPK1:HA alone or in combination with VSV- tagged wild type (WT) CARMA1 or various CARMA1 constructs. The region deleted (Δ) within a construct is indicated. The CC + CARD construct consist only of the coiled-coil and the CARD domain. Cells were lysed and VSV-tagged CARMA1 proteins were immunoprecipitated using anti-VSV-G agarose conjugate. The presence of HPK1 and CARMA1 proteins was visualized by WB.
Next we tested, if TCR stimulation would influence the interaction of HPK1 and CARMA1. We found increased association of HPK1 and CARMA1 after 5 and 15 min of TCR stimulation, which thereafter declined to basal level (Fig. 2B). This stimulation- dependent increase in HPK1-CARMA1 complex formation slightly precedes activation of the IKK complex upon TCR stimulation (27) and suggests a molecular mechanism for HPK1-mediated NF-κB activation in T cells.
To characterize this interaction in more detail, we expressed HPK1 and CARMA1 in epithelial cell lines and found a specific interaction of HPK1 and CARMA1, which was confined to the C-terminal region of HPK1 (data not shown). Next we investigated the interaction of HPK1 with various VSV-tagged constructs of CARMA1 (Fig. 2C). While most of the CARMA1 constructs coimmunoprecipitated HPK1 similar to full-length CARMA1, simultaneous deletion of the coiled-coil motive (ΔCC) and the CARD domain (ΔCARD) clearly impaired interaction with HPK1 (Fig. 2D). Conversely, the coiled-coil and CARD domains alone were still sufficient to pull down low amounts of HPK1. Thus, the HPK1 CARMA1 interaction is mainly mediated by the coiled-coil motive and CARD domain of CARMA1; however, other regions of CARMA1 seem to contribute.
HPK1 Phosphorylates CARMA1 upon TCR Stimulation.
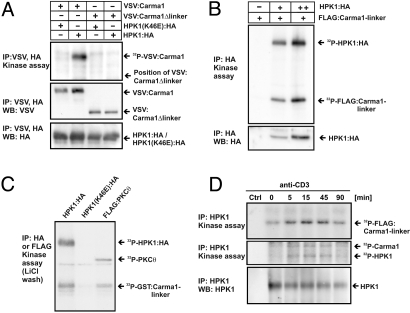
The linker region of CARMA1 separates the CARD and coiled-coil domains from the MAGUK-domain and has to be phosphorylated as a prerequisite for NF-κB activation (8, 10). To investigate whether HPK1 phosphorylates CARMA1, we coexpressed CARMA1 and HPK1 together in 293T cells, immunoprecipitated simultaneously VSV-tagged CARMA1 and HA-tagged HPK1, and performed a kinase assay to test for CARMA1 phosphorylation. Of note, exogenous expression of HPK1 leads to its autocatalytic activation by induced-proximity, which resembles physiological activation of HPK1 upon aggregation and translocation to the immune synapse (20–22). Interestingly, phosphorylation of full length CARMA1 was strongly enhanced in the presence of HPK1, but a CARMA1 construct devoid of the linker region (CARMA1Δlinker) did not serve as a substrate for HPK1 (Fig. 3A). This result implies that HPK1 phosphorylates the linker region of CARMA1. Remarkably, CARMA1 was not phosphorylated in the presence of kinase-inactive HPK1(K46E), which makes unintended coprecipitation of a kinase different from HPK1 unlikely. Thus, HPK1 can be considered as a CARMA1 kinase that phosphorylates CARMA1 depending on the presence of its linker region.
Fig. 3.
HPK1 phosphorylates the CARMA1-linker upon TCR stimulation. (A) VSV-tagged CARMA1 or CARMA1Δlinker were coexpressed with HPK1(K46E):HA or HPK1:HA in 293T cells and immunoprecipitated simultaneously using anti-HA and anti-VSV and subjected to an in vitro kinase assay using 32P-γ-ATP. Phosphorylation of CARMA1 proteins was visualized by autoradiography. (B) Increasing amounts of HPK1:HA were expressed in COS1 cells, immunoprecipitated and subjected to an in vitro kinase assay using 32P-γ-ATP. Purified FLAG:CARMA1-linker (2.5 μg) were added as substrate. Phosphorylation of proteins was visualized by autoradiography. Loading was controlled by WB. (C) HPK1:HA, HPK1(K46E):HA, or FLAG:PKCθ were expressed in 293T cells and immunoprecipitated using anti-HA or anti-FLAG. Immunoprecipitated material was washed three times using 0.5 M LiCl to exclude unspecific bound kinases and subjected to an in vitro kinase assay using 32P-γ-ATP and recombinant GST:CARMA1-linker as a substrate. Phosphorylation was visualized by autoradiography using enhancer plates. The precipitated proteins were below the detection limit of WB. (D) Jurkat T cells were stimulated using anti-CD3, lysed and endogenous HPK1 was immunoprecipitated with anti-HPK1. The capacity of HPK1 to trans-phosphorylate recombinant FLAG:CARMA1-linker as well as endogenous CARMA1 was measured by an in vitro kinase assay and visualized by autoradiography. A non-precipitating antibody was used to control for specificity (Ctrl). Equal HPK1 immunoprecipitation was controlled by WB.
To confirm that HPK1 directly phosphorylates the linker region of CARMA1 we cloned, expressed, and purified FLAG or GST-tagged CARMA1-linker constructs as substrates for in vitro kinase reactions. As expected, the recombinant FLAG-tagged CARMA1-linker served as a phosphorylation substrate for HPK1 (Fig. 3B). The kinase-inaktive HPK1 (K46E) did not phosphorylate the CARMA1-linker (data not shown). To exclude the presence of an accidentally copurified kinase besides HPK1 which might phosphorylate the CARMA1-linker, we repeated the experiment with HPK1:HA, HPK1(K46E):HA or FLAG:PKCθ and subjected the immunoprecipitated proteins to a harsh washing procedure using 0.5 M LiCl. Remarkably, HPK1 as well as PKCθ still showed phosphorylation of a recombinant GST-tagged CARMA1-linker substrate under these conditions (Fig. 3C). After LiCl washing, most of the immunoprecipitated proteins were lost and detection of HPK1 or PKCθ was only possible by autoradiography. Therefore, it is highly unlikely that any kinase different from HPK1 was coimmunoprecipitated in this experiment. These results show that HPK1 phosphorylates the linker region of CARMA1 directly and suggest that this phosphorylation is involved in HPK1-mediated NF-κB activation.
Next, we asked whether HPK1 mediates stimulation-dependent phosphorylation of the linker region of CARMA1, which was reported to be a prerequisite for NF-κB activation (9). To test whether endogenous HPK1 phosphorylates the CARMA1-linker, we performed TCR stimulation and subjected the precipitated HPK1 to a kinase assay with recombinant CARMA1-linker protein. HPK1 activation increases sharply after TCR stimulation in Jurkat T cells (Fig. 3D, middle panel) and leads to phosphorylation of recombinant CARMA1-linker (Fig. 3D, top panel). The stimulation-dependent kinase activity of HPK1 runs in parallel to coimmunoprecipitation of phosphorylated CARMA1 (Fig. 3D, middle panel and data not shown). This result shows that HPK1 phosphorylates the linker of CARMA1 upon TCR stimulation and confirms coprecipitation of endogenous CARMA1 with HPK1 (Fig. 2B).
HPK1 Phosphorylates CARMA1 at Sites Critical for NF-κB Activation.
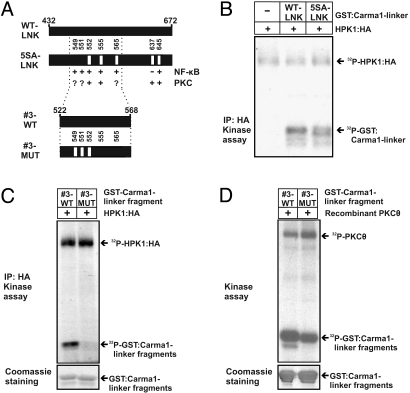
To further delineate HPK1-dependent phosphorylation sites, we generated a mutated GST:CARMA1-linker construct (5SA-LNK) containing 5 amino acid exchanges (S552A, S555A, S565A, S637A, and S645A) within the linker region of CARMA1 (Fig. 4A). These sites were previously reported to be critical for PKCθ-mediated NF-κB activation (8, 10). In an in vitro kinase assay HPK1 phosphorylated the mutated CARMA1-linker construct 5SA-LNK and the wild-type CARMA1-linker (WT-LNK) to similar extents (Fig. 4B). This implies that HPK1 phosphorylates residues within the CARMA1-linker different from those which have been mutated. Of note, mutation of the critical PKCθ-specific residue S552 within construct 5SA-LNK still led to significant HPK1-mediated phosphorylation of the CARMA1 linker. Thus, it is likely that other residues than S552 serve as HPK1-dependent phosphorylation sites.
Fig. 4.
HPK1 phosphorylation sites in CARMA1 are implicated in NF-κB activation, but differ from the known PKCθ consensus sites. (A) Overview of the mutations located within the CARMA1-linker. Shown are the positions of the indicated point mutations within wild type full length CARMA1-linker (WT-LNK) or its mutant bearing the point mutations S552A, S555A, S565A, S637A, S645A (5SA-LNK), or wild-type CARMA1-linker fragment #3 (#3-WT) or its mutant bearing the point mutations S549A, S551A, and S552A (#3-MUT). The involvement of PKCθ in phosphorylation or the impact on NF-κB activation is indicated for each residue by ‘plus’ or ‘minus’ (9). A question mark indicates that no kinase was described yet to phosphorylate the respective residue. Given numbers refer to the amino acid positions of human CARMA1. (B and C) HPK1:HA was expressed in COS1 cells and immunoprecipitated via anti-HA. As substrates for HPK1:HA the GST fusion proteins described in A were expressed, purified and added to an in vitro kinase assay using 32P-γ-ATP. Equal loading of the GST fusion proteins was controlled by Coomassie staining. (D) The experiment was carried out as in (C) but recombinant PKCθ was used to control for phosphorylation of the PKCθ-specific residues S552 and S555 in fragment #3-WT and S555 in fragment #3-MUT.
To further delineate the localization of potential HPK1 phosphorylation sites, we used serially truncated fragments of the CARMA1-linker as substrates in an in vitro kinase assay and found that a central fragment within the CARMA1-linker, termed fragment #3-WT composed of the AS 522–568, was phosphorylated by HPK1 (data not shown). Fragment #3-WT contains the phosphorylation sites S551 and S549 which were previously suggested to play a role in NF-κB activation but did not correspond to PKCθ target sites (8) and could, thus, represent potential HPK1 target sites (Fig. 4A). To test this possibility, we introduced triple point mutations (S549A, S551A, and S552A) into fragment #3-WT to generate the mutated fragment #3-MUT and subjected both the wild-type and the mutated fragment to an in vitro kinase assay with HPK1 (Fig. 4C). In this context the triple mutation abolished phosphorylation by HPK1. In contrast, PKCθ, which can phosphorylate S552 and S555 (9), still phosphorylated both fragments, although the #3-MUT fragment to a lower extent (Fig. 4D). This indicates that positions S552 and S555 within fragment #3-WT and S555 within fragment #3-MUT could serve as PKCθ phosphorylation sites, while HPK1 might phosphorylate S549 and/or S551.
CARMA1 S551A Fails to Support HPK1-Mediated NF-κB Activation.
To show an involvement of S549 or S551 in HPK1-mediated NF-κB activation we mutated both residues individually in full-length CARMA1 and expressed HPK1 or PKCθ in the presence or absence of wild-type or mutated CARMA1 proteins together with an NF-κB-specific reporter plasmid in 293T cells. While HPK1 alone already led to induction of NF-κB, co-expression of wild-type (WT) CARMA1 strongly enhanced NF-κB activation (Fig. S1A). Interestingly, the CARMA1 mutant S549A did also support HPK1-mediated NF-κB activation, while the CARMA1 mutant S551A completely failed to induce NF-κB activation by HPK1. However, PKCθ was able to mediate NF-κB activation via CARMA1 independently of S549 or S551 (Fig. S1B). This result suggests that HPK1-mediated, but not PKCθ-mediated NF-κB activation is dependent on S551 of CARMA1. These findings are consistent with the data of Matsumoto et al., which showed that PMA/CD28-mediated NF-κB activation was largely unaffected by single mutations of S549 or S551 (8). PMA/CD28 bypasses TCR-proximal signaling and, therefore, does not activate HPK1. Hence, no conclusions about the contribution of HPK1 relative to PKCθ can be drawn from the study mentioned above. To investigate a potential overlapping role of these two kinases, we transiently expressed CARMA1 with HPK1 or PKCθ alone or in combination in 293T cells and measured NF-κB activation by cotransfection of a luciferase reporter gene. In line with our previous data HPK1 and PKCθ alone increased CARMA1-dependent NF-κB activity (Fig. S1C). Importantly, combined expression of HPK1 and PKCθ further enhanced NF-κB activation. This further supports our finding that CARMA1 integrates HPK1-mediated as well as PKCθ-mediated signaling events to foster NF-κB activation.
CARMA1 S551 Is Critical for TCR-Mediated NF-κB Activation and IL-2 Production.
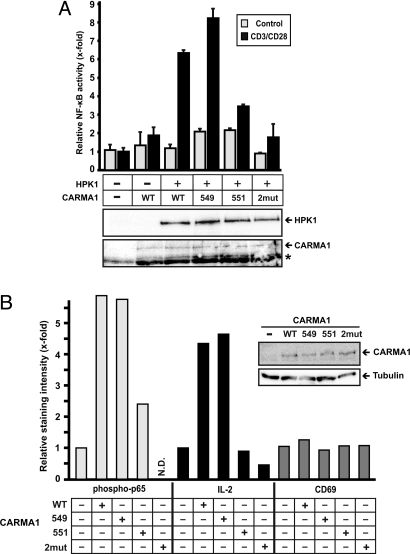
To further delineate the relevance of the identified CARMA1-phophorylation sites we used CARMA1-deficient Jurkat T cells to express WT or mutated CARMA1 proteins with or without HPK1 and analyzed NF-κB activation upon TCR stimulation on the basis of a co-transfected NF-κB reporter gene. In these experiments we used CD3/CD28-stimulation, which involves HPK1 as well as PKCθ to activate NF-κB. CARMA1 deficient Jurkat T cells are completely refractory in response to TCR stimulation. Reconstitution of these cells with WT CARMA1 rescued TCR-induced NF-κB activation, which was strongly enhanced when HPK1 had been co-expressed with CARMA1 (Fig. 5A). TCR stimulation in the presence of the CARMA1 mutant S549A and HPK1 induced NF-κB activation. However, the CARMA1 mutant S551A, and in particular the double mutant S549A/S551A completely failed to support TCR-mediated NF-κB activation in the presence of HPK1. Our results demonstrate that S551 of CARMA1 is a critical residue for TCR-induced NF-κB activation via HPK1 and suggest an auxiliary role for S549.
Fig. 5.
The HPK1-specific residue S551 within the linker of CARMA1 is critical for HPK1-mediated NF-κB activation in T cells. (A) Jurkat T cells with stable expression of CARMA1-specific shRNA (shCARMA1) were transfected with a NF-κB-specific luciferase reporter plasmid and a β-galactosidase expression plasmid for normalization together with or without expression plasmids encoding wild type CARMA1 (WT), CARMA1-S549A (549), CARMA1-S551A (551), CARMA1-S549A/S551A (2mut), and HPK1:HA. Forty-eight h later cells were either left non-stimulated (control) or stimulated with anti-CD3 and anti-CD28 ANC28.1 for 8 h to determine NF-κB activity. The experiment was repeated three times with similar outcome. (B) Jurkat T cells with stable expression of CARMA1-specific shRNA (shCARMA1) were transfected with expression plasmids encoding for CARMA1 proteins as described in D and EGFP. Forty-eight hours later cells were left non-stimulated or stimulated with anti-CD3/CD28 for 30 min (anti-phospho-p65) or 9 h (anit-IL-2 or CD69). For intracellular staining of phospho-p65 and IL-2 cells were fixed and the EGFP positive population was analyzed. Expression of CARMA1 proteins and tubulin was controlled by WB. N.D., not detected. The experiment was repeated three times with similar outcome.
Finally, we investigated the physiological role of the putative HPK1 phosphorylation site S551 in CARMA1 for TCR-mediated NF-κB activation. Again, CARMA1 deficient Jurkat cells were reconstituted with WT CARMA1 or CARMA1 mutants (Fig. 5B). Following CD3/CD28 stimulation phospho-p65 and IL-2 levels were measured by intracellular flow cytometry. Reconstitution with WT or S549A-mutated CARMA1 led to phosphorylation of p65 upon TCR stimulation (Fig. 5B, left side). In contrast, the CARMA1 mutant S551A was less potent and the double mutant S549A/S551A (2mut) completely failed to restore phosphorylation of p65 upon TCR triggering. One of the main NF-κB targets downstream of TCR-signaling is interleukin-2 (IL-2) and CARMA1 deficient T cells fail to express IL-2 upon TCR stimulation (15, 18). Consistent with phosphorylation of p65, CARMA1 deficient Jurkat T cells reconstituted with WT and S549A CARMA1 showed proper induction of IL-2 expression upon TCR stimulation, whereas S551A and S549A/S551A CARMA1 mutants failed to support IL-2 expression (Fig. 5B, Center). Up-regulation of the T-cell activation marker CD69, which is not under NF-κB control showed no significant differences (Fig. 5B, right side), demonstrating specificity of S551 and S549/S551 for the NF-κB pathway. In summary, the CARMA1 mutant S551A leads to decreased NF-κB activation and IL-2 production in CD3/CD28-stimulated Jurkat T cells. These results confirm the physiological relevance of the HPK1-specific residue S551 in CARMA1 upon TCR stimulation.
Discussion
While activation of NF-κB by HPK1 was clearly dependent on its kinase activity (27, 30), the identity of a molecular target was elusive. Here, we further characterize the molecular mechanism of HPK1-mediated NF-κB signaling and identify CARMA1 as an interaction partner and phosphorylation target of HPK1. HPK1 binds CARMA1 in non-stimulated T cells. TCR stimulation leads to an increase in HPK1-CARMA1 complex formation, which peaks 5 min after stimulation (Fig. 2B) and thus, precedes TCR-mediated IKK activation (Fig. 1C). Upon TCR stimulation HPK1 phosphorylates CARMA1 within its linker region and mutants of CARMA1 devoid of residues S551 or S549 and S551 fail to induce HPK1- and TCR-mediated NF-κB activation and IL-2 expression. Our results indicate that CARMA1 serves as a physiological substrate for HPK1 in T cells.
Activation of CARMA1 has been shown to depend on its flexible linker region that needs to be phosphorylated for signal transmission via BCL-10 and MALT1 to the NF-κB regulating IKK complex. Three groups independently reported that this flexible region is a target for PKCβ in B cells and PKCθ in T cells (8, 10, 33). In T cells, activation of NF-κB is also dependent on non-PKCθ phosphorylation sites (8), and it has been suggested that kinases other than PKCθ are involved in TCR-induced CARMA1 activation (11).
In the present study we have shown that residue S551 within the flexible linker region of CARMA1 is critical for HPK1-dependent and TCR-induced NF-κB activation. In addition, residue S549 plays an auxiliary role, since single mutation of S549 does not affect the signaling competence of CARMA1, whereas combined mutations of S549 and S551 in CARMA1 completely abrogates NF-κB activation. Matsumoto and colleagues had shown that residue S551 is not involved in PKCθ-induced NF-κB activation via PMA/CD28-stimulation (8), which is in line with our observation (Fig. S1B). PMA stimulates PKCθ directly, while HPK1 is activated by TCR stimulation (20, 21). Thus, PMA bypasses HPK1 activation and omits contributions of HPK1. In contrast, CD3/CD28 stimulation—which activates HPK1—relies on S551 within CARMA1 to induce NF-κB activation. These results suggest that residue S551 is an HPK1-dependent phosphorylation site and strongly point toward non-redundant functions of HPK1 and PKCθ in the context of physiological TCR stimulation. Thus, TCR-stimulation involving HPK1 in addition to PKCθ might allow for a differential signaling threshold for T-cell activation.
Recent reports suggested HPK1 to be involved in down-regulation of the immune response by phosphorylation of SLP-76 and recruitment of 14–3-3 proteins (24, 25). HPK1(−/−) T cells show hyperproliferation and enhanced cytokine production. The fact that activation of T cells from HPK1(−/−) mice is possible leads to the conclusion that activation of NF-κB can occur in HPK1(−/−) T cells. In contrast, we have shown that transient knock-down of HPK1 in Jurkat T cells [Fig. 1A and (27)] or in primary T cells (Fig. 1C) completely blunts NF-κB activation and HPK1 has been reported to positively regulate NF-κB activity in response to antigen-receptor stimulation (27, 30). Thus, these results argue for compensatory mechanisms which might circumvent the dependency on HPK1 for NF-κB activation in HPK1(−/−) T cells.
The existence of such compensatory mechanisms is supported by increased membrane proximal signaling in HPK1(−/−) T cells (25). The yet not understood elevated ZAP70 activity in HPK1(−/−) T cells leads to enhanced LAT phosphorylation and PLCγ1 activity which results in increased ERK signaling. Interestingly, also VAV tyrosine phosphorylation is enhanced in these cells. It has been shown that VAV activation is essential for PKCθ mediated NF-κB activation (34). This together with the elevated DAG levels derived from increased PLCγ1 activity might strongly activate PKCθ in HPK1(−/−) T cells which might suffice to activate NF-κB without HPK1. This assumption is supported by our finding that long-term knock-down of HPK1 in Jurkat T cells using stably integrated shRNA led to loss of HPK1-dependent IKK activation upon TCR stimulation and showed increased Erk signaling which correlated with elevated expression of the TCR (data not shown). Interestingly, HPK1(−/−) mice generated differently than those published by Shui et al. (25) show elevated TCR levels on the surface of T cells (F.K., unpublished data). It is likely that T cells can compensate for the loss of HPK1 by increased surface TCR levels which lead to enhanced TCR-proximal signaling and, thus, HPK1(−/−) T cells become independent of HPK1-mediated NF-κB activation.
Consequently, it is tempting to speculate that the initial strength of stimulation is crucial for the involvement of HPK1 in NF-κB activation. The less strong a physiological stimulus is the more it would depend on HPK1 for NF-κB activation. In contrast, a strong stimulus, for example, by PMA/ionomycin, would bypass HPK1 and lead to full activation of NF-κB. This might explain why HPK1(−/−) T cells, which show elevated activities for several signaling proteins, have lost their dependency on HPK1 for activation of NF-κB.
Furthermore, HPK1(−/−) mice do not only lack full length HPK1, which positively feeds into the NF-κB pathway, but also do not produce the cleavage product HPK1-C, which is a negative-feedback regulator of NF-κB (27). Thus, one may conclude that hyperproliferation of T cells in the absence of HPK1 is caused by impairment of negative feedback mechanisms involved in downmodulation of T-cell activation (35). In addition, hyperproliferation of HPK1(−/−) T cells can be explained by the recently reported hyperactivation of HPK1(−/−) dendritic cells (DC) (36). The role of HPK1 in DC is less well understood and the function of HPK1 in DC might be more biased toward suppression rather than induction. Further experiments have to be carried out to better understand the physiological role of HPK1 in myeloid cells and particularly in DC. Nonetheless, it cannot be formally ruled out that the hyperproliferation of HPK1(−/−) T cells is not a pure T-cell autonomous phenotype.
In conclusion, we propose a model in which TCR-dependent NF-κB activation requires the phosphorylation of CARMA1 by PKCθ, HPK1 and additional kinases such as casein kinase 1α (37). Our report shows a key role for HPK1-mediated phosphorylation of CARMA1 and identifies residue S551 of CARMA1 to be critical for T-cell activation via HPK1. However, it is likely that HPK1 has a role as modulator rather than as indispensable trigger of TCR signaling. Furthermore, HPK1 might contribute to T-cell activation as well as to subsequent shut down of T-cell activation by phosphorylation of additional components. The identification of additional HPK1 substrates and the generation of knock-in mice expressing catalytically inactive HPK1 might give rise to a more comprehensive picture reflecting the ambivalent signaling properties of HPK1 in T lymphocytes.
Materials and Methods
Antibodies and Reagents.
HRPO-conjugated antibodies (Abs) were purchased from Southern Biotechnology Associates. Antibodies anti-HPK1 (#2, #7, and #9/10) have been described (30). The abs used were anti-T7 (Novagen), anti-FLAG (M2, Sigma), anti-tubulin (Sigma), anti-HA (12CA5), anti-IKKγ (FL-419), anti-IKKα/β and anti-BCL-10 (all Santa Cruz), anti-CARMA1 (ProSci), anti-Erk, anti-human-CD3 (OKT3), anti-phospho-p65, anti-human-IL-2, and anti-human-CD69 (all BD Bioscience). Anti-CARMA1 rabbit antiserum for immunoprecipitation (AL222, provided by M.T.) recognizes the GUK domain of CARMA1. For stimulation we used anti-CD3 (OKT3, plate bound: 10 μg/mL or soluble: 1 μg/mL), anti-CD28 (plate bound: 10 μg/mL or soluble: 1 μg/mL) or anti-CD28 ANC28.1 (soluble, 0.2 μg/mL, Ancell). The expression plasmids: HPK1:HA(wt), HPK1:HA(K46E), and T7:HPK1-C were described previously (29, 30). FLAG:CARMA1-linker (in pEF4, Invitrogen), VSV-tagged CARMA1 (provided by M.T.), CARMA1-mutants and GST:CARMA1-linker constructs (in pGEX-4T2, Amersham) were cloned by standard PCR-techniques. NF-κB reporter plasmids pGL8xNF-κB-fos and pfos-LacZ have been described (30). Transfection efficiency was normalized to LacZ expression. Values depicted give mean and standard deviation of triplicate measurements.
T Cells and Intracellular Staining.
Human peripheral T cells were prepared and cultivated as described in ref. 38. The human Jurkat T-cell clone J16–145 was published elsewhere. Jurkat T cells with stable expression of control shRNA (shControl) or CARMA1-specific shRNA (shCARMA1) were generated by lentiviral transduction (provided by M.T.). Jurkat T cells were stimulated with anti-CD3, anti-CD28, and goat-anti-mouse Abs (1 μg/mL each) for 30 min (anti-phospho-p65 staining) or for 9 h (anti-IL2 staining, with 10 μg/mL brefeldin A added after 1 h). Subsequently, cells were fixed with Phosflow fix-buffer I (BD Biosciences) for 30 min on ice, washed and postfixed with 90% methanol for 60 min at −20 °C. After washing, cells were stained on ice for 90 min in PBS supplemented with 1% FCS.
Transfections, Gene Silencing, and Immunoprecipitation.
COS1 or 293T cells were transfected by Ca2+-phosphate coprecipitation and Jurkat T cells were transfected by electroporation (200 V, 950 μF). Nucleofection (AMAXA) of primary T cells or Jurkat T cells were was used to transfect HPK1-specific (Qiagen) or RelA-specific (Ambion) siRNA. For immunoprecipitation cells were lysed using 1% Nonidet P-40, 20 mM Tris pH 7.4, 150 mM NaCl supplemented with protease and phosphatase inhibitors and 2 μg of antibody coupled to Protein A Sepharose or 5 μL anti-VSV-G agarose conjugate (Sigma) was used. Proteins were resolved by SDS/PAGE and transferred to nitrocellulose membrane (Amersham Pharmacia Biotech) and processed according to the manufacturer's protocol.
In Vitro Kinase Assays.
All in vitro kinase assays were performed as described in ref. 29. A reconstituted in vitro kinase assay was set up using purified, COS1-expressed and immunoprecipitated HPK1:HA and VSV:CARMA1 proteins bound to Sepharose beads. FLAG-CARMA1 linker was recombinantly expressed in 293T cells, purified via anti-FLAG agarose and added to the in vitro kinase assay. Recombinant PKCθ (Upstate) was used according to the manufacturer's instructions.
Supplementary Material
Acknowledgments.
We thank Wolfgang Müller for expert technical assistance, Nina Oberle, and Angelika Schmidt for help with intracellular staining, and Heidi Sauter for excellent secretarial work. This work was supported by the Cooperation Program in Cancer Research of the Deutsches Krebsforschungszentrum (DKFZ) and the Israeli Ministry of Science, Culture and Sport (MOST); Deutsche Krebshilfe; Wilhelm Sander Stiftung, the SFB 405; Tumorzentrum Heidelberg/Mannheim; Swiss National Science Foundation (M.T.); Swiss Cancer League (Oncosuisse) (M.T.); and the Vontobel Stiftung (M.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900457106/DCSupplemental.
References
- 1.Hildeman, et al. Molecular mechanisms of activated T cell death in vivo. Cur Opin Immunol. 2002;14:354–359. doi: 10.1016/s0952-7915(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 2.Karin M, Lin A. NF-kappa B at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 3.Ruland J, Mak TW. From antigen to activation: Specific signal transduction pathways linking antigen receptors to NF-κB. Sem Immunol. 2003;15:177–183. doi: 10.1016/s1044-5323(03)00034-4. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 5.Scheidereit C. I kappa B kinase complexes: Gateways to NF-κB activation and transcription. Oncogene. 2006;25:6685–6705. doi: 10.1038/sj.onc.1209934. [DOI] [PubMed] [Google Scholar]
- 6.Hayden MS, Ghosh S. Signaling to NF-kappa B. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 7.Thome M. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat Rev Immunol. 2004;4:348–359. doi: 10.1038/nri1352. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto, et al. Phosphorylation of CARMA1 plays a critical role in T cell receptor-mediated NF-κB activation. Immunity. 2005;23:575–585. doi: 10.1016/j.immuni.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Rueda D, Thome M. Phosphorylation of CARMA1: The link(er) to NF-κB activation. Immunity. 2005;23:551–553. doi: 10.1016/j.immuni.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Sommer, et al. Phosphorylation of the CARMA1 linker controls NF-κB activation. Immunity. 2005;23:561–574. doi: 10.1016/j.immuni.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Rawlings DJ, Sommer K, Moreno-Garcia ME. The CARMA1 signalosome links the signalling machinery of adaptive and innate immunity in lymphocytes. Nat Rev Immunol. 2006;6:799–812. doi: 10.1038/nri1944. [DOI] [PubMed] [Google Scholar]
- 12.Sun LJ, Deng L, Ea CK, Xia ZP, Chen ZJJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell. 2004;14:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 13.Abbas AK, Sen R. The activation of lymphocytes is in their CARMA. Immunity. 2003;18:721–722. doi: 10.1016/s1074-7613(03)00143-2. [DOI] [PubMed] [Google Scholar]
- 14.Egawa, et al. Requirement for CARMA1 in antigen receptor-induced NF-κB activation and lymphocyte proliferation. Curr Biol. 2003;13:1252–1258. doi: 10.1016/s0960-9822(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 15.Gaide, et al. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-κB activation. Nat Immunol. 2002;3:836–843. doi: 10.1038/ni830. [DOI] [PubMed] [Google Scholar]
- 16.Hara, et al. The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity. 2003;18:763–775. doi: 10.1016/s1074-7613(03)00148-1. [DOI] [PubMed] [Google Scholar]
- 17.Pomerantz JL, Denny EM, Baltimore D. CARD11 mediates factor-specific activation of NF-κB by the T cell receptor complex. EMBO J. 2002;21:5184–5194. doi: 10.1093/emboj/cdf505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, et al. A requirement for CARMA1 in TCR-induced NF-κB activation. Nat Immunol. 2002;3:830–835. doi: 10.1038/ni824. [DOI] [PubMed] [Google Scholar]
- 19.Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–230. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- 20.Liou, et al. HPK1 is activated by lymphocyte antigen receptors and negatively regulates AP-1. Immunity. 2000;12:399–408. doi: 10.1016/s1074-7613(00)80192-2. [DOI] [PubMed] [Google Scholar]
- 21.Liu SK, Smith CA, Arnold R, Kiefer F, McGlade CJ. The adaptor protein Gads (Grb2-related adaptor downstream of Shc) is implicated in coupling hemopoietic progenitor kinase-1 to the activated TCR. J Immunol. 2000;165:1417–1426. doi: 10.4049/jimmunol.165.3.1417. [DOI] [PubMed] [Google Scholar]
- 22.Arnold, et al. Activation of hematopoietic progenitor kinase 1 involves relocation, autophosphorylation, and transphosphorylation by protein kinase D1. Mol Cell Biol. 2005;25:2364–2383. doi: 10.1128/MCB.25.6.2364-2383.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold, et al. Sustained JNK signaling by proteolytically processed HPK1 mediates IL-3 independent survival during monocytic differentiation. Cell Death Diff. 2007;14:568–575. doi: 10.1038/sj.cdd.4402042. [DOI] [PubMed] [Google Scholar]
- 24.Di Bartolo, et al. A novel pathway down-modulating T cell activation involves HPK-1-dependent recruitment of 14–3-3 proteins on SLP-76. J Exp Med. 2007;204:681–691. doi: 10.1084/jem.20062066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shui, et al. Hematopoietic progenitor kinase 1 negatively regulates T cell receptor signaling and T cell-mediated immune responses. Nat Immunol. 2007;8:84–91. doi: 10.1038/ni1416. [DOI] [PubMed] [Google Scholar]
- 26.Schulze-Luehrmann, et al. Hematopoietic progenitor kinase 1 supports apoptosis of T lymphocytes. Blood. 2002;100:954–960. doi: 10.1182/blood-2002-01-0089. [DOI] [PubMed] [Google Scholar]
- 27.Brenner D, Golks A, Kiefer F, Krammer PH, Arnold R. Activation or suppression of NF-κB by HPK1 determines sensitivity to activation-induced cell death. EMBO J. 2005;24:4279–4290. doi: 10.1038/sj.emboj.7600894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner, et al. Caspase-cleaved HPK1 induces CD95L-independent activation-induced cell death in T and B lymphocytes. Blood. 2007;110:3968–3977. doi: 10.1182/blood-2007-01-071167. [DOI] [PubMed] [Google Scholar]
- 29.Kiefer, et al. HPK1, a hematopoietic protein kinase activating the SAPK/JNK pathway. EMBO J. 1996;15:7013–7025. [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold R, Liou J, Drexler HCA, Weiss A, Kiefer F. Caspase-mediated cleavage of hematopoietic progenitor kinase 1 (HPK1) converts an activator of NF kappa B into an inhibitor of NF-κB. J Biol Chem. 2001;276:14675–14684. doi: 10.1074/jbc.M008343200. [DOI] [PubMed] [Google Scholar]
- 31.Brenner D, Krammer PH, Arnold R. Concepts of activated T cell death. Crit Rev Oncol Hematol. 2008;66:52–64. doi: 10.1016/j.critrevonc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 33.Shinohara, et al. PKC beta regulates BCR-mediated IKK activation by facilitating the interaction between TAK1 and CARMA1. J Exp Med. 2005;202:1423–1431. doi: 10.1084/jem.20051591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villalba, et al. A novel functional interaction between Vav and PKCθ is required for TCR-induced T cell activation. Immunity. 2000;12:151–160. doi: 10.1016/s1074-7613(00)80168-5. [DOI] [PubMed] [Google Scholar]
- 35.Acuto O, Di Bartolo V, Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat Rev Immunol. 2008;8:699–712. doi: 10.1038/nri2397. [DOI] [PubMed] [Google Scholar]
- 36.Alzabin S, Bhardwaj N, Kiefer F, Sawasdikosol S, Burakoff S. Hematopoietic progenitor kinase 1 is a negative regulator of dendritic cell activation. J Immunol. 2009;182:6187–6194. doi: 10.4049/jimmunol.0802631. [DOI] [PubMed] [Google Scholar]
- 37.Bidere, et al. Casein kinase 1alpha governs antigen-receptor-induced NF-κB activation and human lymphoma cell survival. Nature. 2008;458:92–96. doi: 10.1038/nature07613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peter, et al. Resistance of cultured peripheral T cells towards activation-induced cell death involves a lack of recruitment of FLICE (MACH/caspase 8) to the CD95 death-inducing signaling complex. Eur J Immunol. 1997;27:1207–1212. doi: 10.1002/eji.1830270523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.