Abstract
Infecting about one-half of the global human population, Helicobacter pylori is well established as the primary cause of gastritis, duodenal ulcer, and gastric cancer. Currently there is no clear information regarding if and how host cells interact with H. pylori, and if such interactions are dependent on the type of gastric disease. Using fluorescently labeled fucose-containing glycoconjugates, we provide evidence observing both the uptake of l-fucose from gastric cancer cells to H. pylori and that human α-l-fucosidase 2 (FUCA2) is secreted only under coculture conditions (i.e., host cells infected with H. pylori). Upon depletion of FUCA2 by RNA interference and detection of translocated CagA (a virulence factor of H. pylori) in host cells, FUCA2 was found to be essential for H. pylori adhesion, in particular to the gastric cancer- and duodenal ulcer-specific strains. Additionally FUCA2 was shown to significantly enhance the expression of Lewis x antigen in H. pylori, which is critical for bacterial cell adhesion in the pathogenesis and defense strategy to escape host surveillance. These findings not only demonstrate an important connection between FUCA2 and the adhesion, growth, and pathogenicity of H. pylori, but also support the idea that FUCA2 is a potential target for clinical diagnosis and therapeutic intervention of H. pylori-related diseases.
About one-half of the global human population is currently infected with Helicobacter pylori, which mainly colonizes the gastric mucosa. Although this pathogen is a leading cause of gastric malignancies (1–3), most infected individuals remain asymptomatic or are affected merely by chronic gastritis (2). Less than 20% of infected patients develop peptic ulcer, gastric cancer, or malignant lymphoma, revealing potential host defense mechanisms against H. pylori pathogenesis. The mechanisms by which H. pylori causes pathology are unknown, but they likely involve how the bacteria interacts with host cells.
Oligosaccharides that are attached to proteins and lipids on cell surfaces are often involved in various types of cell-cell interactions. Fucosylated carbohydrates play an important role in a myriad of biological events, particularly tumor progression and bacterial infections involving Lewis antigens, such as Lewis a (Lea), Lewis b (Leb), Lewis x (Lex), Lewis y (Ley), and their sialylated derivatives. Gastric mucins have many oligosaccharide chains bearing terminal fucose residues, but the function of these residues is not fully understood. Boren et al. (4, 5) reported that Leb antigen expressed on human gastric mucosa is the ligand of H. pylori adhesin BabA, an important protein expressed on the cell surface for bacterial adhesion. Interestingly, the chemical structures of Lea, Leb, Lex, Ley, I-antigen, H type 1, and blood group A antigens are also expressed on the surface of H. pylori, which is hypothesized as a molecular mimicry strategy to evade host surveillance (6–8). H. pylori adhesins, such as BabA, may have evolved an ability to distinguish between host and bacterial ligands based on the differences in their core sugar structures to avoid bacterial autoaggregation (9, 10).
As a first step for successful infection, H. pylori must adhere to the gastric mucosa of epithelial cells. The microbial and host factors that determine the outcome of colonization have been difficult to define, however, in part because of the genetic diversity among H. pylori strains and among humans. The infection process may rely on the pathogen to establish physical contact with the gastric epithelium through H. pylori adhesins. The H. pylori strains that harbor the gene babA2, which encodes the adhesin BabA, are associated with an increased risk for gastric adenocarcinoma (11). In addition, SabA was identified as a sialic acid-binding adhesin that binds several sialylated glycoconjugates, including the tetrasaccharide sialyl Lex, expressed on gastric mucins during chronic H. pylori-mediated inflammation (12).
The Lex structure, but not Leb, is involved in the formation of the adhesion pedestal between H. pylori and gastric epithelium (13, 14). Moreover, a monoclonal antibody against Lex that reacts with the lipopolysaccharides of H. pylori inhibits bacterial adhesion to gastric epithelial cells (15, 16). Further evidence obtained from knockout studies indicates that neither galE (UDP-galactose-4-epimerase) nor rfbM (GDP-mannose pyrophosphorylase) mutant strains (both genes are important for Lex biosynthesis) adhered to human gastric mucosal tissues whereas the Lex-positive parent strain adhered well to these tissues (17). Clinical studies in gastritis patients also yielded a similar conclusion (18). H. pylori strains that strongly express Lex/y are associated with higher-density colonization compared with strains that weakly express Lex/y (18). These results not only suggest the existence of Lex-binding C-type lectins on the surface of gastric epithelial cells (19), but also hint at a link between H. pylori adhesion and inflammation.
The pioneering work by Gordon and coworkers (20) demonstrated that Bacteroides thetaiotaomicron, a commensal bacterium of the distal small intestine, can induce host synthesis of a specific glycan structure that the microbe can then use as a supply of l-fucose residues. l-Fucose is hydrolytically removed and used by the bacteria as a carbon and energy source (20). Thus, we hypothesized that l-fucose plays a similar role in the interaction between H. pylori and gastric epithelial cells. A recent study showed that H. pylori induced host cells to overexpress β1,3-N-acetylglucosaminyltransferase (β3GnT5), which indirectly produced more sialyl Lex (21), suggesting that the pathogen may induce the host to manufacture specific glycans and to activate transcription of several genes simultaneously.
Here, we present evidence that l-fucose was transferred from the surface of human gastric cancer cells to the co-cultured clinical strain of H. pylori. Secreted human α-l-fucosidase 2 (FUCA2) was identified to be the key enzyme responsible for the transfer of l-fucose. The hydrolytic enzyme was found to be essential for H. pylori adhesion to human gastric cancer cells, particularly the H. pylori strains isolated from patients with duodenal ulcer and gastric cancer. Additionally the presence of FUCA2 greatly increased the level of Lex expression. These findings suggest an interesting interplay between H. pylori and gastric epithelial cells. The host cell secretes FUCA2 to restrict cell adhesion of H. pylori, whereas the counteraction of H. pylori is to use the hydrolyzed product (L-fucose) for an additional carbon/energy source and for the production of more Lex to enhance adhesion and escape immune surveillance. In summary, FUCA2 shows great potential as a diagnostic marker and a target for therapeutic treatment.
Results
H. pylori Extracts 6-Azido-l-fucose from Human Epithelial Fucosylated Glycoconjugates.
A considerable proportion of H. pylori in the stomach is located at the epithelial mucosa (22) where there are various fucose-containing oligosaccharides including Lewis antigens. To determine if H. pylori acquires l-fucose from host cells, similar to the interaction between B. thetaiotaomicron and small intestine cells (20), the click chemistry-based fluorogenic labeling method developed by Wong and coworkers (23) was applied to detect fucosylated glycoconjugates. Fucosylated glycoconjugates in cultured whole cells were labeled with tetra-O-acetyl-6-azido-l-fucose. The acetyl groups improve cell permeability of the sugar probe, and they are later removed by non-specific esterases (to yield 6-azido-l-fucose) inside cells before the sugar is incorporated into the biosynthetic pathway (Fig. S1). Human gastric adenocarcinoma epithelial cells (AGS and N87) and pancreatic adenocarcinoma cells (Capan 1, expressing gastric-type mucins) were incubated with tetra-O-acetyl-6-azido-l-fucose for 72 h, intensively washed to remove the unincorporated sugar, and then coupled with a 1,8-naphthalimide alkyne probe by click chemistry for fluorogenic detection. Fluorescence microscopy images indicated that AGS, N87, and Capan 1 cells incorporated the modified l-fucose residue and transformed it into fucosylated glycoconjugates on the cell surface (Fig. S2). Capan 1 and AGS cells were chosen for further analysis due to their relatively high rate of l-fucose incorporation.
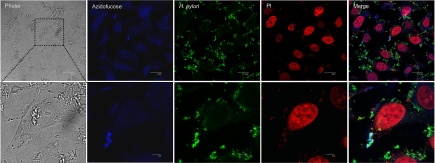
Capan 1 (labeled with blue fluorescent azidofucose) and H. pylori (green labeled with Alexa Fluor 488) cells were found to be co-localized at the epithelial surface (Fig. 1), which increased in a time-dependent manner, indicating that adherent H. pylori takes up 6-azido-l-fucose [Fig. 1 (4 h) and Fig. S3 (8 h)]. Furthermore, H. pylori could also directly take up 6-azido-l-fucose (Fig. S4). Thus, H. pylori potentially obtains l-fucose directly from the plasma membrane of host cells.
Fig. 1.
H. pylori obtains l-fucose from host cell membranes. Capan 1 cells were incubated with 200 μM tetra-O-acetyl-6-azido-l-fucose for 72 h, intensively washed 5 times with PBS, and then infected with H. pylori for 4 h. Cells were fixed, labeled with 1,8-naphthalimide alkyne (blue), stained with H. pylori-specific antibody (Alexa Fluor 488, green) and the nuclei-specific dye propidium iodide (PI, red), and examined by confocal fluorescence microscopy. Overlay appears as light green. (Upper scale bar, 20 μm. Lower scale bar, 5 μm.)
α-l-Fucosidase Is Secreted from Host Cells in Response to H. pylori Infection.
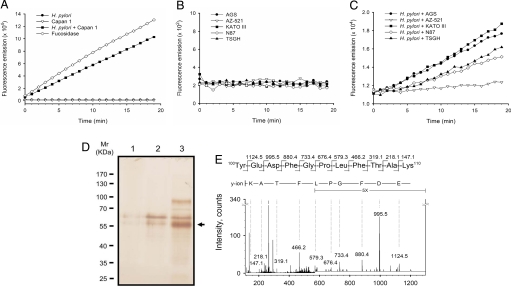
Transfer of l-fucose from host cells to H. pylori requires the presence of an α-l-fucosidase to specifically remove l-fucose from fucosylated glycans. To detect α-l-fucosidase activity, coculture supernatants were concentrated approximately 10-fold and then subjected to an affinity chromatography column packed with α-l-fucosidase inhibitor–immobilized agarose beads [the inhibitor was 1-aminomethyl-1-deoxy-fuconojirimycin (FNJ) (24)]. 4-Methylumbelliferyl-α-l-fucoside (4MU-fucoside) was used as the substrate to measure α-l-fucosidase activity in the resulting fractions. Substantial enzyme activity (309 U/108 host cells; 1 U is defined as hydrolysis of 1 μmol 4MU-fucoside per min at pH 5.5, 25 °C) was detected from the coculture medium 4 h after inoculation at the ideal multiplicity of infection (MOI of ≈200:1). In contrast, no enzyme activity was detected in either uninfected Capan 1 cells or H. pylori alone (Fig. 2A). Similar results were observed in studies of other human gastric adenocarcinoma cell lines including AGS, AZ-521, KATO III, N87, and TSGH 9201 (Fig. 2 B and C). The fractions containing enzyme activity were pooled and then examined by SDS/PAGE. Only 2 major bands were visible in the pooled fraction as compared with individual culture samples from Capan 1 or H. pylori cells. The predominant 55-kDa band (Fig. 2D, arrow) was identified as FUCA2 by in-gel tryptic digestion and LC-MS/MS (Fig. 2E). FUCA2 was proposed to be a secreted hydrolase (residing at chromosome locus 6q24) that is genetically distinct from lysosomal α-l-fucosidase 1 (FUCA1, residing at chromosome locus 1p34) (25). Although the 2 α-l-fucosidases are very similar at the amino acid sequence level (55% identity and 69% similarity), 2 unique peptides of FUCA2 were identified by the LC-MS/MS analysis (Fig. S5). For instance, the fragment ion in the MS/MS spectrum of a doubly charged ion m/z 624.3 corresponded to the peptide sequence, 100YEDFGPLFTAK110 (Fig. 2E), which is unique to FUCA2. It is noted that the secretion of FUCA2 was not a consequence of cell death because cell viability was not affected by H. pylori infection. Additionally, we prepared and characterized human FUCA1 and FUCA2. The result indicated that the modification at C6-position of l-fucose is acceptable to both enzymes and does not affect the kinetic parameters, in comparison with l-fucose-containing substrate or inhibitor (Table S1 and Fig. S6).
Fig. 2.
Activity assay, purification and identification of the secreted α-l-fucosidase from host cells co-cultured with H. pylori. Capan 1 (A) and 5 gastric adenocarcinoma cell lines (B and C) were examined, including AGS, AZ-521, KATO III, N87, and TSGH 9201. Before enzymatic activity measurement, cell culture media were subjected to affinity chromatography using a column packed with FNJ-immobilized Sepharose beads. (A) Measurement of α-l-fucosidase activity was carried out with H. pylori alone (filled circles), Capan 1 alone (inverted open triangles), or the coculture (filled squares) of Capan 1 and H. pylori (MOI ≈200:1). α-l-Fucosidase from Corynebacterium sp. was used as a positive control (open diamond). Furthermore, the activity of the secreted α-l-fucosidases by gastric cancer cell lines was measured either in the absence (B) or presence (C) of H. pylori (MOI ≈200:1). (D) The enzymatically active pooled fractions obtained from the affinity chromatography-based purification were analyzed by SDS/PAGE. The gel was stained with silver nitrate. Lane 1, H. pylori culture supernatant (≈2 × 1010 cells) was used for the purification. Lane 2, Capan 1 cell culture supernatant (≈1 × 108 cells). Lane 3, coculture supernatant of Capan 1 and H. pylori (MOI, ≈200:1). The predominant 55-kDa band, indicated by an arrow, was clearly visualized in Lane 3 as compared with Lanes 1 and 2. (E) Identification of α-l-fucosidase 2 (FUCA2). MS/MS spectrum of the signal [M + 2H]2+ at m/z 644.3 corresponded to the sequence 100YEDFGPLFTAK110 of FUCA2 (the second shaded sequence in Fig. S5). Only the most intense y-series fragment ions were labeled with single letter codes.
Secreted α-l-Fucosidase Controls H. pylori Adhesion.
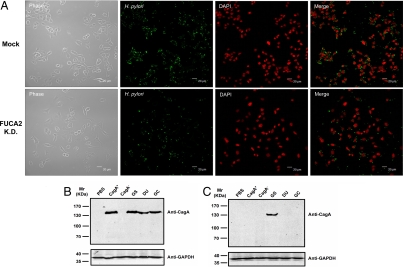
B. thetaiotaomicron produces a fucose-sensing protein that induces the host intestine to increase the expression of fucosylated glycans and secretes bacterial α-l-fucosidase to harvest l-fucose for import and metabolic processing (20). In contrast, FUCA2 is secreted by the host in response to H. pylori infection, releasing l-fucose residues from the host cell surface. Because fucosylated blood group antigens are important for host-microbe interactions, we investigated the potential correlation between FUCA2 and H. pylori adhesion. The attachment of H. pylori was examined using confocal fluorescence microscopy by co-culturing H. pylori in mock-transfected Capan 1 cells or stable FUCA2-knockdown Capan 1 cells (Capan 1-FUCA2 K.D.). With a short-term incubation (4 h), the pathogen attached equally well to both mock-transfected and stable FUCA2-knockdown Capan 1 cells (Fig. S7). With prolonged incubation (8 h), however, H. pylori attachment to Capan 1-FUCA2 K.D. cells decreased by about 50% as compared with mock-transfected cells (Fig. 3A). The number of viable Capan 1 cells did not differ significantly between co-cultures containing mock-transfected or stable FUCA2-knockdown Capan 1.
Fig. 3.
FUCA2 is essential for H. pylori adhesion to host cells. (A) Both mock-transfected Capan 1 cells and Capan 1-FUCA2 K.D. cells were infected with a comparable number of H. pylori, and doubly stained with anti-H. pylori (Alexa Fluor 488, green) and a nuclei-specific dye (DAPI, red). After 8 h of co-culturing, the number of adherent H. pylori was reduced to about 50% in Capan 1-FUCA2 K.D. cells. Phase-contrast photographs indicate the same field. (B and C) Immunoblot analysis of epithelial cells infected with H. pylori using mouse monoclonal anti-CagA. Both mock-transfected Capan 1 (B) and Capan 1-FUCA2 K.D. cells (C) were infected with different H. pylori strains, including CagA+ and CagA−, and various clinical isolates from patients with gastritis (GS), duodenal ulcer (DU), and gastric cancer (GC). PBS represents the negative control. The coculture was maintained for 6–8 h at an MOI of approximately 200:1. CagA (≈140 kDa) was detected when the mock-transfected Capan 1 cells were infected with various strains of H. pylori, except for the CagA− strain (B). In contrast, CagA was not detected in Capan 1-FUCA2 K.D. H. pylori−infected cells with the exception of GS (C).
α-l-Fucosidase Activity Is Critical for H. pylori Attachment in Strains Isolated from Patients with Duodenal Ulcers or Gastric Cancer.
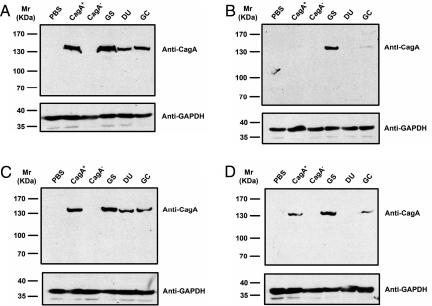
CagA of H. pylori was the first reported bacterial virulence protein translocated by a type IV secretion system (26). Translocated CagA protein can be detected inside host cells soon after H. pylori attachment. The adhesion efficiency of H. pylori can be thus measured by monitoring the level of translocated CagA inside host cells. Four different epithelial cell lines were chosen for the study, namely mock-transfected Capan 1, Capan 1-FUCA2 K.D., Capan 1, and AGS cells. Isogenic vacA (CagA+), cagA (CagA−) mutants, and clinical isolates from patients with gastritis (GS), duodenal ulcer (DU), and gastric cancer (GC) were selected for co-culturing with the 4 epithelial cell lines. The epithelial cell lysates were analyzed by SDS/PAGE 6–8 h after infection and immunoblotted with a CagA-specific monoclonal antibody. CagA (≈140 kDa) was detected when mock-transfected Capan 1 cells were infected with each different H. pylori strain (Fig. 3B). Nonetheless, CagA was not detected upon Capan 1-FUCA2 K.D. infection with the different H. pylori strains, with the exception of GS (Fig. 3C). Likewise, Capan 1 and AGS cells were treated with 100 μM 1-aminomethyl-1-deoxy-fuconojirimycin (FNJ, an α-l-fucosidase inhibitor), followed by infection with each H. pylori strain for 6–8 h. The treatment of Capan 1 or AGS cells with FNJ was found to considerably reduce the transfer of 6-azido-l-fucose from host cells to H. pylori (Fig. S8A). Flow cytometric analysis also demonstrated a similar result [Fig. S8B (for H. pylori) and S8C (for host cells)]. As shown in Fig. 4B, no or little CagA was detected when Capan 1 was treated with FNJ and infected with DU- or GC-specific H. pylori strains. These results are also consistent with those obtained in AGS cells (Fig. 4D). In contrast, CagA was detected in substantial amounts without FNJ treatment (Fig. 4 A and C, corresponding to the results of Capan 1 and AGS cells, respectively). Notably, the level of CagA was not affected by FNJ in the coculture infected with the GS-specific strain, indicating that α-l-fucosidase activity is critical to H. pylori attachment, especially for strains isolated from patients with DU or GC.
Fig. 4.
Immunoblot analysis of epithelial cells infected with H. pylori using mouse monoclonal anti-CagA. Capan 1 and AGS cells were infected with different H. pylori strains, including CagA+ and CagA−, and various clinical isolates from patients with gastritis (GS), duodenal ulcer (DU), and gastric cancer (GC). PBS represents the negative control. The coculture was maintained for 6–8 h at an MOI of approximately 200:1. In the parallel experiments, Capan 1 (A) and AGS cells (C) were infected under the same conditions. The effect of α-l-fucosidase was evaluated by addition of 100 μM FNJ to the co-cultures of Capan 1 (B) and AGS cells (D) with various H. pylori strains.
Furthermore, to confirm if the observed dependence of H. pylori attachment on FUCA2 is related to specific types of gastric disease, a number of DU- and GC-specific strains were clinically isolated and evaluated as described previously. Capan 1 and AGS cells were infected with DU-specific strains of H. pylori in the absence (Fig. S9 A and C) or presence of 100 μM FNJ (Fig. S9 B and D). Five DU strains, DU-152, DU-153, DU-154, DU-155, and DU-157, were further examined. DU-156 was examined previously (Figs. 3 B and C and 4 A–D). Examination of DU-specific strains indicated that CagA was no longer present upon FNJ treatment under coculture conditions that fostered CagA expression.
α-l-Fucosidase Activity Regulates the Expression Level of Lex Antigen.
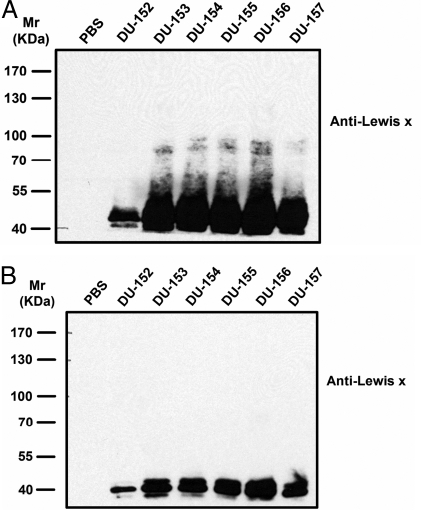
The secretion of FUCA2 directly results in the extracellular production of l-fucose residues that are then incorporated into H. pylori. It is important to investigate if the l-fucose-related biosynthesis is affected at the same time. Mock-transfected Capan 1 and Capan 1-FUCA2 K.D. were both infected with 6 H. pylori DU-specific strains (from DU-152 to DU-157). The H. pylori cell lysates were analyzed by SDS/PAGE 12 h after infection and immunoblotted with a Lex-specific monoclonal antibody. An elevated level of Lex-containing glycoproteins was observed in H. pylori when mock-transfected Capan 1 cells were infected (Fig. 5A), as compared to the limited Lex-expression in the H. pylori cells that were co-cultured with Capan 1-FUCA2 K.D. cells (Fig. 5B). Meanwhile, Capan 1 and AGS cells were treated with 100 μM FNJ, followed by infection with the same H. pylori DU-specific strains for 12 h. The treatment of Capan 1 or AGS cells with FNJ was found to considerably reduce the Lex-expression level in H. pylori (Fig. S10 B and D). In contrast, Lex-containing glycoproteins were detected in substantial amounts without FNJ treatment (Fig. S10 A and C, corresponding to H. pylori-infected Capan 1 and AGS cells, respectively). These results indicated that α-l-fucosidase activity is critical to the level of Lex antigen in H. pylori.
Fig. 5.
Immunoblot analysis of H. pylori-infected Capan 1 cells with mouse monoclonal anti-Lex antigen. Mock-transfected Capan 1 and Capan 1-FUCA2 K.D. cells were infected with different H. pylori strains (from DU-152 to DU-157) that were clinical isolates from 6 different patients with duodenal ulcer (DU). PBS represents a negative control in the absence of H. pylori. The coculture was maintained for 12 h at an MOI of approximately 400:1. In the parallel experiments, mock-transfected Capan 1 (A) and Capan 1-FUCA2 K.D. cells (B) were infected under the same conditions. The bacterial cells were collected and lysed for the Lex analysis. Lex-containing glycoproteins were found to greatly increase in the H. pylori cells co-cultured with mock-transfected Capan 1 cells (A), in contrast to those in the H. pylori cells co-cultured with Capan 1-FUCA2 K.D. cells (B).
Discussion
Helicobacter pylori is a highly successful bacterial pathogen that has colonized the stomach since early human evolution (27). Accumulated evidence has shown that chronic H. pylori infection of the stomach is a major risk factor for GS and DU, as well as for gastric adenocarcinoma and mucosa-associated lymphoma (1). The mechanism of specific colonization from the proximal to the distal stomach probably relies on both intrinsic regional characteristics of the epithelium surface and differential expression of specific cell surface glycoconjugates that are also extrinsically induced by H. pylori at the same time (28–31).
In this report, we provide direct evidence that l-fucose is released from the surface of host cells and acquired by H. pylori. Using rapid affinity column chromatography and tandem mass spectrometric analysis, we identified human FUCA2 as an important enzyme in the hydrolytic removal of l-fucose. FUCA2 is secreted by H. pylori–infected GC cells but not by individual cultures of H. pylori or GC cells. We hypothesized that FUCA2 is secreted outside the cell to remodel the surface carbohydrates, a strategy serving as a host defense mechanism to restrict or even eliminate bacterial colonization. To our surprise, FUCA2 affected H. pylori adhesion and regulated the level of Lex antigen at the same time. The 2 results apparently correlated with each other. As previously mentioned, H. pylori strains strongly expressing Lex/y are linked to higher-density colonization, as compared to strains that weakly produce Lex/y (18). The pathogen thus likely evolves to develop various countermeasures against the host defense, suggesting an opposing interplay between H. pylori and the host. The effect of FUCA2 on H. pylori was examined by 2 studies: RNA interference-mediated depletion of FUCA2 and treatment of H. pylori-infected cells with FNJ, both of which effectively prevented adherence of H. pylori to host cells and significantly reduced the level of Lex-containing glycoproteins in H. pylori. Unlike the lysosomal homologue FUCA1, there are only few studies about FUCA2 that are all related to the genetic analysis. This work defines the function of human FUCA2.
The aforementioned host defense mechanism to exploit α-l-fucosidase for removal of l-fucose residues was not unexpected. β-Hexosaminidase, for instance, was characterized as a peptidoglycan hydrolase that exerts its mycobactericidal effect at the macrophage plasma membrane when secretion from host lysosomes is induced by mycobacteria (32). Moreover, our findings indicate that H. pylori adhesion to host cells is related to the presence of FUCA2. The dependence of FUCA2 correlated with the infection of DU- and GC-specific strains, but not GS-specific strains. Because DU and GC represent relatively long-term chronic inflammations in comparison with GS, it would be interesting to explore the relationship between FUCA2 and exposure time related to H. pylori infection.
All fucosyltransferases (FUTs) use GDP-fucose as the fucose donor in the biosynthesis of fucosylated glycoconjugates. In mammals, there are 2 pathways with respect to the synthesis of GDP-fucose: the de novo pathway and the salvage pathway. The majority of GDP-fucose is derived from the de novo pathway, although this data were obtained using HeLa cells (33). H. pylori also reportedly contained the genes of the de novo pathway, and the 2 corresponding enzymes, GDP-D-mannose 4,6-dehydratase and GDP-l-fucose synthetase, were characterized in detail (34). Our study based on the fluorogenic labeling of l-fucose supported the idea that the salvage pathway also operates in H. pylori. Because l-fucose is the starting sugar in the salvage pathway, the increased influx owing to the secretion of FUCA2 may directly lead to an elevated level of Lex antigen. But the possibility cannot be ruled out that the expression of H. pylori FUT might increase due to the presence of FUCA2 or l-fucose. In the highly competitive microbial ecosystem of the human stomach, where diverse bacterial strains reside, the ability to instruct the host to present a source of utilizable carbon and energy should confer H. pylori a strategic advantage.
L-fucose is a likely source of utilizable carbon and energy because it is always located at the termini of mammalian glycoconjugates. l-fucose is an abundant component of many host gastric mucosa glycoconjugates (35) that are constitutively synthesized by host cells. Thus, the “request” from H. pylori to manufacture fucosylated glycans may require the host to activate translation of 1 or more of its fucosyltransferase proteins. The expression of human FUT3 and FUT6 decreased substantially in response to FNJ treatment (Fig. S11), suggesting that levels of l-fucose may be regulated in host cells to avoid wasting energy. Regulation of l-fucose levels may also minimize the risk of encroachment by the pathogen that utilizes fucosylated glycans as the receptors for their adhesins (36, 37). The level of l-fucose is critical in the commensal relationship between B. thetaiotaomicron and the distal small intestine, where the bacterial protein FucR serves as a molecular sensor to coordinate fucose supply and demand (38). When l-fucose levels are adequate for bacterial needs, fucose-bound FucR promotes expression of bacterial genes required for breakdown of the available l-fucose. On the other hand, when the fucose level is insufficient, FucR in its unbound form promotes transcription of a specific gene to “request” more fucose from the host (38).
The adhesion of H. pylori to host cells is likely mediated by C-type lectins found on the surface mucous cells of the gastric pit (19). Bacterial adhesion to host cells is an important initial event in the pathogenesis of gastric malignancies, and inhibition of bacterial adhesion may prevent certain pathogen-related diseases, such as DU and GC. The current eradication strategy requires a combination of 2 to 3 antibiotics and a proton pump inhibitor (39). As such, it is necessary to develop other therapeutic alternatives due to ever-increasing concerns about antibiotic resistance (40), the lack of protection against re-infection, the necessity of taking a long-term prescription for complete eradication, and the high cost of therapy. Development of an α-l-fucosidase inhibitor as a prophylactic drug or an adjuvant with possible therapeutic applications is certainly considered as a promising approach for the prevention of H. pylori–related diseases.
Materials and Methods
Please note that the following information was described in SI Materials and Methods due to page limitation, including the bacterial strains and cell lines, confocal fluorescence microscopy and imaging, in-gel digestion, and mass spectrometry analysis to identify FUCA2, preparation of human FUCA2, kinetic analysis of FUCA1 and FUCA2, RNA interference, flow cytometric analysis, and immunoblotting of CagA and Lex.
H. pylori and Cancer Cell Coculture Conditions.
H. pylori was grown for 3 days under microaerobic conditions (containing 5% O2, 10% CO2, and 85% N2) at 37 °C on trypticase soy agar II plates supplemented with 5% sheep's blood. The bacterial cells were collected by gentle scrapping with a rubber policeman by centrifugation at 10,000 × g at room temperature for 5 min. The resulting cells were suspended in DMEM at a suitable concentration for further studies without any additives. Cancer cells were grown to confluency in tissue culture dishes. The monolayer of each cancer cell line was washed twice with DMEM. H. pylori was added to cancer cells at an MOI of approximately 200 per cell and incubated at 37 °C with DMEM in a 5% CO2 incubator for a total of 4 or 8 h. An identical amount of H. pylori or cancer cells was cultured individually in a culture dish as the negative control. After 1 h of co-incubation, the H. pylori-cancer cell coculture was washed twice with DMEM to remove unattached H. pylori and debris. DMEM wash medium was prewarmed at 37 °C to avoid stressing either the H. pylori or cancer cells. Tight attachment of H. pylori to cancer cells was achieved after 1 h. After washing, incubation was continued for an additional 3 or 7 h. At the end of the incubation, the coculture was washed 2 times with PBS (PBS, pH 7.4) at 37 °C to remove unattached H. pylori. All of the control experiments were treated in a similar manner. Furthermore, the procedures of confocal fluorescence microscopy and imaging were described in SI Materials and Methods.
Purification and Activity Assay of α-l-fucosidase.
Cell culture supernatants, obtained from (i) culture of H. pylori (≈2 × 1010 cells), (ii) culture of cancer cells (≈1 × 108 cells), or (iii) co-cultured H. pylori-cancer cells (MOI ≈200:1), were incubated in serum-free DMEM for 4 or 8 h. After removing cell debris, the conditioned media containing secreted proteins were collected, concentrated to approximately 10-fold using an Amicon Ultra-15 centrifugal filter (Millipore; 30-kDa cut-off), and dialyzed in 50 mM HEPES pH 8.0. The concentrated solutions were subjected to affinity chromatography with a mini column (3 cm × 0.8 cm, Novagen) that had been packed with FNJ (24)-immobilized agarose (prepared by reacting FNJ with Reacti-Gel CDI Supports, imidazole carbamate containing beaded agarose; Pierce). The column was eluted with 4 mL of 200 mM l-fucose solution. The eluates were concentrated 10-fold using an Amicon Ultra-15 centrifugal filter (Millipore; 10-kDa cut-off). The concentrated materials were stored at −80 °C. All of the purification steps were carried out at 4 °C. α-l-Fucosidase activity was carried out as described (41). Each assay (200 μL) contained 50 mM HEPES (pH 8.0), 30 μM 4MU-fucoside, and 20 μL enzyme (from various sources). α-l-Fucosidase from Corynebacterium sp. was from Sigma used as a positive control. The emission at 465 nm was monitored with an excitation wavelength of 360 nm to measure the release of fluorescent 4-methylumbelliferone at 20 °C.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903286106/DCSupplemental.
References
- 1.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 2.Cave DR. Chronic gastritis and Helicobacter pylori. Semin Gastrointest Dis. 2001;12:196–202. [PubMed] [Google Scholar]
- 3.Sipponen P, Hyvarinen H. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol Suppl. 1993;196:3–6. doi: 10.3109/00365529309098333. [DOI] [PubMed] [Google Scholar]
- 4.Boren T, Normark S, Falk P. Helicobacter pylori: Molecular basis for host recognition and bacterial adherence. Trends Microbiol. 1994;2:221–228. doi: 10.1016/0966-842x(94)90626-2. [DOI] [PubMed] [Google Scholar]
- 5.Ilver D, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 6.Monteiro MA, et al. Expression of histoblood group antigens by lipopolysaccharides of Helicobacter pylori strains from Asian hosts: The propensity to express type 1 blood-group antigens. Glycobiol. 2000;10:701–713. doi: 10.1093/glycob/10.7.701. [DOI] [PubMed] [Google Scholar]
- 7.Monteiro MA, et al. Simultaneous expression of type 1 and type 2 Lewis blood group antigens by Helicobacter pylori lipopolysaccharides. Molecular mimicry between H. pylori lipopolysaccharides and human gastric epithelial cell surface glycoforms. J Biol Chem. 1998;273:11533–11543. doi: 10.1074/jbc.273.19.11533. [DOI] [PubMed] [Google Scholar]
- 8.Monteiro MA, et al. Lipopolysaccharide structures of Helicobacter pylori genomic strains 26695 and J99, mouse model H. pylori Sydney strain, H pylori P466 carrying sialyl Lewis X, and H pylori UA915 expressing lewis B classification of H pylori lipopolysaccharides into glycotype families. Eur J Biochem. 2000;267:305–320. doi: 10.1046/j.1432-1327.2000.01007.x. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson KA. The human gastric colonizer Helicobacter pylori: A challenge for host-parasite glycobiology. Glycobiol. 2000;10:761–771. doi: 10.1093/glycob/10.8.761. [DOI] [PubMed] [Google Scholar]
- 10.Falk P. Why does Helicobacter pylori actually have Lewis antigens? Trends Microbiol. 2001;9:61–62. doi: 10.1016/s0966-842x(00)01935-1. [DOI] [PubMed] [Google Scholar]
- 11.Gerhard M, et al. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci USA. 1999;96:12778–12783. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahdavi J, et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573–578. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng PY, et al. Expression of Lewis b blood group antigen in Helicobacter pylori does not interfere with bacterial adhesion property. World J Gastroenterol. 2003;9:122–124. doi: 10.3748/wjg.v9.i1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor DE, et al. Lack of correlation between Lewis antigen expression by Helicobacter pylori and gastric epithelial cells in infected patients. Gastroenterol. 1998;115:1113–1122. doi: 10.1016/s0016-5085(98)70082-4. [DOI] [PubMed] [Google Scholar]
- 15.Osaki T, et al. Establishment and characterization of a monoclonal antibody to inhibit adhesion of Helicobacter pylori to gastric epithelial cells. J Med Microbiol. 1998;47:505–512. doi: 10.1099/00222615-47-6-505. [DOI] [PubMed] [Google Scholar]
- 16.Appelmelk BJ, Vandenbroucke-Grauls CM. H. pylori and Lewis antigens. Gut. 2000;47:10–11. doi: 10.1136/gut.47.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards NJ, et al. Lewis X structures in the O antigen side-chain promote adhesion of Helicobacter pylori to the gastric epithelium. Mol Microbiol. 2000;35:1530–1539. doi: 10.1046/j.1365-2958.2000.01823.x. [DOI] [PubMed] [Google Scholar]
- 18.Heneghan MA, McCarthy CF, Moran AP. Relationship of blood group determinants on Helicobacter pylori lipopolysaccharide with host Lewis phenotype and inflammatory response. Infect Immun. 2000;68:937–941. doi: 10.1128/iai.68.2.937-941.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cambi A, Koopman M, Figdor CG. How C-type lectins detect pathogens. Cell Microbiol. 2005;7:481–488. doi: 10.1111/j.1462-5822.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 20.Hooper LV, et al. A molecular sensor that allows a gut commensal to control its nutrient foundation in a competitive ecosystem. Proc Natl Acad Sci USA. 1999;96:9833–9838. doi: 10.1073/pnas.96.17.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcos NT, et al. Helicobacter pylori induces β3GnT5 in human gastric cell lines, modulating expression of the SabA ligand sialyl-Lewis x. J Clin Invest. 2008;118:2325–2336. doi: 10.1172/JCI34324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber S, et al. The spatial orientation of Helicobacter pylori in the gastric mucus. Proc Natl Acad Sci USA. 2004;101:5024–5029. doi: 10.1073/pnas.0308386101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawa M, et al. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc Natl Acad Sci USA. 2006;103:12371–12376. doi: 10.1073/pnas.0605418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang CF, et al. Discovery of picomolar slow tight-binding inhibitors of alpha-fucosidase. Chem Biol. 2004;11:1301–1306. doi: 10.1016/j.chembiol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Intra J, et al. Comparative and phylogenetic analysis of α-l-fucosidase genes. Gene. 2007;392:34–46. doi: 10.1016/j.gene.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263–1268. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falush D, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299:1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 28.González-Valencia G, et al. Lewis antigen expression by Helicobacter pylori strains colonizing different regions of the stomach of individual patients. J Clin Microbiol. 2008;46:2783–2785. doi: 10.1128/JCM.02370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falk PG, et al. Creating and maintaining the gastrointestinal ecosystem: What we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998;62:1157–1170. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etzioni A. Adhesion molecules—their role in health and disease. Pediatr Res. 1996;39:191–198. doi: 10.1203/00006450-199602000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Karlsson KA. Microbial recognition of target-cell glycoconjugates. Curr Opin Struct Biol. 1995;5:622–635. doi: 10.1016/0959-440x(95)80054-9. [DOI] [PubMed] [Google Scholar]
- 32.Koo IC, et al. Role for lysosomal enzyme β-hexosaminidase in the control of mycobacteria infection. Proc Natl Acad Sci USA. 2008;105:710–715. doi: 10.1073/pnas.0708110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yurchenco PD, Atkinson PH. Fucosyl-glycoprotein and precursor polls in HeLa cells. Biochemistry. 1975;14:3107–3114. doi: 10.1021/bi00685a011. [DOI] [PubMed] [Google Scholar]
- 34.Wu B, Zhang Y, Wang PG. Identification and characterization of GDP-D-mannose 4,6-dehydratase and GDP-l-fucose synthetase in a GDP-l-fucose biosynthetic gene cluster from Helicobacter pylori. Biochem Biophys Res Commun. 2001;285:364–371. doi: 10.1006/bbrc.2001.5137. [DOI] [PubMed] [Google Scholar]
- 35.Madrid JF, et al. Lectin-gold localization of fucose residues in human gastric mucosa. J Histochem Cytochem. 1998;46:1311–1320. doi: 10.1177/002215549804601111. [DOI] [PubMed] [Google Scholar]
- 36.Benitez JA, et al. Adherence of Vibrio cholerae to cultured differentiated human intestinal cells: An in vitro colonization model. Infect Immun. 1997;65:3474–3477. doi: 10.1128/iai.65.8.3474-3477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brassart D, et al. In vitro inhibition of adhesion of Candida albicans clinical isolates to human buccal epithelial cells by Fuc alpha 1–2Gal beta-bearing complex carbohydrates. Infect Immun. 1991;59:1605–1613. doi: 10.1128/iai.59.5.1605-1613.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hooper LV, Gordon JI. Glycans as legislators of host-microbial interactions: Spanning the spectrum from symbiosis to pathogenicity. Glycobiol. 2001;11:1R–10R. doi: 10.1093/glycob/11.2.1r. [DOI] [PubMed] [Google Scholar]
- 39.Unge P. Antibiotic treatment of Helicobacter pylori infection. Curr Top Microbiol Immunol. 1999;241:261–300. doi: 10.1007/978-3-642-60013-5_13. [DOI] [PubMed] [Google Scholar]
- 40.Glupczynski Y. Antimicrobial resistance in Helicobacter pylori: A global overview. Acta Gastroenterol Belg. 1998;61:357–366. [PubMed] [Google Scholar]
- 41.Gramer MJ, Goochee CF. Glycosidase activities in Chinese hamster ovary cell lysate and cell culture supernatant. Biotech Prog. 1993;9:366–373. doi: 10.1021/bp00022a003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.