Abstract
In advanced breast tumors, protein kinases are upregulated and steroid hormone receptors often function independently of ligand. Herein, we explored mechanisms of ligand-independent progesterone receptor (PR) activity. We showed previously that growth factor-induced phosphorylation of PR Ser-294 blocks PR Lys-388 sumoylation. SUMO-deficient mutant PR-B (K388R) thus provides a model receptor for the study of PR function in the context of high kinase activities. T47D cells stably expressing K388R PR-B exhibited increased ligand-independent proliferation and growth in soft agar relative to cells expressing wt PR-B or phospho-mutant (sumoylated) S294A PR-B. Expression of selected PR target genes (HB-EGF, IRS-1, and STC1) was significantly elevated in cells containing desumoylated (K388R) PR-B. Basal PR transcriptional activity occurred independently of progestins, was increased by activated CDK2, and attenuated by RU486. Notably, ChIP assays demonstrated that K388R PR-B and SRC1 were constitutively recruited to the STC1 promoter in the absence of progestin; PR Lys-388 sumoylation was required for HDAC3 recruitment. Knock-down of STC1 inhibited proliferation of cells expressing K388R PR-B. These data suggest a mechanism whereby phosphorylated, and thus desumoylated, PRs mediate increased expression of growth promoting genes. Our data explain why breast cancer models often remain insensitive to progestins, but are growth-inhibited by antiprogestins, and underscore the need to target PR-B and associated kinase activities as part of breast cancer therapy.
Keywords: HDAC, phosphorylation, sumoylation
Progesterone receptors (PR) mediate lobulo-alveolar proliferation during breast development and contribute to breast cancer progression, in part by synergizing with peptide growth factors (1–3). Regulation of PR activity occurs via the integration of ligand activated rapid signaling events and transcriptional activation (4). Peptide growth factors diminish the requirement for steroid hormone ligands by activation of protein kinases that directly target PR and its co-activators (i.e., a function of rapid signaling events). For example, direct phosphorylation of PR by MAPK or CDK2 alters transcriptional activity in part by modulating other posttranslational modifications including ubiquitination and sumoylation (3, 5). Crosstalk between growth factor pathways and steroid receptors provides an exquisite mechanism for mammary epithelial cells to sense a dynamic range of hormone concentrations and selectively regulate specific promoters.
Sumoylation of steroid hormone receptors represses transcriptional activity (6). We showed that EGF and protein kinase-dependent (MAPK, CDK2) phosphorylation of PR-B on Ser-294 blocks sumoylation of PR Lys-388 (5). Undersumoylated receptors respond to low concentrations of ligand and are transcriptionally hyperactive at a subset of PRE (progesterone response element) containing target genes (HB-EGF). Sumoylation of PR is thus a key modulator of both the progesterone/PR dose–response curve and PR target gene selection. Breast cancer cells containing phosphorylated, undersumoylated PR display increased cell growth in response to progestins, suggesting that modified receptors target growth promoting genes (5).
Accumulating evidence suggests that ligand-independent actions of PR are biologically relevant (7, 8). Herein, we hypothesized that PR sumoylation/desumoylation contributes to the regulation of hormone-independent PR activity. We focused on SUMO-deficient mutant (K388R) PR-B as a model for PR action in breast cancer cells with high kinase activity, where phosphorylation events block PR Lys-388 sumoylation (5).
Results
Sumoylation of PR Represses Basal Proliferation in Breast Cancer Cells.
Sumoylation of PR-B is rapidly induced by both agonist (R5020) and antagonist (RU486) (5). We measured the levels of wt, K388R (SUMO-deficient mutant), or S294A (phospho-mutant) PR-B sumoylation in HeLa cells using the SUMO assay previously described for PR (5). Following 24 h treatment with either progestin (R5020) or vehicle (EtOH), unmodified and upshifted, sumoylated PRs were visualized by Western blotting (Fig. 1A). Consistent with previous reports (5, 9), wt PR-B, but not K388R PR-B, was sumoylated in the presence of exogenous SUMO-1, and PR sumoylation was increased in response to progestin (lane 4). Notably, we detected partial sumoylation of wt PR-B, but not K388R, in the absence of ligand (lane 3) indicating that this modification occurs in unstimulated (serum starved) cells. In a similar experiment, we measured sumoylation of wt PR-B relative to phospho-mutant S294A PR-B (Fig. 1B). Wt PR-B was partially sumoylated within 1 h of R5020 treatment. S294A mutant PR-B cannot undergo Ser-294 phosphorylation, an event that blocks K388 sumoylation (5). Consistent with negative regulation of PR sumoylation via phosphorylation at this site, S294A PR-B displayed increased sumoylation compared to wt PR-B in the absence and presence of R5020.
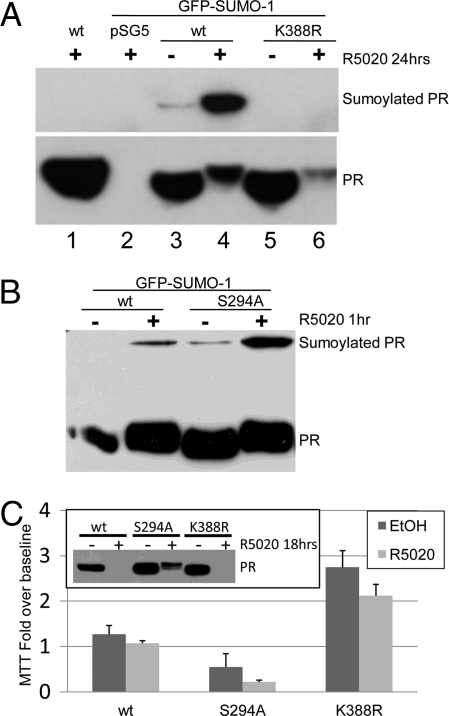
Fig. 1.
Sumoylated PR represses breast cancer cell growth in the absence of progestins. (A) HeLa cells were transfected with either wt or K388R PR-B and either vector or EGFP-SUMO-1, starved for 24 h, treated with R5020 (10−8 M) for 24 h, and Western blotted for PR. (B) HeLa cells were transfected as in (A), except with wt or S294A PR-B, and treated with R5020 for 1 h, and Western blotted for PR. (C) Triplicate cultures of T47D cells stably expressing wt, S294A, or K388R (clone 7) PR-B were subjected to MTT assays under steroid hormone-free conditions supplemented with vehicle (EtOH) or R5020 for 6 days. Bars (y axis) represent fold increases in viable cell number over baseline (±SD). (Inset) T47D cells stably expressing either wt, S294A, or K388R PR-B were starved for 24 h, treated with vehicle or R5020 for 24 h, and Western blotted for PR.
The detection of modest levels of basally sumoylated PRs (Fig. 1A) prompted us to examine the contribution of PR sumoylation to PR-induced breast cancer cell growth, particularly in the absence of ligand. For these experiments, we used T47D breast cancer cells stably expressing either wt, K388R, or S294A PR-B and selected clones for expression of equal receptor levels (Fig. 1C, inset) (5). Cells were cultured in steroid-free media supplemented with either vehicle or R5020 for 6 days and viable cells were quantified by MTT assay as a measure of their basal and progestin-stimulated growth (Fig. 1C). Progestin had no significant effect on the growth of T47D cells. However, surprisingly, multiple clones of T47D cells stably expressing the SUMO-deficient PR-B mutant (K388R) consistently displayed increased basal proliferation relative to wt PR-B expressing cells (Fig. 1C clone 7; [supporting information (SI) Fig. S1A clone 28]). In contrast, cells expressing heavily sumoylated S294A PR-B exhibited decreased basal proliferation relative to cells containing wt PR-B (Fig. 1C). In the absence of progestin, PR-B expression levels remained comparable between stable cell lines (Fig. 1C, inset). Liganded S294A PR-B are stabilized relative to wt or K388R PR (Fig. 1C, inset), consistent with the finding that PR-B Ser-294 phosphorylation augments ligand-induced downregulation by the ubiquitin-proteasome pathway (10).
In MTT assays (6 days), adherent T47D cells are minimally responsive to progestin (Fig. 1C), perhaps owing to the biphasic effects of progestins in short-term 2D cultures (SI Results) (11). We thus performed soft agar assays to examine the contribution of desumoylated PR to long-term (21 days) anchorage independent growth (Fig. S1B). Similar to MTT results, in the absence of added steroid hormone, T47D cells expressing SUMO-deficient mutant PR-B displayed significantly increased colony formation relative to wt PR-B expressing cells. In contrast, basal growth of cells expressing heavily sumoylated S294A PR-B was lower relative to wt controls, suggesting that breast cancer cell proliferation and survival in the absence of progestin is highly sensitive to the degree of PR sumoylation.
Sumoylation of PR-B Represses Transcription of a Subset of Target Genes.
To address the question of a direct role for PR sumoylation/desumoylation in breast cancer cell proliferation in the absence of progestins (Fig. 1C and S1), we investigated the regulation of selected endogenous gene targets by unliganded PR. Stanniocalcin 1 (STC1), a peptide hormone overexpressed in breast cancers, has been shown to increase cell metabolism and tumor growth (12). Gene array analysis of T47D breast cancer cells demonstrated that STC1 expression is induced by the forced expression of unliganded PR-A or PR-B (7). We therefore examined the contribution of sumoylation to PR-B mediated STC1 regulation using real-time PCR. T47D cells stably expressing equal levels (Fig. 1C inset) of either wt, K388R or S294A PR-B were serum starved or treated with R5020 or RU486 for 48 h. In the absence of ligand, cells expressing wt or S294A PR-B exhibited low basal levels of STC1 expression and STC1 mRNA levels remained insensitive to either PR agonist (R5020) or PR antagonist/partial agonist (RU486). However, cells stably expressing K388R PR-B displayed greatly heightened STC1 transcript levels (Fig. 2A clone 7; Fig. S2 clones 24 and 28). Surprisingly, in these cells, addition of either R5020 (agonist) or RU486 (antagonist/partial agonist) for 48 h decreased basal STC1 mRNA expression by unknown mechanisms (see below), but demonstrates the specificity of STC1 regulation by desumoylated receptors. Similar results were observed following 6 h of hormone (Fig. S3). These data suggest that in the absence of ligand, STC1 is positively regulated by desumoylated PR.
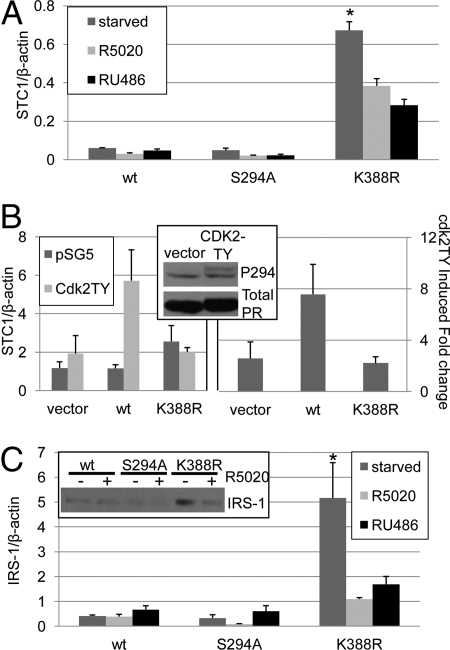
Fig. 2.
Phospho-dependent regulation of PR sumoylation alters target gene levels. (A) T47D cells stably expressing wt, S294A, or K388R PR-B were plated in triplicate cultures and serum starved or treated with R5020 (10−8M) or RU486 (10−7 M) for 48 h. Real-time PCR was performed and STC1 was normalized to β-actin (±SD, *P < 0.0001). (B) HeLa cells in triplicate cultures were transfected with vector, wt or K388R PR-B and either vector or CDK2TY. (Left panel) Real-time PCR was performed and STC1 was normalized to β-actin (±SD). (Right panel) Data from multiple experiments were combined and expressed as CDK2TY induced fold change over vector control (± SEM). (Inset) HeLa cells transfected with wt PR-B and either vector or CDK2-TY were starved for 24 h and Western blotted using phospho-S294 PR and total PR antibodies. (C) Triplicate cultures of T47D cells stably expressing wt, S294A, or K388R PR-B were serum starved or treated with R5020 (10−8 M) or RU486 (10−7 M) for 48 h, real-time PCR was performed, and IRS-1 was normalized to β-actin (±SD, *P < 0.01). (Inset) T47D cells stably expressing wt, S294A or K388R PR-B were serum starved for 24 h and treated with ethanol or R5020 (10−8 M) for 18 h then Western blotted for IRS-1.
PR sumoylation is negatively regulated by growth factors that input to activation of Cdk2 or MAPKs (ERK1/2) or by overexpression of these kinases (5). Although PR are multiply phosphorylated, primarily at Ser residues, the effects of these kinases on PR sumoylation mapped to phosphorylation of PR Ser-294 (5). We therefore tested the sensitivity of PR mediated STC1 expression to activated protein kinases in the absence of progestins. For these experiments, we used PR-null HeLa cells, because of their high transfection efficiency relative to T47D cells. PRs expressed in PR-null HeLa cells closely mimic human breast cancer cells with regard to PR biochemistry (10, 13, 14). To achieve sustained PR phosphorylation, we used an active mutant of Cdk2 (Cdk2-TY) that is resistant to inactivation by Wee-1 kinase-regulated phosphorylation at Thr-14 and Tyr-15 (15). A high percentage of human breast cancers overexpress cyclin E/cdk2 complexes, reviewed by Lopez-Beltran et al. (16), which may usurp the (transient) action of progesterone by directly phosphorylating PR. As predicted, upon transient transfection of Cdk2-TY into HeLa cells, phosphorylation of PR-B Ser-294 occurred in the absence of progestin (Fig. 2B, inset); this event prevents PR sumoylation (5). The regulation of STC1 mRNA was then examined in HeLa cells co-transfected with wt PR-B or K388R PR-B and CDK2-TY (Fig. 2B, left panel). Transient expression of SUMO-deficient PR-B induced a modest increase in STC1 mRNA levels relative to wt or vector controls. However, the co-expression of Cdk2-TY was required for robust activation of STC1 transcription in cells expressing wt PR-B. The Cdk2-TY-dependent fold induction of STC1 expression relative to vector control was averaged from multiple experiments (Fig. 2B. right panel). PR-null cells and cells expressing SUMO-deficient K388R PR-B remained insensitive to activated CDK2, whereas cells expressing wt PR-B exhibited increased STC1 expression in the presence of CDK2-TY. These data suggest that CDK2 induced phosphorylation of wt PR-B favors PR desumoylation, leading to derepression of unliganded wt PRs on the STC1 promoter. K388R PR-B is unable to be sumoylated at Lys-388 and is thus unaffected by Cdk2-TY.
To further explore the contribution of unliganded PRs to target gene regulation and the role of PR sumoylation, we examined insulin receptor substrate 1 (IRS-1) expression. The transcription of IRS-1 is PR-B driven and requires Ser-294 phosphorylation, but also occurs in the absence of progestins (3). IRS-1 is an adaptor molecule in the IGF receptor pathway that is critical for IGF-1 mediated proliferation of breast cancer cells (17). We therefore tested whether IRS-1 expression was sensitive to PR sumoylation using real-time PCR in T47D cells stably expressing either wt, S294A or K388R PR-B. As predicted, IRS-1 expression was relatively insensitive to hormone treatment in cells expressing either wt or S294A PR-B (Fig. 2C). However, similar to the results with STC1 (Fig. 2A), in serum starved (hormone-free conditions) cells, stable expression of K388R PR-B induced high levels of IRS-1 mRNA relative to both wt and S294A PR-B (Fig. 2C clone 7; Fig. S2 clones 24 and 28). Again, treatment with either R5020 or RU486 (48 h) partially blocked IRS-1 induction by SUMO-deficient K388R PR-B, demonstrating the specificity of IRS-1 regulation by unliganded PR-B receptors. These results are supported by Western blots of IRS-1 in T47D cells stably expressing either wt, S294A, or K388R PR-B and treated with or without R5020 (18 h). IRS-1 expression is insensitive to progestins in cells containing wt or S294A PRs, while unliganded SUMO-deficient PR-B mediates increased IRS-1 protein expression levels that are attenuated in the presence of progestin (Fig. 2C inset).
Up-regulation of STC1 and IRS-1 occurred in multiple clones of cells expressing K388R PR (Fig. S2), suggesting that selected genes are dramatically induced by unliganded but phosphorylated PRs. To address the specificity these findings, we tested the basal and hormone-regulated expression of additional progestin regulated genes, including SGK (serum and glucocorticoid regulated kinase) (Fig. S4A). Notably, basal expression of SGK was comparable in all three cell lines (starved condition). Furthermore, R5020 induced similar expression of SGK mRNA in cells expressing either wt or K388R PR-B, whereas PR-null cells remained unresponsive; RU486 alone was without effect. In addition, we examined the regulation of progestin-responsive HB-EGF expression in cells containing either wt or K388R PR (Fig. S4B). HB-EGF mRNA is induced in a PR phosphorylation and sumoylation sensitive manner in response to R5020 (5, 18). As expected, HB-EGF mRNA was induced by R5020, but not RU486, in cells expressing wt PR. SUMO-mutant (K388R) containing cells displayed significantly greater induction of HB-EGF following R5020 treatment relative to cells expressing wt PR. However, in contrast to STC1 and IRS1, basal expression of HB-EGF was insensitive to changes in PR sumoylation and remained comparable between cell lines. Collectively, these data suggest that sumoylated but unliganded PR-B is able to repress transcriptional activity on a subset of promoters (STC1 and IRS1). PR Ser-294 phosphorylation mediates de-repression of these genes via desumoylation; these promoters appear to be further repressed upon ligand addition by unknown mechanisms (addressed below). Other promoters are regulated by sumoylated PRs, but only in the presence of progestin (HB-EGF). Still others are completely “blind” to changes in PR sumoylation (SGK).
Sumoylated PR-B Regulates Gene Expression via Altered Cofactor Recruitment.
We speculated that PR-B sumoylation alters the recruitment of chromatin remodeling and transcriptional complexes to promoter regulatory regions. STC1 expression in cells expressing wt PR-B was particularly sensitive to TSA-mediated inhibition of HDAC activity (SI Results and Fig. S5A), suggesting that HDAC recruitment to the STC1 promoter may contribute to the transcriptional repression observed in these cells. We therefore performed chromatin immunoprecipitation (ChIP) assays as a direct test of HDAC association with the STC1 promoter in the presence of sumoylated PR. T47D cells stably expressing wt or K388R PR-B were subjected to ChIP using antibodies against PR, HDAC3, steroid receptor coactivator 1 (SRC1) and RNA polymerase II (Pol II). In the absence of progestins, both wt and K388R PR-B were detected in the −3550–−3349 (M) region of the STC1 promoter, which contains a PRE half site (Fig. 3A). Interestingly, as we predicted, HDAC3 was preferentially bound to this region of DNA in the presence of unliganded wt PR-B, but not SUMO-deficient K388R PR-B. In contrast, the PR coactivator, SRC1, constitutively associated with this region only in cells expressing K388R PR-B. The specificity of these interactions with DNA is demonstrated, as neither PR nor the associated cofactors were recruited to a control region of the STC1 promoter (−2940–−2734, L) and neither promoter region was amplified in IgG controls. In addition, RNAPolII was detected on STC1 Exon1 DNA in K388R PR-B but not wt PR-B expressing cells (Fig. S5B), consistent with increased mRNA (Fig. 2A). To further examine the ability of PR sumoylation to alter cofactor recruitment to the STC1 promoter, we repeated similar ChIP assays, except using cells stably expressing heavily sumoylated phospho-mutant S294A PR. Consistent with the above results (Fig. 2A), S249A PR was bound to the STC1 promoter in the absence of ligand; HDAC3, but not SRC1, remained associated with this promoter region (Fig. 3B). These data suggest that unliganded but sumoylated PR-B constitutively recruits HDAC3 to the STC1 promoter, inducing a repressive cofactor complex. When sumoylation is prevented, as in the K388R mutation, SRC-1 is recruited constitutively to unliganded receptors and transcription is thus activated.
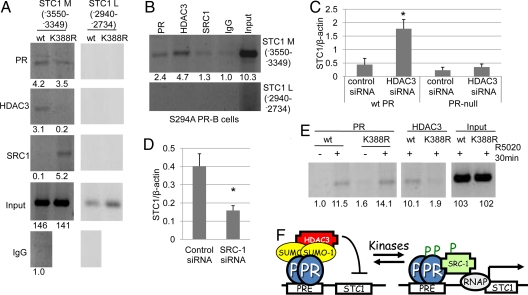
Fig. 3.
PR sumoylation alters the recruitment of cofactors to PR target promoters. (A) T47D cells stably expressing wt or K388R PR-B were serum starved for 48 h and subjected to ChIP assays using antibodies against PR, HDAC3, or SRC1(primers specific for the STC1 promoter). Normal rabbit IgG was used as an isotype control. (B) T47D cells stably expressing S294A PR-B were serum starved for 48 h and subjected to ChIP assays using antibodies against PR, HDAC3, or SRC1 or normal rabbit IgG and primers specific for the STC1 promoter. (C) PR-null HeLa cells and HeLa cells stably expressing wt PR-B were transfected with HDAC3 or negative control siRNA, and starved for 72 h, real-time PCR was performed, and STC1 was normalized to β-actin (±SD, *P < 0.006). (D) T47D cells stably expressing K388R PR-B were transfected with SRC1 or negative control siRNA, real-time PCR was performed, and STC1 was normalized to β-actin (±SD, *P < 0.005). (E) ChIP assays were performed in T47D cells stably expressing either wt or K388R PR-B using antibodies against PR and HDAC3 and primers specific for the HB-EGF promoter. Asterisk denotes statistical significance relative to cells transfected with control siRNA as determined by unpaired Student t tests. Clone 7 T47D K388R PR-B cells were used throughout these experiments. Bands on gels were quantified by densitometry (values are listed below each band). (F) High kinase activity in breast cancer cells blocks PR sumoylation and activates expression of growth promoting genes in the absence of progestins via altered cofactor recruitment.
To confirm the requirement for HDAC3 in SUMO-dependent PR transcriptional repression of the STC1 promoter, we knocked down HDAC3 expression in cells containing wt PR. PR-null HeLa cells or HeLa cells stably expressing wt PR-B were transiently transfected with HDAC3 siRNA or nontargeted control siRNA. Real-time PCR was used to confirm HDAC3 knockdown (Fig. S5C) and assess STC1 transcript levels (Fig. 3C). In cells expressing wt PR-B, HDAC3 knockdown induced a significant increase in STC1 transcription, whereas STC1 levels in PR-null cells remained unaffected by loss of HDAC3. These data support the notion that wt PR-B recruits HDAC3 to the STC1 promoter (in a SUMO-dependent manner) to mediate transcriptional repression (Fig. 3F). Similarly, to confirm the contribution of PR-associated SRC-1 to increased expression of STC1 in cells expressing SUMO-deficient K388R PR-B (Fig. 3A), we used specific siRNA to knockdown expression of SRC-1. T47D cells stably expressing K388R PR-B were transiently transfected with SRC-1 or nontargeted control siRNA (Fig. S5D). As predicted, SRC-1 siRNA, but not control siRNA, reduced STC1 mRNA expression in T47D cells stably expressing SUMO-deficient K388R PR-B (Fig. 3D). These data support the conclusion that constitutive association of unsumoylated PR with SRC-1 can drive transcription (i.e., contribute to the basal expression) of novel PR target genes in the absence of progestins (Fig. 3F).
Finally, we tested HB-EGF as a progestin regulated gene whose basal expression is insensitive to PR sumoylation, but responsive to hormone addition, in part via PR phosphorylation and desumoylation (Fig. S4B). In the presence of R5020, roughly equivalent amounts of both wt and K388R PR-B were recruited to the HB-EGF promoter region −1631–−1266, containing four PRE half sites (Fig. 3E). Interestingly, however, upon R5020 treatment, more HDAC3 was consistently associated with the HB-EGF promoter in cells containing wt PR-B, relative to cells containing K388R PR-B. These data suggest that PR-B sumoylation is required for efficient recruitment of HDAC3 to selected promoters (STC1 and HB-EGF); desumoylated PR-B may fail to maintain a stable complex with HDAC3 resulting in increased gene transcription (Figs. 2A and 3F and Fig. S4B).
Hormone-Independent PR Target Genes Contribute to Breast Cancer Cell Growth.
Multiple PR-target genes likely contribute to increased breast cancer cell growth and survival in cells with under-sumoylated PRs (Fig. 1C and S1) (5). IRS-1 is a key mediator of IGF-1–dependent breast cancer cell proliferation (17). We reasoned that cells expressing K388R PR-B should be more responsive to IGF-1 due to PR-dependent elevated IRS-1 expression. T47D cells expressing either wt, K388R or S294A PR-B were treated for 4 or 5 days with IGF-1, and viable cells were quantified by MTT assay (as in Fig. 1C). Cells expressing wt PR-B were modestly responsive to IGF-1 as measured by a 2- to 3-fold increased cell growth after 4–5 days (Fig. 4A). In contrast, cells expressing SUMO-deficient K388R PR-B demonstrated an increased response to this hormone (5–7-fold), whereas cells containing heavily sumoylated S294A PR remained relatively unresponsive (<2-fold) to IGF-1. These data suggest that the PR sumoylation state in the absence of progestins contributes to the sensitivity of breast cancer cells to IGF-1, in part via IRS-1 transcriptional upregulation (Fig. 2C).
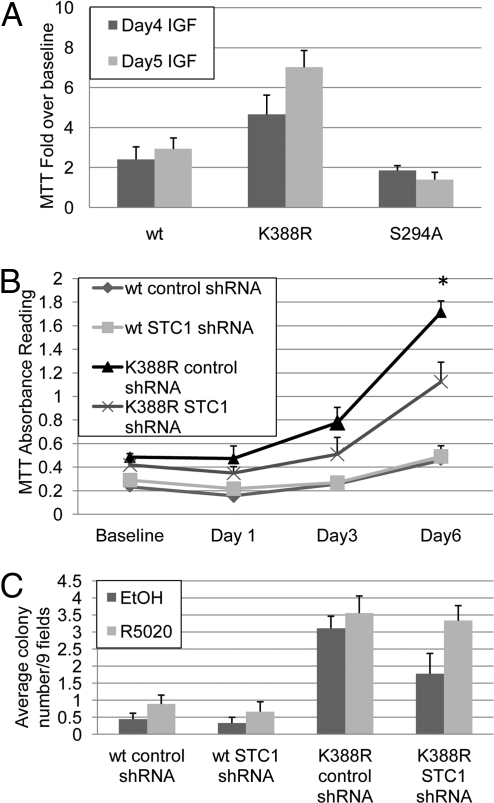
Fig. 4.
STC1 and IRS-1 Contribute to PR mediated breast cancer cell growth in the absence of progestins. (A) T47D cells stably expressing wt, K388R, or S294A PR-B in triplicate cultures were treated for 4–5 days with IGF, and MTT assays were performed. Bars represent IGF induced fold change over baseline (±SD). (B) T47D cells stably expressing wt or K388R PR-B and either control or STC1 shRNA in triplicate cultures were serum starved for 1–6 days and MTT assays were performed (±SD, *P < 0.006). Asterisk denotes significant differences compared to control shRNA expressing cells as determined by unpaired Student t tests. (C) T47D cells stably expressing wt or K388R PR-B and either control or STC1 shRNA were plated in triplicate cultures under serum-free conditions in soft agar containing ethanol or R5020 (10−8 M). After 21 days, colonies were counted and expressed as average colony number per nine fields (±SEM). Clone 7 T47D K388R PR-B cells were used throughout these experiments.
In addition to IRS-1, our studies implicate PR-dependent STC1 up-regulation in the increased basal proliferation of breast cancer cells expressing SUMO-deficient K388R PR-B (Fig. 1C and S1). To directly test the contribution of STC1 to cell proliferation, T47D breast cancer cells expressing either wt or K388R PR-B were engineered to stably express either STC1 shRNA or control shRNA. In both wt and K388R PR-B expressing cell lines, we routinely observed an approximate 50% decrease in STC1 transcript levels relative to control shRNA (Fig. S6). Further knockdown of this transcript was not achievable in stable cell lines, perhaps owing to the pro-proliferative function of this gene (12). Importantly, the stable knockdown of STC1 did not alter PR-B expression in either cell line (Fig. S6 inset). The specificity of STC1 knockdown was confirmed, as measured by constant levels of STC2 (Fig. S7A), a 34% homologous STC family member that is regulated by both progestin (Fig. S7B) and estrogen (19, 20) in breast cancer cells.
We then performed MTT assays in steroid hormone-free conditions using T47D cells stably expressing either wt or K388R PR-B and control shRNA or STC1 shRNA (Fig. 4B). Consistent with previous results (Fig. 1C), cells expressing SUMO-deficient K388R PR-B and control shRNA exhibited significantly increased proliferation (day 6) relative to cells expressing wt PR-B and either shRNA. However, partial knockdown of STC1 shRNA significantly blocked excess growth of cells expressing K388R PR-B. Importantly, targeting shRNA had no effect on cells expressing wt PR (i.e., in which STC1 is not elevated). In a similar MTT assay, an additional cell line containing shRNA targeting a different region of STC1 reduced expression levels by ≈25%, and resulted in an 18% decrease in K388R PR-B cell growth. These data reproduced in soft-agar colony assays (Fig. 4C). Cells expressing wt PR produced very few colonies in serum-free conditions and these were unaltered by stable STC1 knock-down. Again, cells containing K388R PR produced significantly more colonies in steroid hormone free soft-agar relative to cells containing wt PR. However, stable expression of STC1 shRNA blocked almost half of the heightened basal (serum-free) anchorage independent growth induced by K388R PR-B (Fig. 4C). The addition of R5020 reversed this effect, most likely due to regulation of other SUMO-sensitive but progestin-induced genes (i.e., HB-EGF). Together, these data illustrate the remarkable dependence of breast cancer cell growth on ligand-independent but PR-mediated induction of selected target genes (IRS-1 and STC1) in response to changes in PR Ser-294 phosphorylation status and thus the degree of Lys-388 sumoylation (Fig. 3F). We predict that a subset of human breast cancers is driven by this mechanism of PR derepression (discussed below).
Discussion
Progestins are both proliferative and antiproliferative in breast cancer models (21). Our data highlight the concept that the effect of PR activity on breast cancer cell proliferation is context dependent. In unstimulated cells, unliganded PRs are primarily growth suppressive (Fig. 1C and S1A). In the context of high kinase activities, these posttranslationally modified PRs function independently of ligand at selected promoters whose gene products clearly contribute to cell proliferation (Fig. 3F). The regulation of gene expression by phosphorylated and undersumoylated PRs is a novel form of hormone independent PR action that is predicted to contribute to breast cancer cell growth and survival.
PR Sumoylation Favors HDAC Binding but Prevents SRC1 Interaction.
Sumoylated PR regulates selected genes (STC1, HB-EGF) in part by altering cofactor recruitment to the promoter. We speculate that SUMO attachment to PR creates a binding pocket for HDACs. HDAC2 has been shown to mediate sumoylated Elk-1 transcriptional repression (22) and HDAC3 interacts only with the sumoylated form of SREBP-2, repressing its transcriptional activity (23). Upon inspection of the HDAC3 protein sequence we identified a region rich in isoluecine, valine, and leucine residues that is likely to behave as a SUMO interacting motif (SIM). This would allow HDAC3 to associate directly with the DNA bound sumoylated species of PR to alter local histone acetylation, thus creating repressive chromatin structure in the promoter region.
In the absence of ligand, STC1 and IRS-1 are clearly induced by unsumoylated PR. Upon the addition of ligand however, we observed a marked decrease in their expression. Perhaps ligand binding redirects PR species to different promoters (i.e., HB-EGF, SGK, and Muc1) or alters the ability of receptors to form active transcriptional complexes on these promoters, perhaps via additional posttranslational modifications. Notably, we were unable to detect sumoylation (i.e., on other Lys residues) of K388R PR (24). ChIP assays revealed that PR-B is detectable on the STC1 promoter in the presence of either R5020 (for 1 h) or RU486 (for 12 h) (Fig. S8), yet transcription is repressed (Fig. 2A). Perhaps in select promoter contexts, alteration of PR conformation induced by ligand binding facilitates the recruitment of corepressor complexes (i.e., other than HDAC3 containing) that prevent coactivator binding. Alternatively, SRC molecules have been shown to act as corepressors for PR transcriptional activity, most recently on the β-casein promoter (25). We have not ruled out progestin-induced changes in STC1 mRNA stability; studies to further understand the mechanism(s) of progestin and anti-progestin–induced repression of STC1 and IRS-1 expression are currently underway.
PR Sumoylation Mediates Promoter Selectivity.
The number of hormone response elements (HRE) present in the promoter can influence the ability of reporter genes to respond to sumoylation of steroid hormone receptors (6). However, the determinates of endogenous gene promoters that confer regulation by PR sumoylation/desumoylation are unknown. The responsiveness of a promoter to sumoylated PR is likely specified in part by the sequences surrounding PREs. So et al. showed that variations in the nucleotide sequences in GR binding sequences (GBSs), can alter dexamethoasone responsiveness (26). In addition, variation in estrogen response elements (EREs) predict the adoption of specific ER conformations that reveal binding surfaces for coregulatory molecules that dictate coregulator binding preferences (27), or in the case of sumoylated PR, may present SUMO binding surfaces for HDAC recruitment. Alternatively, promoters such as SGK may be insensitive to the recruitment of HDACs by sumoylated PR because their regulatory regions are held in an open chromatin conformation by other cofactors or epigenetic modifications. A better understanding of promoter selection by sumoylated PRs must await the analysis of larger cohorts of genes. These studies may reveal a set of “rules” for promoter sequences and contexts that are susceptible to regulation by sumoylated PRs.
Clinical Implications of PR Sumoylation/Desumoylation.
High intracellular kinase activity is a hallmark of breast cancer with implications for steroid receptor action in women with and without measurable circulating hormone levels. Notably, as with ER-α, PR expression in human breast tumors is predictive of a favorable prognosis. As tumors progress toward hormone-refractory stages, they most often keep their steroid hormone receptors. In the face of elevated signal transduction from tyrosine kinase growth factor receptors, both ER and PR remain highly active, but undergo rapid protein turnover, making them more difficult to detect (10, 28). In contrast to the normal breast where PR-A and PR-B are co-expressed at equal levels, breast cancers often contain aberrant PR-A:PR-B ratios, an early event in breast carcinogenesis (29). As tumors progress, frequent high A:B ratios are most often associated with loss of PR-B protein (rather than gain of PR-A) (30). In fact, PR loss is an excellent marker of heightened protein tyrosine kinase activities and tamoxifen resistance (31). Furthermore, cyclin E/cdk2, a direct input to PR phosphorylation at sites (including Ser-294) that augment both turnover (10) and transcription (32), is associated with breast cancer progression (16). We propose that in the case of PR-B, phosphorylated and undersumoylated receptors turnover rapidly and may be difficult to detect in clinical settings (primarily conducted by immunohistochemistry analyses). These unliganded but hyperactive receptors may contribute to breast cancer proliferation by the novel mechanism of PR derepression detailed herein. Our data explain why breast cancer models often fail to respond to added progestins, but are growth inhibited by anti-progestins, and underscore the need to routinely target PR as part of endocrine-based breast cancer therapy in combination with kinase pathway inhibitors and selective estrogen receptor modulators (SERMS) or aromatase inhibitors. This approach may be especially warranted in apparently steroid hormone receptor negative cases, where phosphorylated receptors may exhibit shortened half-lives but heightened transcriptional activities or derepression at key growth-promoting genes.
Materials and Methods
Cell Culture.
T47D-Y, -YB, -K388R (clones 7, 24, 28) and -S294A cells were previously described (5, 33, 34). HeLa cells were cultured as described previously (10, 13).
Immunoblotting.
SUMO assays were carried out as previously described (5). Membranes were probed with primary antibodies recognizing total PR (MS-298, Lab Vision Corp., Fremont, CA), phospho-S294 PR (Lab Vision Corp.), p44/p42 MAPK (Cell Signaling Technology, Danvers, MA), and IRS-1 (35).
RT-PCR and Real-Time Quantitative PCR.
RNA isolation, cDNA synthesis, and real-time quantitative PCR were performed as previously described (SI Materials and Methods) (4).
Growth Assays.
Soft agar and MTT assays were performed as previously described (35, 36).
Chromatin Immunoprecipitations.
ChIP assays were performed as previously described (37). Antibodies used were PR (H-190), HDAC3, SRC-1, and IgG (Santa Cruz Biotechnology Inc., Santa Cruz, CA) or RNAPolII (Covance, Emeryville, CA).
Supplementary Material
Acknowledgments.
We are grateful to Douglas Yee for the gift of the antibody against IRS-1. This work was supported by National Institutes of Health Grants R01 CA123763 (formerly DK053825) and R21 CA116790 (to C.A.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905118106/DCSupplemental.
References
- 1.Brisken C, et al. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA. 1998;95:5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conneely OM, Jericevic BM, Lydon JP. Progesterone receptors in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2003;8:205–214. doi: 10.1023/a:1025952924864. [DOI] [PubMed] [Google Scholar]
- 3.Qiu M, Lange CA. MAP kinases couple multiple functions of human progesterone receptors: Degradation, transcriptional synergy, and nuclear association. J Steroid Biochem Mol Biol. 2003;85:147–157. doi: 10.1016/s0960-0760(03)00221-8. [DOI] [PubMed] [Google Scholar]
- 4.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27:466–480. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniel AR, Faivre EJ, Lange CA. Phosphorylation-dependent antagonism of sumoylation derepresses progesterone receptor action in breast cancer cells. Mol Endocrinol. 2007;21:2890–2906. doi: 10.1210/me.2007-0248. [DOI] [PubMed] [Google Scholar]
- 6.Holmstrom S, Van Antwerp ME, Iniguez-Lluhi JA. Direct and distinguishable inhibitory roles for SUMO isoforms in the control of transcriptional synergy. Proc Natl Acad Sci USA. 2003;100:15758–15763. doi: 10.1073/pnas.2136933100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobsen BM, Schittone SA, Richer JK, Horwitz KB. Progesterone-independent effects of human progesterone receptors (PRs) in estrogen receptor-positive breast cancer: PR isoform-specific gene regulation and tumor biology. Mol Endocrinol. 2005;19:574–587. doi: 10.1210/me.2004-0287. [DOI] [PubMed] [Google Scholar]
- 8.Richer JK, et al. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Hafiz H, Takimoto GS, Tung L, Horwitz KB. The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J Biol Chem. 2002;277:33950–33956. doi: 10.1074/jbc.M204573200. [DOI] [PubMed] [Google Scholar]
- 10.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci USA. 2000;97:1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groshong SD, et al. Biphasic regulation of breast cancer cell growth by progesterone: Role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1) Mol Endocrinol. 1997;11:1593–1607. doi: 10.1210/mend.11.11.0006. [DOI] [PubMed] [Google Scholar]
- 12.Chang AC, Jellinek DA, Reddel RR. Mammalian stanniocalcins and cancer. Endocr Relat Cancer. 2003;10:359–373. doi: 10.1677/erc.0.0100359. [DOI] [PubMed] [Google Scholar]
- 13.Qiu M, Olsen A, Faivre E, Horwitz KB, Lange CA. Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endocrinol. 2003;17:628–642. doi: 10.1210/me.2002-0378. [DOI] [PubMed] [Google Scholar]
- 14.Shen T, Horwitz KB, Lange CA. Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol Cell Biol. 2001;21:6122–6131. doi: 10.1128/MCB.21.18.6122-6131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krek W, Nigg EA. Mutations of p34cdc2 phosphorylation sites induce premature mitotic events in HeLa cells: Evidence for a double block to p34cdc2 kinase activation in vertebrates. EMBO J. 1991;10:3331–3341. doi: 10.1002/j.1460-2075.1991.tb04897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Beltran A, MacLennan GT, Montironi R. Cyclin E as molecular marker in the management of breast cancer: A review. Anal Quant Cytol Histol. 2006;28:111–114. [PubMed] [Google Scholar]
- 17.Byron SA, et al. Insulin receptor substrates mediate distinct biological responses to insulin-like growth factor receptor activation in breast cancer cells. Br J Cancer. 2006;95:1220–1228. doi: 10.1038/sj.bjc.6603354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniel AR, et al. Linkage of progestin and epidermal growth factor signaling: Phosphorylation of progesterone receptors mediates transcriptional hypersensitivity and increased ligand-independent breast cancer cell growth. Steroids. 2007;72:188–201. doi: 10.1016/j.steroids.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouras T, et al. Stanniocalcin 2 is an estrogen-responsive gene coexpressed with the estrogen receptor in human breast cancer. Cancer Res. 2002;62:1289–1295. [PubMed] [Google Scholar]
- 20.Charpentier AH, et al. Effects of estrogen on global gene expression: Identification of novel targets of estrogen action. Cancer Res. 2000;60:5977–5983. [PubMed] [Google Scholar]
- 21.Lange CA, et al. Progesterone receptor action: Translating studies in breast cancer models to clinical insights. Adv Exp Med Biol. 2008;630:94–111. [PubMed] [Google Scholar]
- 22.Yang SH, Sharrocks AD. SUMO promotes HDAC-mediated transcriptional repression. Mol Cell. 2004;13:611–617. doi: 10.1016/s1097-2765(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 23.Arito M, Horiba T, Hachimura S, Inoue J, Sato R. Growth factor-induced phosphorylation of srebps inhibits sumoylation, thereby stimulating the expression of their target genes, LDL uptake and lipid synthesis. J Biol Chem. 2008;283:15224–15231. doi: 10.1074/jbc.M800910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Man JH, et al. PIAS3 induction of PRB sumoylation represses PRB transactivation by destabilizing its retention in the nucleus. Nucleic Acids Res. 2006;34:5552–5566. doi: 10.1093/nar/gkl691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boonyaratanakornkit V, et al. Keystone Symposia on Molecular Cell Biology, Nuclear Receptors: Orphan Brothers and Steroid Sisters. Silverthorne, CO: Keystone Symposia; 2008. The role and mechanims of progesterone receptor crosstalk with signal transduction pathways; p. 109. [Google Scholar]
- 26.So AY, Cooper SB, Feldman BJ, Manuchehri M, Yamamoto KR. Conservation analysis predicts in vivo occupancy of glucocorticoid receptor-binding sequences at glucocorticoid-induced genes. Proc Natl Acad Sci USA. 2008;105:5745–5749. doi: 10.1073/pnas.0801551105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall JM, McDonnell DP, Korach KS. Allosteric regulation of estrogen receptor structure, function, and coactivator recruitment by different estrogen response elements. Mol Endocrinol. 2002;16:469–486. doi: 10.1210/mend.16.3.0814. [DOI] [PubMed] [Google Scholar]
- 28.Chu I, et al. Src promotes estrogen-dependent estrogen receptor alpha proteolysis in human breast cancer. J Clin Invest. 2007;117:2205–2215. doi: 10.1172/JCI21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mote PA, Bartow S, Tran N, Clarke CL. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat. 2002;72:163–172. doi: 10.1023/a:1014820500738. [DOI] [PubMed] [Google Scholar]
- 30.Graham JD, et al. Characterization of progesterone receptor A and B expression in human breast cancer. Cancer Res. 1995;55:5063–5068. [PubMed] [Google Scholar]
- 31.Arpino G, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: Association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97:1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 32.Pierson-Mullany LK, Lange CA. Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2. Mol Cell Biol. 2004;24:10542–10557. doi: 10.1128/MCB.24.24.10542-10557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lange CA, Richer JK, Shen T, Horwitz KB. Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogen-activated protein kinase pathways. J Biol Chem. 1998;273:31308–31316. doi: 10.1074/jbc.273.47.31308. [DOI] [PubMed] [Google Scholar]
- 34.Sartorius CA, et al. New T47D breast cancer cell lines for the independent study of progesterone B- and A-receptors: Only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 1994;54:3868–3877. [PubMed] [Google Scholar]
- 35.Sachdev D, et al. A chimeric humanized single-chain antibody against the type I insulin-like growth factor (IGF) receptor renders breast cancer cells refractory to the mitogenic effects of IGF-I. Cancer Res. 2003;63:627–635. [PubMed] [Google Scholar]
- 36.Ostrander JH, Daniel AR, Lofgren K, Kleer CG, Lange CA. Breast tumor kinase (protein tyrosine kinase 6) regulates heregulin-induced activation of ERK5 and p38 MAP kinases in breast cancer cells. Cancer Res. 2007;67:4199–4209. doi: 10.1158/0008-5472.CAN-06-3409. [DOI] [PubMed] [Google Scholar]
- 37.Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol. 2008;22:823–837. doi: 10.1210/me.2007-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.