Abstract
The Ediacara biota include macroscopic, morphologically complex soft-bodied organisms that appear globally in the late Ediacaran Period (575–542 Ma). The physiology, feeding strategies, and functional morphology of the modular Ediacara organisms (rangeomorphs and erniettomorphs) remain debated but are critical for understanding their ecology and phylogeny. Their modular construction triggered numerous hypotheses concerning their likely feeding strategies, ranging from micro-to-macrophagus feeding to photoautotrophy to osmotrophy. Macrophagus feeding in rangeomorphs and erniettomorphs is inconsistent with their lack of oral openings, and photoautotrophy in rangeomorphs is contradicted by their habitats below the photic zone. Here, we combine theoretical models and empirical data to evaluate the feasibility of osmotrophy, which requires high surface area to volume (SA/V) ratios, as a primary feeding strategy of rangeomorphs and erniettomorphs. Although exclusively osmotrophic feeding in modern ecosystems is restricted to microscopic bacteria, this study suggests that (i) fractal branching of rangeomorph modules resulted in SA/V ratios comparable to those observed in modern osmotrophic bacteria, and (ii) rangeomorphs, and particularly erniettomorphs, could have achieved osmotrophic SA/V ratios similar to bacteria, provided their bodies included metabolically inert material. Thus, specific morphological adaptations observed in rangeomorphs and erniettomorphs may have represented strategies for overcoming physiological constraints that typically make osmotrophy prohibitive for macroscopic life forms. These results support the viability of osmotrophic feeding in rangeomorphs and erniettomorphs, help explain their taphonomic peculiarities, and point to the possible importance of earliest macroorganisms for cycling dissolved organic carbon that may have been present in abundance during Ediacaran times.
Keywords: erniettomorphs, rangeomorphs, Fractofusus, Pteridinium
The late Ediacaran Period marks the rapid diversification of globally widespread macroscopic organisms: the soft-bodied and morphologically complex Ediacara biota. For the first time in Earth history, organisms constructed macroscopic body plans allowing for moderate levels of ecological interactions and competition for nutrients and substrate availability (1–3). The iconic, serially quilted erniettomorphs and fractally branched rangeomorphs (4) are the most poorly understood members of the Ediacara biota because of their unique construction, unknown from any Phanerozoic/modern groups (Fig. 1) (5). Erniettomorphs are characterized by highly repeatable tubular units (6), whereas rangeomorphs consist of repeatedly branched frondlets (7). Their body plans highlight a modularity in which individual units are assembled into complex, species-specific arrangements (4, 8, 9). However, the purpose of this growth arrangement has yet to be fully explored.
Fig. 1.
Modular Ediacara fossils. (A) Three incomplete specimens of the erniettomorph fossil Pteridinium composed of tubular modular units. (B) Pteridinium with nine modular units (right side of fossil). (C) Erniettomorph Ernietta with module infilling. (D) Magnified section of the specimen in the boxed section in C, with arrows highlighting sediment infill. (E) Rangeomorph fossil Rangea with fractal modules (bracket on the right). (F) Rangea with three primary fractal modules (large modules on the left) and three smaller subsidiary modules tucked in between the larger modules. (G) Rangeomorph Fractofusus with 16 fractal modules on either side of the longitudinal midline. Bracket displaying one module. G is provided by G.M. Narbonne. (Scale bar: 1 cm.)
The likely feeding strategy of these modular Ediacara organisms has been a topic of considerable controversy. Interpretations varied from micro-to-macrophagus feeding, photoautotrophy, saprotrophy, and parasitism, to symbiosis (5, 10). However, a macrophagus interpretation for erniettomorphs and rangeomorphs is contradicted by their lack of any macroscopic feeding structures (e.g., tentacles, zooids, or oral openings), and rangeomorph photoautotrophy is inconsistent with their range of habitats, which extended to the aphotic zone. Most recently, it has been hypothesized that the fractal branching of rangeomorph modular units served to increase the surface area to volume (SA/V) ratio to facilitate direct nutrient absorption (osmotrophy) of dissolved organic carbon (DOC) (8, 11), which is presumed to have been abundant in Ediacaran deep oceans (12–14).
Osmotrophy in erniettomorphs and rangeomorphs requires a high SA/V ratio to allow for rapid and effective transport of nutrients directly through the integument. Today, true osmotrophy is restricted to microscopic bacteria with sizes typically <100 μm, although osmotrophic “giants,” such as Thiomargarita and Epulopiscium (Table 1), have cell sizes up to 600 and 750 μm, respectively (15, 16). However, these prokaryotic giants are dwarfed by even the smallest erniettomorphs and rangeomorphs, raising the question of whether the inferred trophic models of these Ediacara fossils can be reconciled with their large body size. Thus, the goal of this study was to simulate various morphological changes in the overall construction of modular Ediacara organisms to test whether it is possible for them to attain SA/V ratios on the same order as those seen in modern osmotroph bacteria.
Table 1.
Gross estimates of surface area and SA/V ratios of modern osmotrophic unicellular megabacteria
| Giant bacteria | Length, mm | Width, mm | SA, mm2 | Volume, mm3 | SA/V, mm2/mm3 | Ref. |
|---|---|---|---|---|---|---|
| Achromatium oxaliferum | 0.100 | 0.0450 | 1.98E-1 | 1.06E-4 | 1.9E+3 | 19 |
| Beggiatoa spp. | 0.160 | 0.0500 | 2.65E-1 | 2.09E-4 | 1.3E+3 | 19 |
| Epulopiscium fishelsoni | 0.600 | 0.0800 | 1.14E+0 | 3.00E-3 | 3.8E+2 | 16 |
| Prochloron spp. | 0.030 | 0.0300 | 2.83E-3 | 1.41E-5 | 2.0E+2 | 19 |
| Sporospirillum praeclarum | 0.100 | 0.0040 | 1.71E-2 | 8.38E-7 | 2.0E+4 | 19 |
| Staphylothermus marinus | 0.015 | 0.0150 | 2.83E-3 | 1.77E-6 | 1.6E+3 | 15 |
| Thiomargarita namibiensis | 0.750 | 0.7500 | 1.77E+0 | 2.21E-1 | 8.0E+0 | 16 |
| Thiomargarita namibiensis | 0.001* | 8.82E-4 | 2.0E+3 | 16 | ||
| Thiovulum majus | 0.025 | 0.0250 | 1.96E-3 | 8.18E-6 | 2.4E+2 | 19 |
*An estimate of the effective SA/V ratio of Thiomargarita by assuming a 1-μm-thick layer of metabolically active cytoplasm surrounding a metabolically inactive central vacuole (16).
The use of strictly osmotrophic megabacteria as ecological analogues for rangeomorphs and erniettomorphs represents a conservative choice for comparative purposes. Should the hypothesis that rangeomorphs and erniettomorphs represent eukaryotic organisms be true, it is likely that these organisms would be able to supplement osmotrophy by endocytosis, thus reducing the strict SA/V threshold suggested by giant bacteria.
Computer modeling of individual erniettomorph and rangeomorph structural modules was performed to evaluate how SA/V ratios would change with the addition, inflation, or vacuolization of individual modules. If the hypothesis that these organisms fed through direct diffusion is correct, the SA/V ratio should place a strong physiological constraint on the morphologies of erniettomorphs and rangeomorphs.
Modeling Parameters and Justification
Modeling Erniettomorphs.
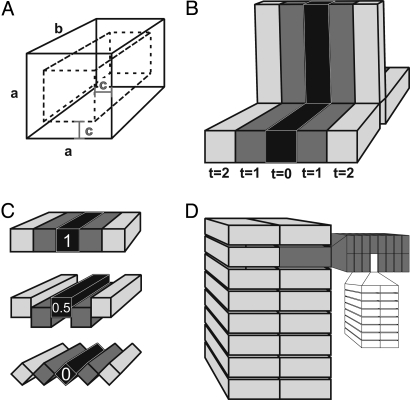
We chose Pteridinium (Fig. 1 A and B) as a representative erniettomorph for modeling purposes because this organism is an optimal form to model, and there are published specimen measurements for comparison with modeling results (6). The modular units of Pteridinium are assumed to have been tubes with a square cross-section and assembled into three petaloids (vanes). Complete Pteridinium specimens are spindle-shaped, tapering at both ends and suggesting a bipolar growth strategy (Fig. 1A). The earliest ontogenetic sequence of module addition is difficult to interpret from Pteridinium, because even the smallest complete specimen consists of 37 modules per petaloid (6). However, the comparison of numerous complete specimens suggests that increases in size result from the addition of new modules (6). The uncertainty surrounding module addition in juvenile specimens, both in terms of initial starting morphologies and whether module triplets were added unipolarly or bipolarly, simultaneously or sequentially in a continual spiral, does limit the faithfulness of the model. However, the most likely hypothesis was followed, with simulations constructed to add modular units to all three petaloids simultaneously (as triplets) and bipolarly (Fig. 2B). Modular units were assumed to have a square cross-section and modeled to evaluate changes in SA/V ratios associated with the size (length and width), number, hollowness, and degree of overlap between units (Fig. 2). Unit size and number of units were varied individually and incrementally, as guided by specimen measurements reported previously (6): width (a: 2.2–4 mm, varied at 0.2-mm intervals; Fig. 2A), length (b: 40–85 mm, varied at 5-mm intervals; Fig. 2A), and number of units (40–85 triplets, varied at five-triplet intervals; Fig. 2B). The thickness of the active biological organic matter was evaluated for a module of average size (length: 55 mm, width: 3.2 mm; n = 11) from the starting point of a full module lacking any internal hollowness (c = 1.6 mm; Fig. 2A), and it was incrementally reduced in thickness (c = 1 mm, 0.5 mm, 0.1 mm, 0.01 mm; Fig. 2A). A distally closed vs. open termination of the modular unit was compared (Fig. 2A), with the assumption that a distally open tube (i.e., a cylinder) would allow for additional nutrient exchange along the exposed internal surface of the module. The effect of modular packing was compared based on the amount of overlap shared with neighboring modules (Fig. 2C overlap: 1, 0.5, or 0). Thus, a total of six parameters (a, b, c, hollowness, overlap, and termination) determined size, shape, and hollowness of Pteridinium. All simulations were performed by using a code written in SAS/IML (Interactive Matrix Language in SAS 9.1 for Windows; SAS). The program outputs surface, volume, and SA/V ratio values for modeled organisms as a function of the parameters. The modeled results were compared with measurements of complete Pteridinium fossils from the Kliphoek Member of the Dabis Formation, Kuibis Subgroup, Nama Group reported in Grazhdankin and Seilacher (6). The Kliphoek Member contains a large number of Pteridinium of varying sizes and module numbers, therefore allowing for empirical constraints on the parameters modeled.
Fig. 2.
Modeling parameters of erniettomorphs and rangeomorphs. (A) Width (a), length (b), and hollowness (c) of an erniettomorph module. (B) Bipolar addition of erniettomorph module triplets during growth (t = 0, 1, 2…). (C) Overlap (1 = full, 0 = none) between erniettomorph modules was simulated to compare tightly packed vs. loosely overlapping modules. (D) Fractal subdivision of a rangeomorph module.
Modeling Rangeomorphs.
The rangeomorph frondlet was modeled as a series of eight identical rectangular units that branched fractally (Figs. 1 E–G and 2D). The initial assumption of a nonbranched rectangular unit similar to an erniettomorph module (fractal state 0) was subsequently repeatedly branched (fractal states 1–4) to represent the fractal orders (7), with each fractal branching oriented perpendicular to the previous one. Each increasingly smaller modular unit was considered free from adjourning modules (Fig. 1F) (7, 17). The modeled frondlets were then used as building blocks to construct rangeomorph vanes. Fractofusus was selected as a template for modeling rangeomorphs because its morphology consists entirely of rangeomorph frondlets (Fig. 1G) and because a large population of specimens (n = 66) have been made available (18). Gehling and Narbonne (18) proposed three different constructions for Fractofusus, with two, three, or four identical vanes of rangeomorph frondlets. The two-vaned model was preferred because it represents a more truthful resemblance to the preserved fossils and also because additional vanes would not significantly change the SA/V ratios. Because a large number of specimens with a significant range in size were reported (18), thus providing a robust assessment of growth in Fractofusus, the model focused only on the effects of fractal division on SA/V and did not directly simulate growth. All parameters (specimen length and width, module length and width, and number of modules) were taken directly from empirical measurements (18), and therefore were not simulated in the same manner as Pteridinium. The thickness of the module was assumed to be the same as the width of the second-order branches (Fig. 2D) (7).
Comparison with Osmotrophic Bacteria.
To evaluate the possibility of osmotrophy, the modeling results are compared with gross SA/V ratios of modern megabacteria (Table 1). Bacteria are strictly osmotrophic; thus, their size is typically limited by SA/V ratios. Certain megabacteria adopt strategies (e.g., vacuolization in Thiomargarita) to overcome the SA/V constraints to attain a large size. If we consider the effect of vacuolization, the effective SA/V ratios (excluding metabolically inactive vacuoles) of vacuolized megabacteria are much greater than the gross SA/V ratios (Table 1, Thiomargarita full vs. vacuolized). We chose the gross SA/V ratios of modern megabacteria as an analog to evaluate the possibility of osmotrophy in erniettomorphs and rangeomorphs, which may also have inactive inclusions. Estimated gross SA/V ratios of eight modern osmotrophic megabacteria were compiled from the literature (15, 16, 19).
Results
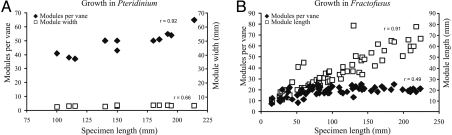
Theoretical models were constructed to explore the effects of length, width, and number of modules on the surface area of the Pteridinium modular unit (Fig. S1A). Increasing the length or width of individual modules or the number of modules clearly led to greater overall surface area of the modeled Pteridinium organism. However, small variations in the width of individual modules had a strong negative effect on the SA/V ratio, whereas module lengths and the addition of new modules had no net effect on the SA/V ratio, because both the surface area and volume increased proportionally (Fig. S1B). Growth by addition of new modules rather than by inflation of a set number of modules conserved the overall SA/V ratio and would be expected to dominate Pteridinium growth should a high SA/V ratio be important. Whereas both module width and number of modules showed positive, statistically significant, rank correlation with overall specimen size, the increase in size was clearly accomplished primarily by addition of modules (Fig. 3A). That is, module width increased only subtly with the overall increase in specimen size, whereas the number of modules nearly doubled when comparing the smallest versus the largest specimens.
Fig. 3.
Growth variations in Pteridinium and Fractofusus. (A) Plot of the number of modules and the maximum module width vs. the overall length of each of the 11 complete Pteridinium specimens from Namibia (6). The plot shows that Pteridinium grew mostly by module addition, not module inflation. (B) Plot of the number of modules and the maximum module length (equal to half of specimen width) vs. the overall length of each of the 66 complete Fractofusus specimens from Newfoundland (18), showing that growth was through module inflation, with minor contribution from module addition during early growth. Spearman Rank Correlation values (r) for Pteridinium: length vs. module width: r = 0.66 (P = 0.03); length vs. number of modules: r = 0.92 (P < 0.0001); for Fractofusus: length vs. module width: r = 0.91 (P < 0.0001); length vs. number of modules: r = 0.49 (P < 0.0001).
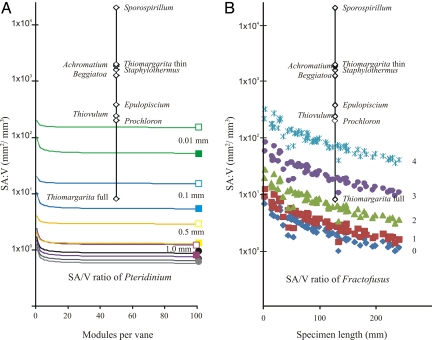
To set the boundaries on possible SA/V ratios for Pteridinium, the modular sizes of the smallest (length: 37 mm, width: 2.4 mm), average (length: 55 mm, width: 3.2 mm), and largest (length: 82 mm, width: 3.6 mm) modules from 11 complete specimens from Namibia were modeled with growth from 1 to 100 module triplets through module addition (Fig. 4A, circles). The largest drop in SA/V ratio occurred when the second and third triplets of modular units were simultaneously added bipolarly with a high degree of overlap (Fig. 2C), because the new bracketing modules effectively share several surfaces no longer in contact with the medium, while tripling the volume. Models that incorporated limited to no overlap (overlap = 0 in Fig. 2C) between adjacent modules were not affected by this initial drop in SA/V ratio. In all cases, the SA/V ratio leveled off at around 1 mm2/mm3 (Fig. 4A, circles), one to four orders of magnitude smaller than the gross SA/V ratios of modern giant osmotrophic megabacteria (Fig. 4A, diamonds). Variations in module size (kept within the boundaries of real samples) only led to SA/V changes within the same order of magnitude. Reducing the thickness of metabolically active tissue from 1 mm to 0.01 mm in average-sized modules, however, drastically reduced the volume and resulted in SA/V ratios for Pteridinium that are on the same order of magnitude as several large, osmotic-feeding bacteria (Fig. 4A). Another means of increasing the overall surface area without changing the volume is to allow for open-ended modules in which the internal surface area of each unit is also available for nutrient diffusion (Fig. 4A, open boxes).
Fig. 4.
Effect of hollowness in Pteridinium (A) and fractal branching in Fractofusus (B) on their SA/V ratios. (A) Estimated SA/V ratios of a Pteridinium specimen consisting of 100 triplets of the smallest (length b = 37 mm, width a = 2.4 mm; black circle), average (b = 55 mm, a = 3.2 mm; dark gray circle), and largest (b = 82 mm, a = 3.6 mm; light gray circle) modules that have full overlap. Modular sizes based on measurements of Pteridinium specimens from Namibia (6). Colored squares represent the effect of hollowness (purple: thickness of metabolically active material, c = 1 mm; yellow, c = 0.5 mm; blue, c = 0.1 mm; and green, c = 0.01 mm). Filled squares represent instances where modules are terminally closed, whereas open squares represent distally open modules in which the internal surface area of modules could also be used for diffusion. All square-symbol models used 100 module triplets with an average module size and full overlap. (B) Increase in Fractofusus SA/V ratios with incremental fractal branching (blue diamonds, fractalless modules; red squares, one order of fractal branching; green triangles, two orders of fractal branching; purple circles, three orders of fractal branching; and blue stars, four orders of fractal branching). Modular sizes based on measurements of Fractofusus specimens from Newfoundland (18). Diamonds in A and B represent SA/V ratios of modern megabacteria. Note Thiomargarita full vs. thin distinguishes between gross SA/V ratio (the entire cell was biologically active) vs. effective SA/V ratios (active organic matter was restricted to a 0.5-μm peripheral layer). Only gross SA/V ratios are presented for all other megabacteria.
The most prominent difference between Pteridinium and Fractofusus models is the subdivision of the module through fractal branching. Modeling shows that each fractal division of rangeomorph frondlets resulted in an increase in the SA/V ratio by a factor of 1.4 to 3.7 (Fig. 4B and Fig. S2). Within the same degree of fractality, the variation in surface area from the smallest to the largest specimens of Fractofusus could be as much as two orders of magnitude (Fig. S2A), but the SA/V ratio decreased by an order of magnitude from the smallest to the largest specimens (Fig. S2B). The SA/V scaling factor in Fractofusus (0.695; Fig. S3) more closely resembles the ideal SA/V scaling factor of 0.67 associated with direct diffusion when compared with a scaling factor of 0.75 attributed to the effect of an internal distribution network (20). This result may also imply that nutrient distribution within rangeomorphs was also guided by direct diffusion.
Ediacaran Osmotrophic Feeding.
Osmotrophy is an effective means of gathering nutrients in microscopic organisms. Osmotrophy relies on a large SA/V ratio so as to ensure proper diffusion throughout the entire organism or cell (21). In microscopic organisms, ensuring a large SA/V ratio is achieved easily. As organisms increase in size, SA/V drops and osmotrophy alone becomes insufficient to meet nutrient demands. Thus, strictly osmotrophic organisms tend to be microscopic (e.g., bacteria), although some macroscopic animals, including sponges, corals, brachiopods, bryozoans, molluscs, and echinoderms, use osmotrophic feeding on DOC as a supplemental food source (22–26).
Modular erniettomorphs and rangeomorphs are constructionally unique, adopting macroscopic morphologies that are uncommon or even unknown from modern or fossil metazoans (4). However, it is possible to consider that rangeomorphs and erniettomorphs attempted to maximize their ability to absorb nutrients via osmosis through Gould's (27) three means by which organisms reduce the effects of disproportional volume increase relative to surface area.
1. The differential increase of surfaces by allometrically changing shape but not morphological complexity through flattening in one dimension, as seen in plant leaves or tapeworms. Both rangeomorphs and erniettomorphs used this strategy to maintain SA/V ratios.
To grow larger, rangeomorphs and erniettomorphs could increase their size through inflation and/or addition of new modules. Although inflationary growth results in a reduction in the SA/V ratio, the addition of new modules has no effect on the SA/V ratio, because the amount of surface area added to the organism is directly proportional to the amount of volume that is also added (Fig. 4A and Fig. S1B). Our results show that the erniettomorph Pteridiunium, which lacked any small-scale branching or convolutions, grew nearly exclusively by module addition (Fig. 3A), whereas the rangeomorph Fractofusus appears to have grown by module addition early in the ontogeny, followed by proportional modular inflation (Fig. 3B).
Constructional flattening is remarkably common and widespread among other Ediacara organisms, and it has been suggested as a means of conserving the SA/V ratio to allow for effective transfer of oxygen and DOC through the integument (11, 28). A sheet-like morphology is ideal for reducing the effect of increasing volume on the SA/V ratio, because the addition of modules along a vane effectively flattens the organism. Furthermore, the proliferation of multifoliate rangeomorph and erniettomorph fronds, such as Rangea, Swartpuntia, and Pteridinium, with several identical sheet-like petaloids encircling a central stalk similar to a revolving door (8) represents an alternative means of extracting nutrients from multiple orientations while maintaining a viable SA/V ratio.
2. The differential increase of surfaces by complication of structure through branching, folding, or convolutions, as seen in our lung alveoli or intestinal villi. Fractal branching in the rangeomorph Fractofusus represents an effective means of increasing SA/V ratios (Fig. 4B and Fig. S2) and compensating for its inflational growth mode (Fig. 3B).
In contrast to erniettomorphs, rangeomorphs, such as Fractofusus, grew mainly by module inflation (Fig. 3B). Despite a significant increase in surface area with growth, the net result was a staggering reduction in SA/V ratio (Fig. 4B and Fig. S2). This decrease in SA/V ratio is compensated for by fractal branching of rangeomorph modules. Modular growth made further orders of fractal branching physiologically beneficial, because extremely small modules (fractal order >2) resulting from repeated branching without a size increase would approach the boundary layer limit. Our modeling results (Fig. 4B) show that fractal branching in rangeomorphs results in significant increases in SA/V ratios that are comparable to some giant bacteria. Insofar as modern giant bacteria have intracellular vacuoles, fractal branching alone might not have been sufficient for rangeomorphs to attain osmotrophic SA/V ratios. Thus, rangeomorphs would need to further increase their SA/V ratios through the inclusion of metabolically inactive material for them to be strictly osmotrophic.
3. The incorporation of inactive matter within the volume occupied, thereby restricting the effective volume used by the organism. An extreme example of this strategy is the modern giant sulfur bacteria Thiomargarita (15). Thiomargarita cells are up to 750 μm in diameter, but they contain a large central vacuole that restricts the cytoplasm to a 0.5- to 2-μm-thick layer, therefore strategically reducing the overall active organic volume (Table 1). It appears that rangeomorphs, and particularly erniettomorphs, would also need to use this strategy to achieve osmotrophic SA/V ratios.
As shown in Fig. 4, variations in the length, width, size, or number of modules did not raise the SA/V ratio sufficiently to allow a strictly osmotrophic feeding strategy for erniettomorphs, and fourth-order fractality in rangeomorphs barely led to osmotrophic SA/V ratios. Thus, the presence of a metabolically inactive material within erniettomorph modules may represent the only viable means of satisfying the minimum osmotrophic SA/V ratios, as determined from megabacteria (Fig. 4A and Table 1). Without metabolically inactive inclusions, it is morphologically impossible for modular erniettomorphs to have SA/V ratios similar to osmotic bacteria and, by extension, for erniettomorphs to be effective osmotrophs.
How plausible is the hypothesis of metabolically inactive inclusions in erniettomorph and possibly rangeomorph modules? Because Ediacara fossils are typically preserved as casts, molds, or two-dimensional impressions (10), it is difficult to infer their internal body structure with any certainty. However, several taphonomic clues suggest that much of the internal body cavity of erniettomorph and rangeomorph may have been filled with metabolically inactive material (inorganic, organic, or fluid). Three-dimensional preservation of Ediacara fossils in the Kuibis Formation of Namibia and the Trepassey Formation of Newfoundland gives a rare glimpse at the complete three-dimensional form of these organisms. Unlike the two-dimensional impressions, three-dimensional casting requires sediment to be introduced into the body cavity of the organism. Variations in the preservation of the tubular modular units in Swartpuntia, from positive structures filled with sediment to empty, deflated units in negative relief, suggest that either the termination of the modular units was open to allow for sediment to be present in the quilts during life, or it was damaged to allow postmortem introduction of sediment into the quilts (29). Regardless, it seems unlikely that Ediacara modules were fully filled with solid tissue. In fact, Ediacara-like fossils from the Dengying Formation of the Yangtze Gorges area, South China, appear to have had distal openings during life (30), and modular units of Pteridinium from the Spitskop Member of the Urusis Formation (31) also show evidence of distal openings. However, many three-dimensionally preserved erniettomorph and rangeomorph modules are filled with sediment but show no evidence of opening or damaging. Thus, it is possible that they incorporated sediments during growth, similarly to modern xenophyophores, which uptake sediments and organic waste within their body to increase mechanical strength and SA/V ratios (32–34). Similar conclusions about sand ingestion were reached concerning Ernietta (Fig. 1 C and D) and other Ediacara organisms (35, 36). The proposition that erniettomorph and rangeomorph modules were filled with metabolically inert material is therefore not novel; however, there was little evidence until now as to the adaptational advantages of such a morphological construction.
Conclusions
Theoretical growth modeling and specimen-based SA/V ratio studies of the rangeomorphs and erniettomorphs have demonstrated that the growth strategies used by these organisms allowed them to maintain high SA/V ratios despite their macroscopic size. This is consistent with morphological expectations for osmotrophs. However, the two groups used different growth strategies.
Erniettomorphs used modular addition, which allows for surface area to keep up with volume increases. Our estimates, based on theoretical modeling and fossil specimens from the Nama Group, show that erniettomorphs were incapable of attaining the SA/V ratios necessary for strict osmotrophy if their body was completely filled with metabolically active material. However, by reducing the volume of biologically active material (through inclusion of sands or vacuoles in the internal space of erniettomorph modules) and by restricting biologically active material to a thin layer of distally open tubes (so that an inner surface can aid diffusion), it is possible for erniettomorphs to have been macroscopic osmotrophs.
In contrast, rangeomorphs grew by inflation and addition of modules and through repetitive branching of the module. Fractal branching played an important role in increasing rangeomorph SA/V ratios. Combined with possible inclusion of metabolically inactive material, fractal branching in rangeomorphs resulted in SA/V ratios on the same order as those expressed in giant bacteria, suggesting that a diffusion-based feeding strategy may have been feasible for rangeomorphs as well.
Finally, giant sulfur bacteria, such as Thiomargarita, thrive along the coastal area of Namibia, where constant upwelling allows for greater access to DOC and nutrients. Such nutrient-rich areas may be modern-day analogs to Ediacaran deep oceans (e.g., Avalon), where contour currents may have continuously replenished nutrients and oxygen supplies (37). Strong convection aids molecular diffusion across the boundary layer. These ecological factors, in tandem with morphological changes to conserve and increase SA/V ratios postulated here, may have played a role in facilitating osmotrophy of erniettomorphs and rangeomorphs, suggesting that it may be more than coincidental that the earliest rangeomorphs occurred in DOC-rich deep waters.
Supplementary Material
Acknowledgments.
We thank J. Gehling, G. Narbonne, the Briggs Paleontology-Evolution group at Yale, and two anonymous reviewers for useful comments and for providing illustrations used in this paper. We also thank C. Hoffmann for introducing us to the Namibian fossils and M. Côté for technical assistance. Research was supported by National Sciences and Engineering Research Council of Canada and Bateman Fellowships (to M.L.), the NASA Exobiology and Evolutionary Biology Program and the National Science Foundation Sedimentary Geology and Paleobiology Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904836106/DCSupplemental.
References
- 1.Clapham ME, Narbonne GM. Ediacaran epifaunal tiering. Geology. 2002;30:627–630. [Google Scholar]
- 2.Clapham ME, Narbonne GM, Gehling JG. Paleoecology of the oldest-known animal communities: Ediacaran assemblages at Mistaken Point, Newfoundland. Paleobiology. 2003;29:527–544. [Google Scholar]
- 3.Bambach RK, Bush AM, Erwin DH. Autecology and the filling of ecospace: Key metazoan radiations. Palaeontology. 2007;50:1–22. [Google Scholar]
- 4.Seilacher A. The nature of vendobionts. In: Vickers-Rich P, Komarower P, editors. The Rise and Fall of the Ediacaran Biota. London: Geological Society; 2007. pp. 387–397. Special Publication 286. [Google Scholar]
- 5.Xiao S, Laflamme M. On the eve of animal radiation: Phylogeny, ecology and evolution of the Ediacara biota. Trends Ecol Evol. 2009;24:31–40. doi: 10.1016/j.tree.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Grazhdankin D, Seilacher A. Underground Vendobionta from Namibia. Palaeontology. 2002;45:57–78. [Google Scholar]
- 7.Narbonne GM. Modular construction of early Ediacaran complex life forms. Science. 2004;305:1141–1144. doi: 10.1126/science.1099727. [DOI] [PubMed] [Google Scholar]
- 8.Laflamme M, Narbonne GM. Ediacaran fronds. Palaeogeogr Palaeoclimatol Palaeoecol. 2008;258:162–179. [Google Scholar]
- 9.Laflamme M, Narbonne GM. Competition in a Precambrian world: Palaeoecology of Ediacaran fronds. Geol Today. 2008;24:182–187. [Google Scholar]
- 10.Narbonne GM. The Ediacara biota: Neoproterozoic origin of animals and their ecosystems. Annu Rev Earth Planet Sci. 2005;33:421–442. [Google Scholar]
- 11.Sperling EA, Pisani D, Peterson KJ. Poriferan paraphyly and its implications for Precambrian paleobiology. In: Vickers-Rich P, Komarower P, editors. The Rise and Fall of the Ediacaran Biota. London: Geological Society; 2007. pp. 355–368. Special Publication 286. [Google Scholar]
- 12.Rothman DH, Hayes JM, Summons RE. Dynamics of the Neoproterozoic carbon cycle. Proc Natl Acad Sci USA. 2003;100:8124–8129. doi: 10.1073/pnas.0832439100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fike DA, Grotzinger JP, Pratt LM, Summons RE. Oxidation of the Ediacaran ocean. Nature. 2006;444:744–747. doi: 10.1038/nature05345. [DOI] [PubMed] [Google Scholar]
- 14.McFadden KA, et al. Pulsed oxidation and biological evolution in the Ediacaran Doushantuo Formation. Proc Natl Acad Sci USA. 2008;105:3197–3202. doi: 10.1073/pnas.0708336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz HN, et al. Dense populations of a giant sulfur bacterium in Namibian shelf sediments. Science. 1999;284:493–495. doi: 10.1126/science.284.5413.493. [DOI] [PubMed] [Google Scholar]
- 16.Schulz HN, Jorgensen BB. Big bacteria. Annu Rev Microbiol. 2001;55:105–137. doi: 10.1146/annurev.micro.55.1.105. [DOI] [PubMed] [Google Scholar]
- 17.Narbonne GM, Laflamme M, Greentree C, Trusler P. Reconstructing a lost world: Ediacaran rangeomorphs from Spaniard's Bay, Newfoundland. J Paleontol. 2009;83:503–523. [Google Scholar]
- 18.Gehling JG, Narbonne GM. Spindle-shaped Ediacara fossils from the Mistaken Point assemblage, Avalon Zone, Newfoundland. Can J Earth Sci. 2007;44:367–387. [Google Scholar]
- 19.Angert ER, Clements KD, Pace NR. The largest bacterium. Nature. 1993;362:239–246. doi: 10.1038/362239a0. [DOI] [PubMed] [Google Scholar]
- 20.West WB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–126. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- 21.Hurd CL. Water motion, marine macroalgal physiology, and production. J Phycol. 2000;36:453–472. doi: 10.1046/j.1529-8817.2000.99139.x. [DOI] [PubMed] [Google Scholar]
- 22.Roditi HA, Fisher NS, Sanudo-Wilhelmy SA. Uptake of dissolved organic carbon and trace elements by zebra mussels. Nature. 2000;407:78–80. doi: 10.1038/35024069. [DOI] [PubMed] [Google Scholar]
- 23.Yahel G, Sharp JS, Marie D, Hase C, Genin A. In situ feeding and element removal in the symbiont-bearing sponge Theonella swinhoei: Bulk DOC is the major source for carbon. Limnol Oceanog. 2003;48:141–149. [Google Scholar]
- 24.Baines SB, Fisher NS, Cole JJ. Uptake of dissolved organic matter (DOM) and its importance to metabolic requirements of the zebra mussel, Dreissena polymorpha. Limnol Oceanog. 2005;50:36–47. [Google Scholar]
- 25.de Goeij JM, van Duyl FC. Coral cavities are sinks of dissolved organic carbon (DOC) Limnol Oceanog. 2007;52:2608–2617. [Google Scholar]
- 26.de Goeij JM, Moodley L, Houtekamer M, Carballeira NM, van Duyl FC. Tracing 13C-enriched dissolved and particulate organic carbon in the bacteria-containing coral reef sponge Halisarca caerulea: Evidence for DOM feeding. Limnol Oceanog. 2008;53:1376–1386. [Google Scholar]
- 27.Gould SJ. Allometry and size in ontogeny and phylogeny. Biol Rev. 1966;41:587–638. doi: 10.1111/j.1469-185x.1966.tb01624.x. [DOI] [PubMed] [Google Scholar]
- 28.Runnegar B. Oxygen requirements, biology and phylogenetic significance of the late Precambrian worm Dickinsonia, and the evolution of the burrowing habit. Alcheringa. 1982;6:223–239. [Google Scholar]
- 29.Narbonne GM, Saylor BZ, Grotzinger JP. The youngest Ediacaran fossils from Southern Africa. J Paleontol. 1997;71:953–967. doi: 10.1017/s0022336000035940. [DOI] [PubMed] [Google Scholar]
- 30.Xiao S, Shen B, Zhou C, Xie G, Yuan X. A uniquely preserved Ediacaran fossil with direct evidence for a quilted bodyplan. Proc Natl Acad Sci USA. 2005;102:10227–10232. doi: 10.1073/pnas.0502176102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Droser ML, Gehling JG, Jensen SR. Assemblage palaeoecology of the Ediacara biota: The unabridged edition? Palaeogeogr Palaeoclimatol Palaeoecol. 2006;232:131–147. [Google Scholar]
- 32.Tendal OS. A monograph of the Xenophyophoria (Rhizopodea, Protozoa) Galathea Rep. 1972;12:7–99. [Google Scholar]
- 33.Tendal OS. Phylum Xenophyophora. In: Margulis L, Corliss JO, Melkonian M, Chapman DJ, editors. Handbook of Protoctista: The Structure, Cultivation, Habitats and Life Histories of the Eukaryotic Microorganisms and Their Descendants Exclusive of Animals, Plants and Fungi: A Guide to the Algae, Ciliates, Foraminifera, Sporozoa, Water Molds and the Other Protoctists. Boston: Jones and Bartlett Publishers; 1990. pp. 135–138. [Google Scholar]
- 34.Seilacher A, Grazhdankin D, Legouta A. Ediacaran biota: The dawn of animal life in the shadow of giant protists. Paleontol Res. 2003;7:43–54. [Google Scholar]
- 35.Seilacher A. Vendobionta and Psammocorallia: Lost constructions of Precambrian evolution. J Geol Soc London. 1992;149:607–613. [Google Scholar]
- 36.Dzik J. Organic membranous skeleton of the Precambrian metazoans from Namibia. Geology. 1999;27:599–522. [Google Scholar]
- 37.Wood DA, Dalrymple RW, Narbonne GM, Gehling JG, Clapham ME. Paleoenvironmental analysis of the late Neoproterozoic Mistaken Point and Trepassey formations, southeastern Newfoundland. Can J Earth Sci. 2003;40:1375–1391. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.