Abstract
Therapeutics for cerebral ischemia/hypoxia, which often results in ischemic stroke in humans, are a global unmet medical need. Here, we report that bryostatin-1, a highly potent protein kinase C (PKC) activator, interrupts pathophysiological molecular cascades and apoptosis triggered by cerebral ischemia/hypoxia, enhances neurotrophic activity, and induces synaptogenesis in rats. This postischemic therapeutic approach is further shown to preserve learning and memory capacity even 4 months later as well as long-term memory induced before the ischemic event. Our results of electromicroscopic and immunohistochemical analyses of neuronal and synaptic ultra-structure are consistent with a PKC-mediated synaptic remodeling and repair process that confers long-lasting preservation of spatial learning and memory before and after the cerebral ischemic/hypoxic event, suggesting a previously undescribed therapeutic modality for cerebral ischemia/hypoxia and ischemic stroke.
Keywords: bryostatin, protein kinase C, stroke therapy, synaptogenesis
Cerebral ischemia/hypoxia, which often results in stroke, is the third leading cause of death and the most common cause of long-term disability in the developed countries. Decades of intensive searching for neuroprotective drugs against cerebral ischemia/ischemic stroke have met many preclinical but not clinical successes (1–8). The only option currently available for the treatment of ischemic stroke is the thrombolytic therapy (such as, the recombinant tissue plasminogen activator), which must be administered within 3 h after the episode to achieve early arterial recanalization. Several factors most likely contribute the translational failures. Targeting mainly the pathological cascades induced by ischemia/hypoxia (arresting the pathological pathway), such as extracellular build up of excitatory aminio acids and intracellular Ca2+ elevation, is most effective when the blockers are given at or immediately before an ischemic event. Furthermore, the targeted neurotransmitters, Ca2+, and many enzymes involved in the ischemic/hypoxic responses are also critical for neuronal functions so that their effective blockade cannot be maintained without producing serious adverse effects or toxicity. In addition, the short-term effectiveness of agents arresting the pathological pathway as determined in most preclinical studies, does not guarantee a cure, because such drug candidates may delay but not prevent the pathological impact (9).
We have recently found that, instead of only arresting the pathological cascades in cerebral ischemia, postischemic (24 h after) bryostatin-1, a relatively isoform-selective protein kinase C (PKC) activator with antiapoptotic and synaptogenesis-facilitating properties (10–13), rescues rats from ischemic/hypoxic impairment of spatial learning and memory (14), evaluated about 2 weeks after the end of a 5-week treatment.
Results
Here, we investigated therapeutic effects of postischemic bryostatin-1 in two paradigms: one involving long-term anterograde, and the other retrograde efficacies. Four groups of rats were used: ischemia, ischemia+bryostatin-1, brayostatin-1 only, and sham-operated control.
Bryostatin-1 Produces a Lasting Rescue of Spatial Learning and Memory from Global Cerebral Ischemic Impairment.
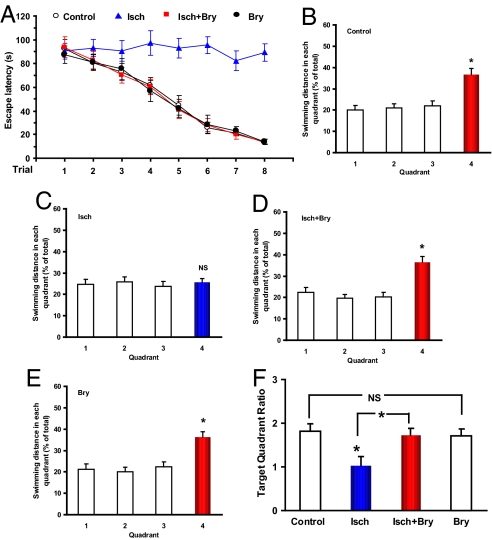
First, we determined whether postischemic bryostatin-1 would produce a persistent (e.g., many months) functional recovery of the neural circuits and synapses. Bryostatin-1 was administered (15 μg/m2, i.v., two doses/week for 5 weeks) with the first dose given 24 h after the end of the cerebral ischemic/hypoxic event in rats (14). Spatial learning and memory abilities were tested 4 months after the last bryostatin-1 dose. There was a significant learning difference among the four groups (Fig. 1). The ischemic group showed a severe learning impairment (escape latency over trials: F7,63 = 0.393, P > 0.05) and an impaired learning as compared with the controls (F1,127 = 52.613, P < 0.001). The other three groups all learned the task (P < 0.001 over trials). The bryostatin-1 treatment greatly improved the learning (ischemic group with bryostatin-1 vs. ischemic rats: F1,127 = 66.106, P < 0.001), to the level of the control (ischemic with bryostatin-1 vs. control: F1,127 = 0.434, P > 0.05). There were no significant differences between the control and bryostatin-1-only groups (bryostatin-1 vs. control: F1,127 = 0.952, P > 0.05).
Fig. 1.
Lasting effects of postischemic bryostatin-1 on cerebral ischemia/hypoxia-induced impairment of spatial learning and memory. (A) Spatial water maze performance of rats over training trials (means ± SEM.; 2 trials/day for 4 days; group difference: F3,255 = 29.888, P < 0.001). (B–E) Results of the probe test after the training trials. Quadrant 4 was the target quadrant where the hidden platform was placed during the training trials. (F) Target quadrant ratios (dividing the target quadrant distance by the averaged nontarget quadrant values during the probe test; group difference: F3,31 = 4.096, P < 0.05). Eight rats/group; Bry, bryostatin-1; Isch, cerebral ischemia. *, P < 0.05. NS: P > 0.05.
The rats in the ischemic group did not show a target preference in the memory recall test (F3,31 = 0.169, P > 0.05), whereas the other three groups all showed a target quadrant preference (all P < 0.05; see Fig. 1). There was a significant difference in the target quadrant ratio among the groups (Fig. 1). Significant difference existed between the control and ischemic (F1,15 = 7.446, P < 0.05) and between the ischemic and ischemic with bryostatin-1 (F1,15 = 7.300, P < 0.05). No significant difference was found between the control and ischemic with bryostatin-1 (F1,15 = 0.140, P > 0.05) and between the control and bryostatin-1-only (F1,15 = 0.212, P > 0.05). Importantly, a visible platform test after the probe test revealed no significant difference among the 4 groups (F3,31 = 0.172, P > 0.05), indicating no significant group differences in sensorimotor ability and escape motivation.
Bryostatin-1 Rescues Hippocampal Neurons from Global Cerebral Ischemia-Induced Cell Death.
It should be noted that infarct volume is commonly measured in studies that involve focal ischemia/stroke, but not global cerebral ischemia—the model that was used in the present study. The majority of brains in the present studies showed neither hippocampal atrophy nor lateral ventricle enlargement. No macroscopic changes were observed in the ischemia+bryostatin-1, control, and bryostatin-1 groups.
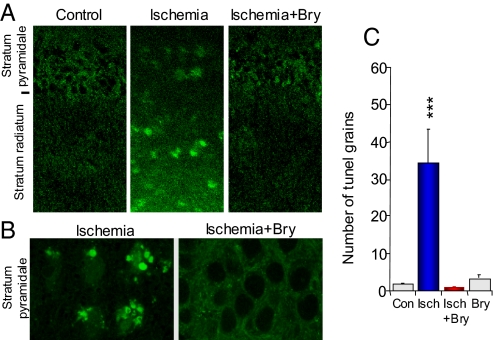
The global ischemia/hypoxia-induced impairment of spatial learning and memory results from a gradual loss of synapses, apoptotic cell death, and an insufficient synaptic repairing/neurotrophic activity in the dorsal hippocampus CA1 area (14). All of these consequences were effectively rescued by the bryostatin-1 treatment. Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) (15) detects DNA strand breaks in apoptosis (programmed cell death) at the single cell level. Five weeks after global ischemia/hypoxia, TUNEL-positive staining was found in the stratum pyramidale and the stratum radiatum in the hippocampal CA1 area (Fig. 2 A and B), reflecting translocation of TUNEL-labeled DNA strand breaks from the nuclei to the dendritic processes of the pyramidal neurons. Quantitative analysis (Fig. 2C) showed significant differences among the groups (F3,116 = 10.974, P < 0.001). Ischemia/hypoxia increased apoptosis in the dorsal hippocampal CA1 area (P < 0.001; coefficient of error or C.E. = 1.53), a response blocked by the postischemic bryostatin-1 treatment (P < 0.001; C.E. = 1.08). Bryostatin-1 alone did not significantly change TUNEL staining.
Fig. 2.
Bryostatin prevents apoptotic cell death induced by ischemia/hypoxia. Low (A) and high (B) magnification of apoptotic cell death in CA1 hippocampal area, detected by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) and visualized by a confocal microscope (a double-blind study). (C) Quantification of TUNEL staining in stratum radiatum (n = 3 animals; n = 30 confocal images). Con, control; Bry, bryostatin-1; Isch, cerebral ischemia; ***, P < 0.001.
Bryostatin-1 Induces Synaptogenesis and Prevents Global Cerebral Ischemia-Induced Synaptic Loss.
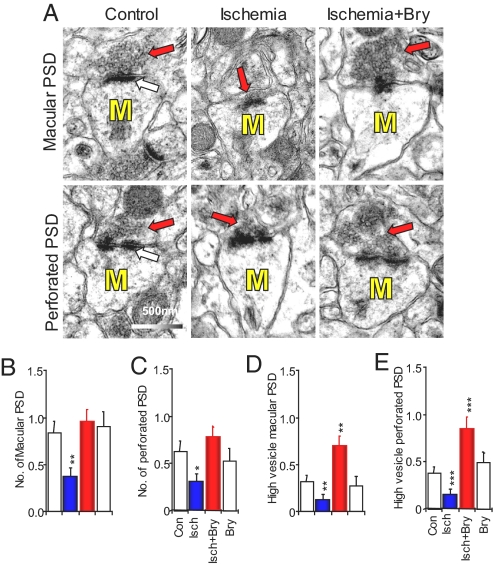
Using immunohistochemistry and confocal and electron microscopy, we previously demonstrated the decrease in the number of total dendritic spines induced by global ischemia/stroke in hippocampi with and without macroscopic changes. Bryostatin-1 prevented the loss of dendritic spines induced by global ischemia/stroke (12). Long-term memory is stored in the brain by the formation of mushroom-shape dendritic spines-containing perforated postsynaptic density (PSDs) (Fig. 3A) (16). Because the number of presynaptic boutons was not altered by memory, the memory-increased mushroom spines form multiple synapses with preexisting axonal boutons that already synapse with other dendritic spines (10, 17). The increase in the number of multiple synapse boutons results in an increase in presynaptic vesicle concentration (10).
Fig. 3.
Bryostatin restores the decreases in mushroom-shape dendritic spines and presynaptic vesicles in axonal boutons. (A) Electron micrographs of mushroom spines (M) showing macular (Upper) and perforated (Lower) postsynaptic densities (PSD; white arrows) and presynaptic vesicles (red arrows). (B–C) The total numbers of mushroom spines containing macular and perforated PSDs. (D–F) The numbers of axonal boutons with high concentration of presynaptic vesicles. Con, control; Bry, bryostatin-1; Isch, cerebral ischemia; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Our present EM data show that the bryostatin-1 treatment prevented the ischemic loss of the preexisting ultra-structures for long-term associative memory, that is, perforated PSD mushroom spines that form synapses with an axonal bouton containing a high volume of presynaptic vesicles (10). Double-blind electron microscopy was used to investigate the effects of ischemia/hypoxia and/or bryostatin-1 on the number of mushroom spines and the number of axonal boutons with high concentration of presynaptic vesicles (n = 48–62 electron micrographs; see Fig. 3). There were significant differences among the groups for the numbers of macular PSD mushroom spines (F3,232 = 4.577, P < 0.01; see Fig. 3 A and B) and perforated PSD mushroom spines (F3,232 = 4.988, P < 0.01; see Fig. 3 A and C). Global ischemia/hypoxia decreased the number of macular (P < 0.01; C.E. = 0.97) and perforated (P < 0.05; C.E. = 1.02) PSD mushroom spines. The loss of the mushroom spine synapses was prevented with the postischemic bryostatin-1 treatment, for both the macular (P < 0.001; C.E. = 0.44) and perforated (P < 0.001; C.E. = 0.54) PSDs. Bryostatin-1 alone did not affect the number of mushroom spines. Fig. 3 A and D showed that there were significant differences among the groups for the number of axonal boutons with dense presynaptic vesicles that formed synapses with macular PSDs (F3,232 = 7.769, P < 0.001). Fig. 3 A and E showed similar effect for the number of dense presynaptic vesicles that formed synapses with perforated PSDs (F3,232 = 8.095, P < 0.001). The global cerebral ischemia/hypoxia reduced the number of axonal boutons containing dense presynaptic vesicles and forming synapses with macular (P < 0.01; C.E. = 1.02) and perforated (P < 0.001; C.E. = 0.97) PSD mushroom spines. Bryostatin-1 not only rescued the decreases in presynaptic vesicles induced by ischemia/hypoxia but also increased presynaptic vesicles as compared with the controls for macular (P < 0.01; C.E. = 0.36) and perforated (P < 0.001; C.E. = 0.50) PSD mushroom spines. Bryostatin-1 alone had no effect on the density of presynaptic vesicles. The EM results are consistent with our previous observation using immunohistochemistry of synaptophysin, a presynaptic vesicle membrane protein, visualized by a confocal microscope (14).
Bryostatin-1 Rescues Learned Spatial Experiences from Global Cerebral Ischemic Impairment.
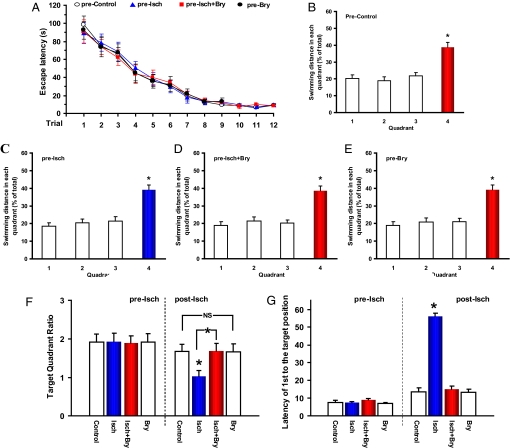
In the second paradigm, we investigated whether bryostatin-1 could rescue previously learned experience. Separate groups of rats were intensively trained in a water maze. One day after the probe test, rats were randomly divided into four groups: sham-operated control, ischemic-hypoxic, ischemic-hypoxic with bryostatin-1 treatment, and bryostatin-1-only. Before the ischemia/treatment, all these rats learned the spatial task (Fig. 4) without significant learning difference among the groups. All of the groups showed preference to the target quadrant in the memory probe test (all P < 0.005; see Fig. 4). Two sets of data were obtained in the probe test for evaluating memory of the learned experience: the target quadrant ratios and the latency of the first crossing the square position of the removed platform (the same 1-min trial with 60 s scored if a rat did not reach the position in 60 s). There were no significant differences in the target quadrant ratios among the groups (F3,31 = 0.009, P > 0.05) and in the latency of the first crossing the position of the removed platform (F3,31 = 0.939, P > 0.05) before the ischemia/treatment.
Fig. 4.
Rescuing learned experience with postischemic bryostatin-1. (A) Spatial water maze performance of rats over training trials (2 trials/day for 6 days) before the ischemia/treatment (means ± SEM.; trials: F11,383 = 40.483, P < 0.001; groups: F3,383 = 0.315, P > 0.05). (B–E) Results of the probe test after the training trials before the ischemia and/or treatment. Quadrant 4 was the target quadrant. (F) Target quadrant ratios before (pre-Isch) and after (post-Isch) the ischemia and/or treatment. (G) The latency of the first crossing the target location before (pre-Isch) and after (post-Isch) the ischemia and/or treatment. Eight rats/group; Bry, bryostatin-1; Isch, cerebral ischemia. *, P < 0.05. NS: P > 0.05.
Cerebral ischemia was then induced as described previously (14), 24 h after the probe test. A brief period of hypoxia was induced after recovery from the surgery. The bryostatin-1 treatment (i.v., 15 μg/m2, two doses/week for 5 weeks) was then started, with the first dose given 24 h after the end of the ischemic/hypoxic episode. A second probe test was performed 2 weeks after the last bryostatin-1 dose. The rats in the ischemic group did not show a target preference in the second memory probe test (F3,31 = 0.213, P > 0.05), indicating an impairment of previously formed memory, whereas the other three groups showed a target quadrant preference (all P < 0.005). There was a significant difference in the target quadrant ratios among the groups (F3,31 = 3.222, P < 0.05; see Fig. 4F), between the control and ischemic rats (F1,15 = 7.329, P < 0.05) and between the ischemic and ischemic with bryostatin-1 (F1,15 = 7.297, P < 0.05), but no significant differences between the control and ischemic rats with bryostatin-1 (F1,15 = 0.060, P > 0.05) and between the control and bryostatin-1-only (F1,15 = 0.032, P > 0.05). There was also a group difference in the latency of the first crossing the position of the removed platform in the second probe test (F3,31 = 107.113, P < 0.001; see Fig. 4G). Excluding the ischemia group, there was no significant difference in this latency among the remaining three groups (F2,23 = 0.247, P > 0.05). After the probe test, a visible platform test revealed no significant difference among the groups (F3,31 = 0.218, P > 0.05), indicating no significant group differences in sensorimotor ability and escape motivation.
Discussion
The results presented in this paper showed that the postischemic bryostatin-1 treatment not only rescues the ability of rats to learn and memorize the spatial maze, but also rescues/protects previously learned spatial experience. This result illustrates the great therapeutic potential for agents, such as bryostatin-1, that possess both antiapoptotic and synaptic remodeling/repairing-enhancing properties in postischemic therapy. Bryostatin-1, at low concentrations, selectively activates PKCε, while down-regulating PKCδ (18). The data are also consistent with the critical role of specific PKC isozymes, such as PKCε, in spatial learning and memory and synaptic remodeling (19, 20). PKCδ, on the other hand, is possibly involved in ischemic/reperfusion injury (21). Thus, consistent with biochemical changes of pre- and postsynaptic neurotrophic and other proteins (14), including spinophilin, synaptophysin, and BDNF, bryostatin-1 prevents synaptic loss, induces synaptogenesis, produces an antiapoptotic effect, and increases cell survival and activity of brain-derived neurotrophic factor (BDNF) in the dorsal hippocampal CA1 area as shown in our previous study (14).
We interpret these data to suggest that the bryostatin-1-induced rescuing effects most likely represent a true “cure” because they remain long after the end of the treatment and include learned experience. The observation is consistent with a recent report that BDNF, not nerve growth factor, produces a protective and restorative effect against synaptic loss in rodent and primate models of Alzheimer's disease (22). The results thus strongly suggest that bryostatin-like compounds may be developed as effective therapeutics for the treatment of ischemic/hypoxic neurological disorders and ischemic stroke (23, 24).
Materials and Methods
Animals, Cerebral Ischemia/Hypoxia, and Drug Treatments.
Adult Wistar rats (male, 225–250 g) were housed in a temperature-controlled (20–24 °C) room for at least 1 week before experimentation, allowed free access to food and water, and kept on a 12-h light/dark cycle. They were randomly assigned to different groups. Global cerebral ischemia was induced in vivo by the two-artery occlusion (2-VO) method, combined with a controlled period of hypoxia. The bilateral common carotid arteries (from ventral side of the neck) were tied with silk threads, under an appropriate level of pentobarbital anesthesia (60 mg/kg, i.p., with additional supplemental doses when necessary). The 2-VO occlusion led to a chronic reduction in cerebral blood flow to 70% of the original flow rate in these young rats. Rectal temperature was monitored throughout the surgery and maintained at 37.5 °C by using a heat lamp. At the end of surgery and under appropriate anesthesia, rats received ketoprofen (5 mg/kg, s.c.) for postsurgical pain relief and 5 ml saline to compensate for loss of water and minerals. A 7-day recovery period from surgery was allowed before the period of hypoxic exposure (about 14 min in a jar with blowing in low oxygen of about 5%). The combined cerebral ischemia/hypoxia resulted in a loss of righting reflex for about 10 min. Control rats were subjected to an identical procedure, except that the arteries were not occluded and the oxygen level was not reduced in the same jar.
Bryostatin-1 (15 μg/m2) was administered via a tail vein (two doses/week, for 10 doses), starting 24 h after the end of the ischemic/hypoxic event.
All procedures were conducted according to National Institutes of Health Animal Care and Use Committee guidelines and were approved by the Ethical Committee of the Institute.
Spatial Learning and Memory.
The ability of the rats in spatial learning (2 trials/day for 4 days or 6 days) and memory (a probe test of 1 min, 24 h after the last learning trial) was evaluated as the functional endpoint, with the first training started 9 days after the last dose of bryostatin-1. The delay between the last bryostatin-1 dose and the first spatial maze training trial appears appropriate for a separation of the acute memory action of bryostatin-1 from their effects on neurorepairing after cerebral ischemia.
Water maze acquisition was used in this study because it was most closely associated with functions of the dorsal hippocampal CA1 pyramidal cells. Rats were moved to the test room in their home cages at least 1 h before daily trials. The maze pool had a diameter of 152 cm and height of 60 cm and was filled with 40 cm H2O (22 ± 1 °C). The maze was divided into four quadrants. Rats were trained for 4 days (two trials/day) to find a hidden platform (9 cm diameter), centered in one of the quadrants and submerged about 2 cm below the water surface. At the start of all trials, rats were placed individually in the water facing the maze wall, using different starting positions each trial, and allowed to swim until they find the platform, where they remained for 20 s, before being returned to their home cages. A rat that failed to find the platform within 2 min was guided there by the investigator, with 120 s scored. The swim path was recorded with a video-tracking system, which computed latency to the platform, swim distance, and percentage of time spent in the quadrants. After the training trials, a probe trial (a quadrant test or retention trial) was given with the platform removed to assess memory retention for its location by the distance the rat moved in the quadrants. The video-tracking system tracked the animal's movements in each quadrant during a period of 1 min.
Visible Platform Test.
After completion of the spatial memory procedure, all rats were tested on a visible platform task (with the platform marked with a pole that protruded 9 inches above the water surface but at a new location) to evaluate their sensorimotor abilities and escape motivation differences.
Staining for Apoptotic Cell Death.
Three animals were randomly selected to fix as described (14). Under anesthesia (Choral hydrate; Sigma-Aldrich; 400 mg/kg body weight, i.p.), animals were perfused by gravity through the heart with PBS (PBS) at room temperature to wash out the blood and then fixed with approximately 150 mL of 4% paraformaldehyde in PBS at room temperature. Brains were removed and postfixed with 4% paraformaldehyde for 15 min at 4 °C and then kept in PBS at 4 °C. Thereafter, right hippocampi were sectioned with a vibratome at 400 μm and further fixed with 4% paraformaldehyde in PBS at room temperature for 1 h and resectioned to 35 μm.
Sections at 35-μm thickness at about 800 μm apart along dorsal hippocampi (three sections/animal) were used to detect apoptotic cells with terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL; R&D Systems) by free floating technique. The staining was modified from the standard procedure as described in the TUNEL labeling kits. Sections were washed with deionized (DNase-free) water (at room temperature, three×, 5 min each) and treated with proteinase K (1:200) in deionized water for 2 h at 37 °C. After washed with deionized water (at room temperature, three times 5 min each), sections were placed in TdT labeling buffer for 5 min at room temperature. Sections were incubated with the labeling reaction mix (TdT dNTP mix, Mn2+, and TdT enzyme; all 1:50 in TdT labeling buffer) in a humidity chamber, slightly shaked with a rocking platform shaker, at room temperature over night. Sections were stored in TdT stop buffer for 10 min at room temperature and then washed three times with PBS (room temperature) for 5 min each. Sections were incubated with Strep-Fluor solution (1:50) in PBS at room temperature for 3 h and washed two times with 0.1% Tween 20 in PBS at room temperature for 5 min each. After washed with deionized water at room temperature for 5 min, sectioned were mounted with Vectashield mounting medium containing DAPI to counterstain nuclei (Vector Lab). Sections were imaged with a Zeiss 510 confocal scanning microscope. Confocal images were obtained from strata radiatum of hippocampal CA1 area (3–4 images/section; randomly selected throughout the CA1 area) and then analyzed with the ImageJ program (http://rsb.info.nih.gov/ij/).
Electron Microscopy.
Electron microscopy was performed as previously described as above with 400 mL of the fixative (2% glutaradehyde and 3% paraformadehyde) in PBS at room temperature. Thereafter, right hippocampi were isolated from the brains of randomly selected rats (n = 3 rats), and dorsal hippocampi were sectioned with a vibratome at 400 μm and re-sectioned to 100 μm (three sections from each animals and ≈800 μm apart). Sections were washed three times with cacodylate buffer (pH 7.4), postfixed in 1% OsO4 for 1 h, and then rinsed with distilled water. The slices were dehydrated in a graded ethanol series, followed by resin embedding and then sectioning using standard procedures. Ultra-thin sections (90 nm), randomly selected and cut from 100 μm thickness hippocampal slices, were stained with uranyl acetate and lead acetate and viewed at 100 kV in a JEOL 200CX electron microscope. Data were analyzed by double-blind quantification (unknown treatment and animal subject) from electron micrographs (10 μm × 10 μm). Morphology of mushroom-shape dendritic spines, presynaptic axonal boutons, and synapses were quantitatively analyzed according to Hongpaisan and Alkon (10). The criteria of mushroom spines are dendritic spines with (cross-sectionally visualized) diameter >600 nm. Increased presynaptic vesicles in axon boutons were determined by an increase in the frequency to find axon boutons with presynaptic vesicles occupied more than 50% cross section. Each 90-nm section that was analyzed was taken at random from the CA1 area. Adjacent sections were not used.
Statistical analysis was performed using the analysis of variance (ANOVA), followed by the Newman-Keuls multiple comparisons test, or using the Student's t test for paired and unpaired data wherever appropriate. P < 0.05 is considered significant. Coefficient of error was defined as the standard deviation divided by the mean.
Footnotes
The authors declare no conflict of interest.
References
- 1.Marler JR. NINDS clinical trials in stroke. Lesions learned and future directions. Stroke. 2007;38:3302–3307. doi: 10.1161/STROKEAHA.107.485144. [DOI] [PubMed] [Google Scholar]
- 2.Savitz SI, Fifher M. Future of neuroprotection for acute stroke: In the aftermath of the SAINT trials. Ann Neurol. 2007;61:396–402. doi: 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]
- 3.Pollack A. AstraZeneca stroke drug fails in a clinical trial. NY Times. 2006 Oct 27; Section B, p 5. [Google Scholar]
- 4.Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: Two decades of success and failure. J Am Soc Exp Neuro Ther. 2004;1:36–45. doi: 10.1602/neurorx.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark WM, Wechsler LR, Sabounjian LA, Schwiderski UE. Citicoline Stroke Study Group. A phase III randomized efficacy trial of 2000 mg citicoline in acute ischemic stroke patients. Neurology. 2001;57:1595–1602. doi: 10.1212/wnl.57.9.1595. [DOI] [PubMed] [Google Scholar]
- 6.Yam PS, Dunn LT, Graham DI, Dewar D, McCulloch J. NMDA receptor blockade fails to alter axonal injury in focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:772–779. doi: 10.1097/00004647-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Davis SM, et al. Selfotel in acute ischemic stroke: Possible neurotoxic effects of an NMDA antagonist. Stroke. 2000;31:347–354. doi: 10.1161/01.str.31.2.347. [DOI] [PubMed] [Google Scholar]
- 8.Mohr JP, et al. Meta-analysis of oral nimodipine trials in acute ischemic stroke. Cerebrovasc Dis. 1994;4:197–203. [Google Scholar]
- 9.Valtysson J, Hillered L, Andine P, Hagberg H, Persson L. Neuropathological endpoints in experimental stroke pharmacotherapy: The importance of both early and late evaluation. Acta Neurochir. 1994;129:58–63. doi: 10.1007/BF01400874. [DOI] [PubMed] [Google Scholar]
- 10.Hongpaisan J, Alkon DL. A structural basis for enhancement of long-term associative memory in single dendritic spines regulated by PKC. Proc Natl Acad Sci USA. 2007;104:19571–19576. doi: 10.1073/pnas.0709311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennings H, et al. Bryostatin 1, an activator of protein kinase C, inhibits tumor promotion by phorbol esters in SENCAR mouse skin. Carcinogenesis. 1987;8:1343–1346. doi: 10.1093/carcin/8.9.1343. [DOI] [PubMed] [Google Scholar]
- 12.Sun M-K, Alkon DL. Bryostatin-1: Pharmacology and therapeutic potential as a CNS drug. CNS Drug Rev. 2006;12:1–8. doi: 10.1111/j.1527-3458.2006.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alkon DL, Epstein H, Kuzirian A, Bennett MC, Nelson TJ. Protein synthesis required for long-term memory is induced by PKC activation on days before associative learning. Proc Natl Acad Sci USA. 2005;102:16432–16437. doi: 10.1073/pnas.0508001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun M-K, Hongpaisan J, Nelson TJ, Alkon DL. Post-stroke neuronal rescue and synaptogenesis mediated in vivo by PKC in adult brains. Proc Natl Acad Sci USA. 2008;105:13620–13625. doi: 10.1073/pnas.0805952105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavrieli Y, et al. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagerl UV, Kostinger G, Anderson JC, Martin KA, Bonhoeffer T. Protracted synaptogenesis after activity-dependent spinogenesis in hippocampal neurons. J Neurosci. 2007;27:8149–8156. doi: 10.1523/JNEUROSCI.0511-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van der Zee EA. Associative learning elicits the formation of multiple-synapse boutons. J Neurosci. 2001;21:5568–5573. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szallasi Z, Smith CB, Petti GR, Blumberg PM. Differential regulation of protein kinase C isozymes by bryostatin-1 and phorbol 12-myristate 13-acetate in NIH 3T3 fibroblasts. J Biol Chem. 1994;269:2118–2124. [PubMed] [Google Scholar]
- 19.Alkon DL, Sun M-K, Nelson TJ. PKC signaling deficits: A mechanistic hypothesis for the origins of Alzheimer's disease. Trends Pharmacol Sci. 2007;28:51–60. doi: 10.1016/j.tips.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Bank B, DeWeer A, Kuzirian AM, Rasmussen H, Alkon DL. Classical conditioning induces long-term translocation of protein kinase C in rabbit hippocampal CA1 cells. Proc Natl Acad Sci USA. 1988;85:1988–1992. doi: 10.1073/pnas.85.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bright R, Steinberg GK, Mochly-Rosen D. δPKC mediates microcerebrovascular dysfunction in acute ischemia and in chronic hypertensive stress in vivo. Brain Res. 2007;1144:146–155. doi: 10.1016/j.brainres.2007.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagahara AH, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alhzeimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 24.Lee J-M, Grabb MC, Zipfel GJ, Choi DW. Brain tissue response to ischemia. J Clin Invest. 2000;106:723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]