Abstract
Akt1 is implicated in cell metabolism, survival migration, and gene expression; however, little is known about the role of specific Akt isoforms during inflammation in vivo. Thus, we directly explored the roles of the isoforms Akt1 and Akt2 in acute inflammation models by using mice deficient in either Akt1 or Akt2. Akt1−/− mice showed a markedly reduced edema versus Akt2−/− and WT controls, and the reduced inflammation was associated with a dramatic decrease in neutrophil and monocyte infiltration. The loss of Akt1 did not affect leukocyte functions in vitro, and bone marrow transplant experiments suggest that host Akt1 regulates leukocyte emigration into inflamed tissues. Moreover, carrageenan-induced edema and the direct propermeability actions of bradykinin and histamine were reduced dramatically in Akt1−/− versus WT mice. These findings are supported by in vitro experiments showing that Akt1 deficiency or blockade of nitric oxide synthase markedly reduces histamine-stimulated changes in transendothelial electrical resistance of microvascular endothelial cells. Collectively, these results suggest that Akt1 is necessary for acute inflammation and exerts its actions primarily via regulation of vascular permeability, leading to edema and leukocyte extravasation.
Keywords: endothelium, nitric oxide
Acute inflammatory reactions are characterized by increased postcapillary venule permeability to fluid and plasma proteins and leukocyte emigration into tissues. In nonimmune models, acute inflammation induced by agents such as carrageenan causes an immediate increase in vascular permeability, triggered by proinflammatory mediators such as bradykinin, histamine, tachykinins, complement and nitrogen oxide species. This occurs simultaneously with the recruitment of neutrophils (0–6 h), followed by monocytes and macrophages (<24 h) to the site of inflammation. Several classes of antiinflammatory drugs including nonsteroidal antiinflammatory agents have been shown to be active in this model of inflammation (1).
The phosphoinositide 3-kinase (PI3K)/Akt (also known as protein kinase B) signaling pathway is involved in regulating the inflammatory response. Several studies have reported the importance of the PI3K/Akt pathway in inflammation-mediated diseases, such as rheumatoid arthritis (2), multiple sclerosis (3), asthma (4), chronic obstructive pulmonary disease (5), psoriasis (6), and atherosclerosis (7). In particular, there is good evidence that PI3K-γ, predominantly expressed in leukocytes, is critical to the inflammatory response and, more specifically, to leukocyte functions (8, 9). PI3K-γ−/− neutrophils exhibit impaired chemotactic responses toward IL-8, N-formyl-Met-Leu-Phe (fMLP), complement component C5 activated, and macrophage inflammatory protein-1α in vitro, whereas mice lacking PI3K-γ show defective migration of macrophages and neutrophils to sites of infection and defects in adaptative immunity in vivo (8, 9). However, the role of Akt1 in inflammation is less well studied.
Akt is a multifunctional kinase implicated in a broad range of cellular functions, including survival, proliferation, gene expression, and migration of cells of most lineages; thus, Akt plays a central role in both physiological and pathological signaling mechanisms (10). In mammals, there are three different Akt isoforms, Akt1, Akt2, and Akt3, products of different genes, which share >85% sequence homology. Recent studies of Akt knockout mice have shown that despite significant sequence homology the three Akt isoforms have nonredundant functions. Although Akt1-deficient mice exhibit overall growth impairment (11–13), Akt2 knockout mice have impaired glucose tolerance and insulin resistance (14), and Akt3 null mice display a selective reduction in brain size (15, 16). In the vascular system, Akt1-deficient mice have defective placental angiogenesis associated with a reduction in endothelial nitric oxide synthase (eNOS) phosphorylation on serine 1176 (13). Akt1 is the main Akt isoform expressed in endothelial cells, and the loss of Akt1 results in reduced nitric oxide (NO) release and impaired postnatal angiogenesis in some (13, 17, 18), but not all (19), models of angiogenesis. In addition, breeding Akt1-deficient mice with atherosclerotic mice enhances atherosclerosis and promotes coronary disease primarily due to Akt localized in vascular cells not circulating cells (7). However, the role of specific Akt isoforms in the inflammatory process is still largely unknown.
To explore directly the role of Akt in acute inflammation, we used mice deficient in either Akt1 or Akt2 and examined the extent of inflammation in vivo. Here, we show that the loss of Akt1, but not Akt2, dramatically suppresses microvascular permeability and leukocyte recruitment to sites of inflammation in models of inflammation and in response to histamine and bradykinin. This effect is due to Akt1 in the host, not circulating, cells, and in vitro experiments show the critical role of the Akt/eNOS pathway and vascular endothelial (VE)-cadherin in promoting histamine-mediated junctional permeability. Thus, inhibition of Akt1 may be beneficial in conditions of exaggerated microvascular leakage.
Results
Loss of Akt1, but Not Akt2, Markedly Reduces Edema Formation and Neutrophil and Monocyte Infiltration.
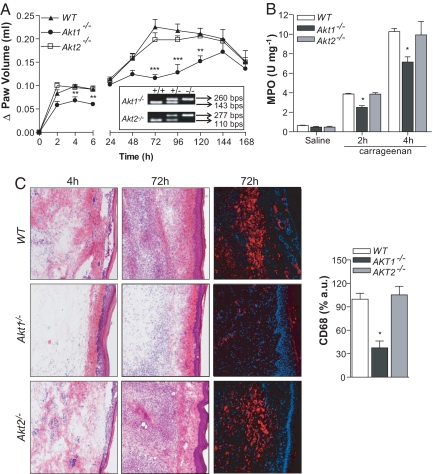
To evaluate if the genetic loss of Akt influences acute inflammation, WT, Akt1−/−, and Akt2−/− mice were injected with carrageenan, and the time course of paw edema was assessed. As seen in Fig. 1A, WT and Akt2−/− mice developed severe paw swelling in the early (0–6 h) and later phases (>24 h) of the response, whereas Akt1−/− mice showed reduced paw edema throughout the study. During the evolution of an acute inflammatory response, peripheral blood neutrophils are the first responder cells to arrive, followed by mononuclear cells. Thus, to evaluate neutrophil infiltration into the paw, myeloperoxidase (MPO) assays were performed on the inflamed tissue in the early phase of the response. Akt1−/− mice showed a significant reduction in MPO activity compared with WT and Akt2−/− mice at 2 and 4 h after carrageenan injection (Fig. 1B). Histological assessment of the inflamed paw clearly showed a reduced number of infiltrating leukocytes in Akt1−/− mice compared with WT and Akt2−/− mice at early and late time points after carrageenan injection (Fig. 1C). Immunofluorescent staining for CD68, a monocyte/macrophage marker, after 72 h showed a dramatic reduction in monocyte/macrophage infiltration in Akt1−/− mice compared with that in WT and Akt2−/− mice (Fig. 1C). Thus, Akt1 is necessary for the development of the acute inflammatory response.
Fig. 1.
Paw edema is markedly reduced in Akt1−/− mice in both early and late phases of inflammation. (A) Akt1−/− mice display reduced edema in the first phase (0–6 h) and in the second phase (>24 h) (n = 6 per group). (B) Myeloperoxidase (MPO) activity, an index of the neutrophil influx into the paw 2 and 4 h after subplantar injection of carrageenan (2% wt/vol) into the paw. Akt1−/− mice display a reduction in tissue MPO levels at 2 and 4 h time points (n = 5 per group). (C) Hematoxylin and eosin staining of carrageenan-injected paws at 4 (left) and 72 h (center) time points. Immunostaining of WT and Akt1−/− histological paw sections for CD68, a monocyte/macrophage marker, at 72 h after carrageenan injection (right). The Akt1−/− mice show a marked reduction in leukocyte infiltration in both early and late phases of paw edema compared with those of WT and Akt2−/− mice. Data are expressed as mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (Magnification, 10×.)
Akt1 Is Not Required for Neutrophil Migration and Monocyte Activation.
To delineate if the loss of Akt influenced leukocyte function, vascular function, or both during acute inflammation, we initially studied the importance of Akt in bone marrow neutrophils (BMNs) and bone-marrow-derived monocytes (BMDMs) (Fig. S1). Previous work has shown that the chemotactic response to G-protein-coupled receptors (GPCRs) agonists is correlated with PI3K-dependent activation of Akt (20). Thus, we evaluated the role of Akt1 in neutrophil migration in vitro using the GPCR agonists fMLP and IL-8. The loss of Akt1 did not influence the chemotactic response of BMNs induced by fMLP or IL-8 (Fig. S1 A and B). Western blot analysis for activation of fMLP-stimulated activation of ERK p42/44 and p38 MAPK revealed only a slight reduction in p42/p44 phosphorylation, with no effect on fMLP-induced p38 activation in Akt1−/− cells (Fig. S1C). We next focused on the role of Akt1 in BMDMs. We have reported previously that the migratory capacity of BMDMs toward monocytes chemotactic protein-1 was not impaired by the loss of Akt1 (7). Here, we show that absence of Akt1 did not affect the number of baseline circulating monocytes (physiological) or the number of monocytes 72 h after carrageenan administration (pathologic) (Fig. S2) and that Akt1−/− BMDMs expressed the same patterns of adhesion and lineage-specific molecules (CD11a, CD11b, P-selectin glycoprotein ligand-1, and F4/80) as did WT BMDMs (Fig. S1D). To address the role of Akt1 in host cell responses, WT, Akt1−/−, and Akt2−/− BMDMs were stimulated with cytokine-rich exudates recovered from the air pouch of WT mice 24 h after carrageenan injection, and we measured BMDM cytokine levels. As seen in Fig. S1E, treatment of BMDMs with exudates increased the mRNA levels of IL-1β, IL-6, and TNF-α; however, they were not different among the groups nor were the levels of COX-2 by Western blot analysis (Fig. S1F). Thus, the loss of Akt in BMNs and BMDMs does not influence their migratory activity or responsiveness to chemokines.
Host Vascular Akt1 Regulates Leukocyte Recruitment in Response to Carrageenan.
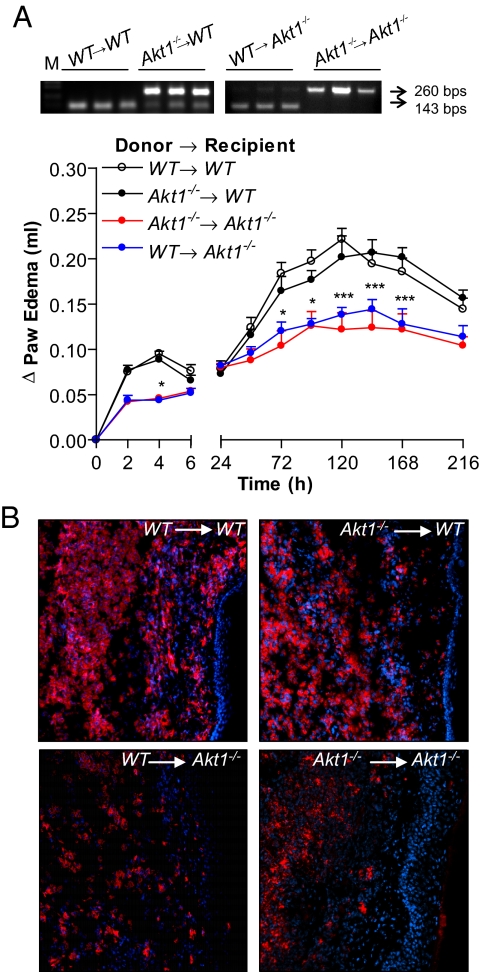
To investigate the role of leukocyte versus host vascular Akt1 in the integrated inflammatory response in vivo, we performed bone marrow transplantation (BMT) experiments. The WT and Akt1−/− mice were lethally irradiated and engrafted with bone marrow from WT mice (WT → WT; WT → Akt1−/−) or Akt1−/− mice (Akt1−/− → Akt1−/−; Akt1−/− → WT) and left to reconstitute for 5 weeks. After this time, mice were injected with carrageenan, and paw edema was assessed. As seen in Fig. 2A, transfer of Akt1−/− bone marrow (BM) into WT hosts had no effect on the magnitude and duration of the edema formation compared with mice engrafted with WT BM, and the transfer of WT BM into Akt1−/− mice did not alter the profile of the paw edema versus the Akt1−/− → Akt1−/− group (Fig. 2A). This was confirmed histologically after staining for CD68+ macrophages in inflamed tissues (Fig. 2B). Collectively, the above in vitro and in vivo data argue against the importance of Akt1 in inflammatory cells.
Fig. 2.
Host vascular Akt1 drives leukocyte recruitment in response to carrageenan. (A) Carrageenan-induced paw edema was investigated in lethally irradiated WT mice injected with bone marrow from Akt1−/− mice (filled circle) or WT mice (open circle) as a control and lethally irradiated Akt1−/− mice injected with bone marrow from WT mice (blue circle) or Akt1−/− mice (red circle; n = 7 per group). (B) CD68 immunostaining of paw sections 72 h after carrageenan injection. (Magnification, 10×.)
Dissection of Changes in Vascular Permeability versus Neutrophil Infiltration in Akt1−/− Mice.
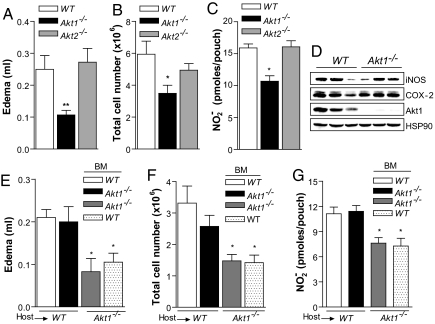
To dissect the role of Akt1 in modulating microvascular permeability versus leukocyte recruitment in vivo, we created air pouches on the backs of the mice, which permit independent quantification of edema and cellular infiltrates after instillation of carrageenan into the pouch. Notably, edema formation was markedly blunted in Akt1−/− mice compared with that in WT and Akt2−/− mice (Fig. 3A), as was neutrophil infiltration 4 h after carrageenan injection (Fig. 3B). Because eNOS is an Akt1 substrate (21, 22) activated by inflammatory mediators, we measured nitrite (NO2−) accumulation, a stable breakdown product of NO, 4 h after intrapouch carrageenan injection. The Akt1−/− mice showed a significant reduction in NO2− content in the exudate compared with those in the WT and Akt2−/− mice (Fig. 3C); however, the induction of inducible nitric oxide synthase and COX-2 in neutrophils was similar in WT and Akt1−/− mice (Fig. 3D; from three mice per genotype). To confirm the role of host Akt1 in leukocyte transmigration and edema formation, we used the carrageenan air pouch models in BMT mice. The extent of edema and the number of neutrophils recovered were markedly reduced in Akt1−/− → Akt1−/− and WT → Akt1−/− mice compared with those in WT → WT and Akt1−/− → WT mice, suggesting that host Akt1, presumably vascular Akt1, is necessary for edema formation and leukocyte influx at the site of inflammation (Fig. 3 E–G). We have reported previously that the loss of Akt1 does not affect TNF-α-induced adhesion molecules expression such as ICAM-1 and VCAM (7). Here we showed that Akt1−/− mouse lung endothelial cells (MLECs) expressed the same levels of E-selectin of WT MLECs, suggesting that the absence of Akt1 does not affect the expression of adhesion molecules induced by TNF-α (Fig. S4 and SI Materials and Methods). Western blot analysis of neutrophils recovered from the pouch confirmed the engraftment of Akt1−/− BM in WT mice and WT BM in Akt1−/− mice (Fig. S3; four mice per group).
Fig. 3.
Dissection of acute permeability versus neutrophil infiltration in Akt1−/− mice. The Akt1−/− mice display a marked reduction in (A) edema formation, (B) neutrophil infiltration, and (C) NO2− production in air pouch inflammation induced by carrageenan (1% wt/vol) compared with those of the WT control. (D) Twenty-four hours after intrapouch injection of carrageenan, neutrophils are recovered and evaluated for COX-2 and inducible nitric oxide synthase expression by Western blot analysis (n = 3, per group). The carrageenan air pouch (4 h) in chimeric mice did not show differences in edema formation, neutrophil accumulation, or NO2− levels between WT mice engrafted with WT (open bar) and Akt1−/− (filled bar) bone marrow or between Akt1−/− mice engrafted with WT (gray bar) and Akt1−/− (dotted bar; n = 5 per group). The data shown represent the mean ± SEM. *, P < 0.05; **, P < 0.01
Evidence That Akt1 Is Essential for Acute Changes in Vascular Permeability.
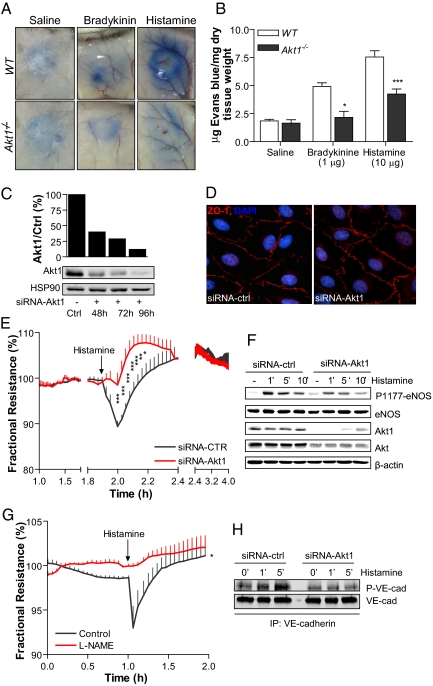
Carrageenan triggers the release of kinins and histamine as mediators of vascular leakage during inflammation. To directly test if Akt1 is necessary for vascular leakage, we examined the propermeability actions of bradykinin and histamine in a modified Miles assay in WT and Akt1−/− mice. After intradermal injection of bradykinin and histamine, the extravasation of Evan's blue (a tracer that binds albumin as index of protein leakage into tissue) was increased in WT mice but blunted in Akt1−/− mice (Fig. 4 A and B), suggesting that the loss of Akt1 attenuates the propermeability effect of bradykinin and histamine in vivo. To dissect the effects of histamine on the endothelial barrier from the changes in blood flow that occur during inflammation in vivo, we investigated the role of Akt1 in histamine-induced permeability by measuring transendothelial electrical resistance (TEER) in human dermal microvascular endothelial cells (HDMECs) in vitro. The HDMECs were transfected with control siRNA or Akt1 siRNA to reduce the levels of Akt1. As shown in Fig. 4C, siRNA against Akt1 results in a reduction in Akt1 protein levels in HDMECs by up to 80% when compared with negative control siRNA, and the knockdown of Akt1 did not grossly alter the morphology and the junctional integrity of endothelial cells, as demonstrated by immunofluorescence staining for zona occludins-1 (Fig. 4D) and baseline TEER levels (2,207 ± 138.2 and 2,081 ± 108.9 Ω for control siRNA versus Akt siRNA, respectively; n = 4 independent experiments in triplicate). Treatment of HDMECs with histamine acutely reduced TEER (expressed as fractional resistance; Fig. 4E, black line), and this effect recovered back to baseline levels within 20 min and was stable for close to 2 h. In contrast, transfection of Akt1 siRNA markedly impaired histamine-induced changes in TEER and improved barrier function (Fig. 4E, red line). Because Akt can phosphorylate eNOS and eNOS-derived NO regulates aspects of vascular permeability, we examined histamine-stimulated eNOS phosphorylation in siRNA-treated cells. Histamine stimulated a time-dependent increase in eNOS phosphorylation on serine 1177, an effect reduced by Akt1 siRNA. To test whether the inhibition of NO production would affect histamine-induced changes in TEER, we used N(G)-nitro-l-arginine methyl ester (l-NAME) as a well characterized inhibitor of nitric oxide synthase (NOS). As shown in Fig. 4G, the histamine-induced TEER was blunted in the presence of l-NAME, suggesting that Akt activation of eNOS contributed to changes in vascular permeability. To further determine if Akt regulates aspects of histamine coupling to VE-cadherin, an important component of paracellular signaling in endothelial cells, we examined the effects of Akt1 silencing on histamine-induced phosphorylation of VE-cadherin. The HDMECs were transfected with control or Akt1 siRNA (72 h) and then stimulated with histamine (10 μM for 1 and 5 min), and cell lysates were subjected to immunoprecipitation with a VE-cadherin antibody. As shown in Fig. 4H, tyrosine phosphorylation of VE-cadherin in response to histamine was blunted in Akt1-deficient endothelial cells compared with that in the control. Collectively, these data point out the importance of the Akt1/eNOS/VE-cadherin pathway in promoting acute changes in endothelial permeability induced by histamine, in vivo and in vitro.
Fig. 4.
Propermeability actions of bradykinin and histamine are blunted in Akt1−/− mice and endothelial cells in vitro. Evans blue dye (30 mg/kg) was injected in the tail vein and (A) saline, bradykinin, or histamine was immediately injected intradermally. (B) Quantification of Evans blue dye. Data represent four mice per group and were calculated as microgram of dye per gram of dry tissue weight. *, P < 0.05. (C) The expression of Akt1 in human dermal microvascular endothelial cells (HDMECs) tranfected with siRNA against Akt1. Data are expressed as mean ± SD of five separate experiments. (D) Immunofluorescence analysis of interendothelial contacts of HDMECs. Representative staining of HDMECs treated with Akt1 and control siRNA, showing the distribution of zona occludins-1. (E) Transendothelial electrical resistance (TEER) measurements of postconfluent HDMECs transfected with control (black line) or Akt1 siRNA (red line) are expressed as fractional resistance (%) of the TEER basal values. The HDMECs were stimulated with histamine (10 μM), and TEER was measured over time. (F) Histamine-induced endothelial nitric oxide synthase (eNOS) phosphorylation on serine 1179 was analyzed by Western blot in HDMECs transfected with control siRNA or with siRNA against Akt1. A representative immunoblot of three separate experiments is shown. (G) Change in fractional resistance of HDMECs incubated with N(G)-nitro-l-arginine methyl ester (l-NAME; 100 μM) for 30 min or vehicle followed by histamine stimulation (10 μM). (H) The HDMECs were transfected with Akt1 or control siRNA and 72 h later were stimulated with histamine (10 μM; 1 and 5 min). At the indicated times, vascular endothelial (VE)-cadherin immunoprecipitates were analyzed by immunoblotting using either antibodies against phosphorylated tyrosine or VE-cadherin. The data are representative of four independent experiments; every experiment was performed in triplicate. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by two-way ANOVA, Bonferroni posttest (n = 12).
Discussion
The major finding of this study is that the loss of Akt1 in vivo markedly suppresses acute changes in microvascular permeability induced by carrageenan, bradykinin, and histamine. The reduction in permeability reduces the influx of neutrophils and monocytes at the site of injury, and this phenotype is specifically attributable to Akt1 expressed in host vasculature and not in inflammatory cells. Moreover, these in vivo results are supported by in vitro studies showing a critical role for the Akt1–eNOS axis and VE-cadherin in regulating histamine-stimulated changes in junctional permeability. Thus, our data suggest that inhibitors of the PI3K/Akt signaling pathway may be beneficial in conditions of enhanced vascular leakage associated with acute inflammatory responses.
A growing body of evidence suggests a role for PI3K/Akt signaling in the regulation of acute and chronic inflammatory processes, and compounds that antagonize the PI3K or Akt isoforms are promising drug targets for the modulation of inflammatory and autoimmune disorders and cancer and cardiovascular diseases (23). A hallmark of the inflammatory process is an increase in microvascular permeability, which facilitates the delivery of protein and cells from the vascular space to extravascular tissues. In these models, bradykinin, histamine, prostaglandins, and endothelial-derived NO have been characterized as mediators of permeability in the early phase. These agonists that promote edema bind to their cognate receptors and activate signaling pathways that result in the development of transient changes in focal discontinuities between adjacent endothelial cells. In particular, histamine acts primarily to alter sites of VE-cadherin-mediated cell–cell adhesion associated with an increase in centripetal tension, thus pulling on the endothelial junctions. Here, we show that in two models of inflammation the loss of Akt1 markedly suppresses the development of edema and the direct propermeability effects of bradykinin and histamine, suggesting a critical role for Akt1, but not Akt2, in microvascular leakage. Our data are supported by recent experiments showing the importance of Akt1 in VEGF-A-induced acute permeability (17, 24–26), tumor permeability (27), and the coupling of Akt to eNOS signaling (22, 28, 29) and results showing less eNOS-derived NO in exudates of Akt1−/− mice. Moreover, the in vivo experiments were supported by the in vitro data showing that histamine-induced changes in electrical resistance were blunted in HDMECs treated with siRNA against Akt1 or with the NOS inhibitor l-NAME. Our data are consistent with a report showing that the first phase of carrageenan-induced paw edema is reduced dramatically in eNOS−/− mice and in C57BL/6J mice locally administered with LY294002, a pan-inhibitor of PI3K, suggesting a critical role for PI3K/Akt/eNOS-derived NO in the early changes in microvascular permeability (29). Others have shown that histamine release from perivascular mast cells contributes to the early phase of inflammation and that NO can reduce the degranulation of mast cells (30). However, in different paradigms, NO has been shown to participate in inflammation downstream of mast cell degranulation (17, 22, 24–26, 28, 29).
In principle, the changes in permeability in both Akt−/− and eNOS−/− mice could be attributed to altered blood flow, effects of the genes on endothelial barrier function, or both. A critical role for eNOS and Akt in blood flow is appreciated (31, 32); however, our in vitro experiments in cultured endothelial cells point toward a critical autocrine role of the Akt–eNOS axis in regulating junctional integrity, independent of hemodynamics. That VE-cadherin is critical for the stability of adherent junctions by binding catenins and thereby mediating attachment to the cortical cytoskeleton is well established. Histamine activation of endothelial cells causes an increase in monolayer permeability in vitro, and this effect is accompanied by tyrosine phosphorylation of VE-cadherin and the partial dissociation of VE-cadherin from the cytoskeleton (33). Here, we show that histamine-induced phosphorylation of VE-cadherin is reduced dramatically in Akt1-siRNA-trasfected HDMECs compared with that of the control, suggesting that Akt either via eNOS or independent of eNOS regulates VE-cadherin signaling, leading to changes in permeability.
In conclusion, the loss of Akt1, but not Akt2, suppresses acute inflammatory responses to carrageenan, histamine, and bradykinin, and this is likely due to the absence of Akt1 in the host, but not in bone-marrow-derived mononuclear, cells. Mechanistically, Akt1 is critical for promoting microvascular leakage in response to inflammatory stimuli such as histamine and thus exerts a controlling influence over the magnitude of this response. Thus, the specific inhibition of Akt1 could provide a therapeutic approach to reduce vascular permeability and excessive leukocyte migration during histamine-mediated acute inflammatory reaction or perhaps during conditions of excessive vascular leakage.
Materials and Methods
Animals.
The Akt1−/− and Akt2−/− mice were generated as described in refs. 12 and 14. Congenic male, 8- to 14-week-old Akt1 knockout mice and their WT littermates were used. Also, F5 and F6 generation, male, 8- to 14-week-old Akt2−/− knockout mice and their WT littermates were used. The number of blood cells was determined by using an automated cell counter (Hemavet Multispecies Hematology System; Drew Scientific). All experiments were approved by the Institutional Animal Care and Use Committee of Yale University.
Induction of Edema in the Mouse Paw.
Male Akt1−/−, Akt2−/−, and littermate mice were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). Each group of animals received subplantar injection of 50 μL of saline or 50 μL of λ-carrageenan (2% wt/vol in saline; Sigma) into the left footpad (34).
Carrageenan Air Pouch.
Air pouches were generated by s.c. injecting 3 mL of sterilized air (filtered through a 0.2-μm Millipore filter) into the back of mice on days 0 and 3. On day 6, 0.5 mL of carrageenan suspension (1% wt/vol) in sterile saline was injected into the pouch. Air pouches were washed 2 or 4 h later with 1 mL of PBS containing 3 mM EDTA (35).
Myeloperoxidase Activity Measurement.
Tissue MPO activity was measured as an index of neutrophil recruitment at 2 and 4 h after carrageenan injection as described in ref. 36.
Bone Marrow Transplantation.
Eight-week-old male C57BL/6 and Akt1−/− mice were lethally irradiated with 900 rads (9 Gy) from a X-ray source 4 h before transplantation. Bone marrow was collected from femurs of donor C57BL/6 and AKT1−/− mice by flushing with sterile medium (RPMI medium 1640, 2% FBS, 5 U/mL heparin, 50 U/mL penicillin, and 50 μg/mL streptomycin). Each recipient mouse was injected with 5 × 106 bone marrow cells through the tail vein. Five weeks after BMT, peripheral blood was collected from the tail vein for PCR analysis of bone marrow reconstitution. Carrageenan-induced paw edema was performed in fully chimeric mice as described above.
Modified Miles Assay.
Under anesthesia, mice were injected intravenously with Evans blue (30 mg/kg; Sigma-Aldrich). Bradykinin (1 μg/20 μL; Sigma-Aldrich), histamine (0.1 mg/20 μL; Sigma-Aldrich), or saline was injected s.c. into the dorsal skin, respectively. After 30 min, mice were euthanized and perfused, and the skin was removed, oven-dried at 55 °C, and weighed. Evans blue was then extracted from the skin using 500 μL of formamide for 24 h at 55 °C. Evans blue extravasation into the skin was measured spectrophotometrically at 630 nm using a standard curve of Evans blue in formamide.
Cell Resistance Measurement.
The HDMEC barrier function was assayed by measuring the resistance of a cell-covered electrode by using an ECIS instrument (Applied BioPhysics). An 8W10E plate was incubated for 15 min with l-cysteine (10 mM) solution, followed by gelatin 0.1% for 30 min. Cells were plated on the electrode at 4 × 104 cells per well. The day after being plated, the cells were transfected with siRNA for Akt1 or control siRNA. Seventy-two hours later, HDMECs were exposed to histamine (10 μM), and the resistance was monitored. In another set of experiments, HDMECs were incubated for 30 min with l-NAME (100 μM), a NOS inhibitor, followed by the acute stimulation with histamine (10 μM).
Statistical Analysis.
Data are expressed as mean ± SEM. The level of statistical significance was determined by ANOVA, followed by Bonferroni's t test for multiple comparisons or Student's t test using the GraphPad Prism software.
Supplementary Material
Acknowledgments.
We thank Morris Birnbaum for the Akt1- and Akt2-deficient mice. This work was supported in part by grants from the National Institutes of Health (R01 HL64793, R01 HL61371, R01 HL57665, and P01 HL70295), Contract N01-HV-28186 (National Heart, Lung, and Blood Institute–Yale Proteomics Contract) to W.C.S., and a fellowship from Phillip Morris USA to C.F.-H.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904073106/DCSupplemental.
References
- 1.di Meglio P, Ianaro A, Ghosh S. Amelioration of acute inflammation by systemic administration of a cell-permeable peptide inhibitor of NF-κB activation. Arthritis Rheum. 2005;52:951–958. doi: 10.1002/art.20960. [DOI] [PubMed] [Google Scholar]
- 2.Camps M, et al. Blockade of PI3Kγ suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 3.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 4.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro SD. COPD unwound. N Engl J Med. 2005;352:2016–2019. doi: 10.1056/NEJMe058044. [DOI] [PubMed] [Google Scholar]
- 6.Schon MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Hernando C, et al. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch E, et al. Central role for G protein-coupled phosphoinositide 3-kinase γ in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki T, et al. Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 10.Paez J, Sellers WR. PI3K/PTEN/AKT pathway. A critical mediator of oncogenic signaling. Cancer Treat Res. 2003;115:145–167. [PubMed] [Google Scholar]
- 11.Chen WS, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 13.Yang ZZ, et al. Protein kinase Bα/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 14.Cho H, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 15.Easton RM, et al. Role for Akt3/protein kinase Bγ in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tschopp O, et al. Essential role of protein kinase Bγ (PKBγ/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–2954. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- 17.Ackah E, et al. Akt1/protein kinase Bα is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somanath PR, Chen J, Byzova TV. Akt1 is necessary for the vascular maturation and angiogenesis during cutaneous wound healing. Angiogenesis. 2008;11:277–288. doi: 10.1007/s10456-008-9111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meili R, et al. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 1999;18:2092–2105. doi: 10.1093/emboj/18.8.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimmeler S, Dernbach E, Zeiher AM. Phosphorylation of the endothelial nitric oxide synthase at Ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett. 2000;477:258–262. doi: 10.1016/s0014-5793(00)01657-4. [DOI] [PubMed] [Google Scholar]
- 22.Fulton D, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wetzker R, Rommel C. Phosphoinositide 3-kinases as targets for therapeutic intervention. Curr Pharm Des. 2004;10:1915–1922. doi: 10.2174/1381612043384402. [DOI] [PubMed] [Google Scholar]
- 24.Aramoto H, Breslin JW, Pappas PJ, Hobson RW, II, Duran WN. Vascular endothelial growth factor stimulates differential signaling pathways in in vivo microcirculation. Am J Physiol Heart Circ Physiol. 2004;287:H1590–H1598. doi: 10.1152/ajpheart.00767.2003. [DOI] [PubMed] [Google Scholar]
- 25.Six I, Kureishi Y, Luo Z, Walsh K. Akt signaling mediates VEGF/VPF vascular permeability in vivo. FEBS Lett. 2002;532:67–69. doi: 10.1016/s0014-5793(02)03630-x. [DOI] [PubMed] [Google Scholar]
- 26.Vogel C, et al. Flt-1, but not Flk-1 mediates hyperpermeability through activation of the PI3-K/Akt pathway. J Cell Physiol. 2007;212:236–243. doi: 10.1002/jcp.21022. [DOI] [PubMed] [Google Scholar]
- 27.Phung TL, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bucci M, Roviezzo F, Cicala C, Sessa WC, Cirino G. Geldanamycin, an inhibitor of heat shock protein 90 (Hsp90) mediated signal transduction has anti-inflammatory effects and interacts with glucocorticoid receptor in vivo. Br J Pharmacol. 2000;131:13–16. doi: 10.1038/sj.bjp.0703549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bucci M, et al. Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proc Natl Acad Sci USA. 2005;102:904–908. doi: 10.1073/pnas.0408906102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvemini D, Masini E, Anggard E, Mannaioni PF, Vane J. Synthesis of a nitric oxide-like factor from l-arginine by rat serosal mast cells: Stimulation of guanylate cyclase and inhibition of platelet aggregation. Biochem Biophys Res Commun. 1990;169:596–601. doi: 10.1016/0006-291x(90)90372-t. [DOI] [PubMed] [Google Scholar]
- 31.Luo Z, et al. Acute modulation of endothelial Akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. J Clin Invest. 2000;106:493–499. doi: 10.1172/JCI9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, et al. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci USA. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andriopoulou P, Navarro P, Zanetti A, Lampugnani MG, Dejana E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler Thromb Vasc Biol. 1999;19:2286–2297. doi: 10.1161/01.atv.19.10.2286. [DOI] [PubMed] [Google Scholar]
- 34.Henriques MG, et al. Mouse paw edema. A new model for inflammation? Braz J Med Biol Res. 1987;20:243–249. [PubMed] [Google Scholar]
- 35.Edwards JC, Sedgwick AD, Willoughby DA. The formation of a structure with the features of synovial lining by subcutaneous injection of air: An in vivo tissue culture system. J Pathol. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- 36.Posadas I, et al. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br J Pharmacol. 2004;142:331–338. doi: 10.1038/sj.bjp.0705650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.