Abstract
Corticotropin-releasing factor (CRF), encoded by the CRH gene, is a key integrator of stress responses, and, as such, CRH gene variation may contribute to individual differences in susceptibility to stress-related pathology. In rhesus macaques, a single nucleotide polymorphism (SNP) is found within the CRH promoter (−248C→ T). Here, we assessed whether this variant influenced stress responding and, because increased CRF system activity drives alcohol drinking in rodents, we examined whether it predicted voluntary alcohol consumption as a function of prior stress exposure. Using a hypothalamic nuclear extract, we showed that the −248 T allele resulted in increased DNA protein interactions relative to the C allele. In vitro, the T allele resulted in CRH promoter activity that was higher following both stimulation with forskolin and treatment with dexamethasone. Endocrine and behavioral responses to social separation stress (release of ACTH and cortisol, and suppression of environmental exploration, respectively) were higher among carriers of the T allele, particularly among those exposed to early adversity in the form of peer rearing. We also found that T allele carriers with a history of early life adversity consumed more alcohol in a limited-access paradigm. Our data suggest that CRH promoter variation that confers increased stress reactivity increases the risk for alcohol use disorders in stress-exposed individuals.
Keywords: corticotropin-releasing factor, CRH promoter
Corticotropin-releasing factor (CRF or CRH), encoded by the CRH gene, is a key integrator of stress adaptation. In response to stress, extrahypothalamic and hypothalamic CRF systems act in concert to mount adaptive behavioral, autonomic, and endocrine responses (1). In the adrenal gland, secretion of glucocorticoids is stimulated by adrenocorticotropic hormone (ACTH), which is released from adenohypophyseal corticotrophs in response to CRF (2). Glucocorticoids then interact with specific cytosolic receptors (glucocorticoid receptors, or GR) in central and peripheral tissues (3), enabling life-preserving adaptation to stress. In parallel, release of CRF from extrahypothalamic brain regions, such as the locus coeruleus, BNST, and amygdala, mediate autonomic and behavioral responses to stress (4) that underlie vigilance, fear, and emotionality (5–7).
The CRF system is critical for survival, but chronic overactivity can lead to stress-related pathologies (8–10). Dysregulation of this system has been linked to a variety of stress-related psychiatric disorders, including depression, PTSD, and alcohol dependence (11–14). Studies in both non-primate and primate species have shown that an up-regulated CRF system can produce anxiety- and/or depression-like phenotypes (15–18), and rodent studies show that it leads to escalated alcohol drinking (19–23). Based on these findings, CRF1 antagonists have been proposed for the treatment of stress-related disorders, including alcohol dependence (24, 25). Genetic variation that drives further recruitment of the CRF system in response to stress or alcohol exposure would be expected to increase sensitivity to the negative reinforcing effects of alcohol (relief drinking). Individuals carrying such alleles might not only be more vulnerable to developing alcohol dependence, but may be particularly good candidates for treatment with CRF1 antagonists.
We have identified a putatively functional SNP in the proximal regulatory region of the rhesus CRH gene. This region is highly conserved (26, 27), and transcription factor-binding sites and interactions among bound transcription factors are well-characterized (28). CRH expression is largely driven through a cAMP response element (CRE, located between −228 to −221 bps), and interactions of this site with the adjacent GR response element (GRE, located between - 278 to −251 bps) are important in regulating expression of CRH during periods of stress (28). Whereas in hypothalamus, glucocorticoids inhibit CRH transcription, high levels of glucocorticoids induce CRH transcription in some extrahypothalamic tissues, such as the amygdala (26, 27). Suppression of hypothalamic CRH is critical to negative feedback control of the HPA axis, whereas glucocorticoid-mediated increases in amygdalar CRH expression are proposed underlie behavioral sensitization to stress (7). Genetic variation within the proximal regulatory region of the CRH gene would likely disrupt its transcriptional regulation and would, therefore, moderate susceptibility to stress-related pathology. Here, we examined whether a SNP in this region of the rhesus macaque CRH promoter (CRH-248 C → T) influenced CRH promoter activity in vitro, and whether it predicted phenotypic variation in vivo.
During periods of stress, hypothalamic CRF activates the HPA axis, while extrahypothalamic CRF systems suppress exploration of a novel environment (5). In the laboratory, rhesus macaques will decrease the degree to which they explore their environments during exposure to various types of stressors (29), and pharmacological studies demonstrate this to be rescued by CRF receptor antagonism (30, 31). We examined whether CRH −248 C → T would confer differences in HPA axis reactivity and levels of environmental exploration during periods of social separation stress. Because exposure to early stress in the form of peer rearing increases alcohol consumption in adult rhesus macaques (32–35) and increased CRF system activity drives alcohol drinking in rodents (25), we examined whether −248 C → T would influence alcohol consumption as a function of early stress exposure.
Results
Comparative Genome Information.
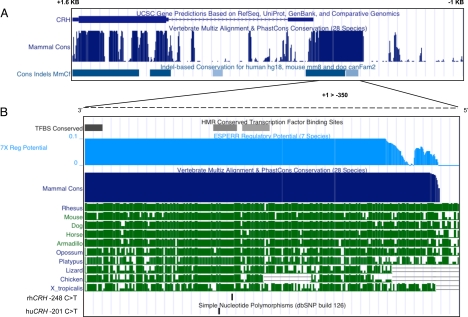
In silico analyses indicated that the proximal promoter for the CRH gene is highly conserved among placental mammalian species and that is under purifying selection (Fig. 1A). Further analyses indicated conserved transcription factor-binding sites and a high degree of regulatory potential for this region (Fig. 1B). There is a high degree of conservation in invertebrates as well (Fig. 1B). The CRH −248 C → T SNP and a human CRH promoter SNP (rs28364015, −201 C → T) are both present within this highly conserved regulatory region (Fig. 1B).
Fig. 1.
Comparative genome information for the CRH gene from the UCSC Genome Browser Gateway (http://genome.ucsc.edu). (A) Coding regions for CRH are shown in addition to vertebrate conservation and Indel-based Conservation for the region −1 KB to + 1.6 KB from the transcription start site. (B) Conserved transcription factor-binding sites, regulatory potential and vertebrate conservation are shown for the CRH proximal promoter (−350 KB - +1). Also shown is the location of the rhesus −248 C → T SNP and a human SNP reported in this region (−201 C → T, rs28364015).
Functional Characterization of CRH −248 C → T.
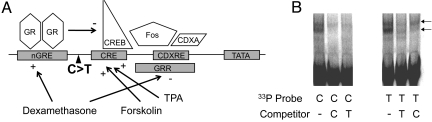
The −248 C → T SNP was located between the CRE and the nGRE, a region through which various agents act to stimulate or diminish CRH expression (Fig. 2A). Gel shift assays performed using hypothalamic nuclear extract (generated from IVB cells) demonstrated DNA-protein interactions to differ in assays performed with −248 T probes (Fig. 2B).
Fig. 2.
Location of the CRH −248 C → T SNP and effects on DNA-protein interactions. (A) Schematic of CRH promoter regulation and the transcription factors whose interactions are essential for regulatory control of CRH transcription, as reproduced from Nicholson et al., 2004 (46). The location of −248 C → T within this regulatory region is indicated. (B) Gel shift assay result, showing altered DNA-protein interactions with −248 T allele probes in experiments performed using nuclear extract from a hypothalamic cell line. Arrows to the right of the gel image indicate complexes that differ as a function of the −248 T allele.
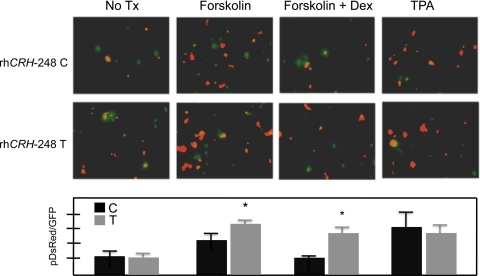
To test whether transcriptional activity was altered as a consequence of this SNP, reporter assays were performed in HT22 cells transfected with −248 C or T pDsRed constructs (Fig. 3). There were effects of treatment [F (3, 18) = 9.6, P = 0.0005] and a treatment by genotype interaction [F (3, 18) = 4.2, P = 0.02]. Reporter activity in forskolin and TPA-treated cells was significantly higher than that observed in untreated cells or in forskolin-stimulated cells treated with the Type II GR receptor agonist, dexamethasone (Tukey-Kramer, P < 0.05). Under basal conditions or in response to TPA, there were no effects of genotype. Following forskolin stimulation, cells expressing the T allele constructs exhibited higher reporter activity than did those expressing the C allele (Tukey-Kramer, P < 0.05) and, while dexamethasone treatment reversed forskolin-stimulated promoter activity in cells expressing the C allele constructs (Tukey-Kramer, P < 0.05), this was not true in those transfected with constructs expressing the T allele (n.s.). (No treatment: C, n = 4, T, n = 4; forskolin: C, n = 4, T, n = 4; forskolin + dex: C, n = 3, T, n = 3; TPA: C, n = 2, T, n = 2).
Fig. 3.
Functional consequences of the CRH −248 C → T SNP. Reporter assay result, comparing promoter activity of −248 C and T constructs at baseline and following treatment with forskolin, forskolin + dexamethasone, and TPA. Constructs were co-transfected with a GFP reporter, and an expression value was obtained by dividing intensity of pDsRed by that for GFP. GFP and dsRed images were merged by overlaying the GFP image with the dsRed image (both at 40% transparency). *, P < 0.05.
Stress Responsivity.
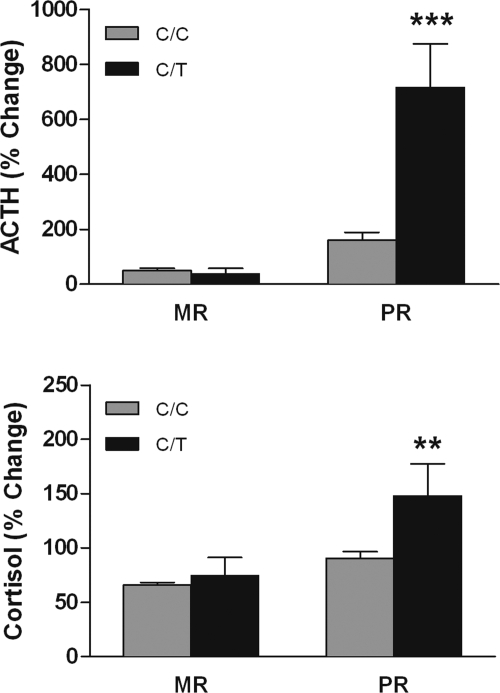
There were main effects of both rearing history and genotype on HPA axis responses to stress (Fig. 4). Infants carrying the −248 T allele exhibited higher stress-induced ACTH [F (1, 149) = 48.1, P < 0.0001, Tukey-Kramer, P < 0.05] and cortisol release [F (1, 140) = 8.5, P = 0.004, Tukey-Kramer, P < 0.05]. Peer reared infants also exhibited higher ACTH [F (1, 149) = 60.6, P < 0.0001, Tukey-Kramer, P < 0.05] and cortisol release [F (1, 140) = 18.8, P < 0.0001, Tukey-Kramer, P < 0.05] in response to stress. There was an interaction between genotype and rearing condition on ACTH [F (1, 149) = 52.15, P < 0.001] and cortisol [F (1, 140) = 4.6, P < 0.04] responses to social separation stress. Among subjects carrying the −248 T allele, those that also had been peer reared exhibited more marked increases in ACTH and cortisol responding to acute stress (Tukey-Kramer, P < 0.05). Genotype accounted for 14% of the variance in ACTH (34% in PR subjects) and 5% of the variance in cortisol (13% in PR subjects).
Fig. 4.
Interaction between rhCRH genotype (C/C vs. C/T) and early rearing history (MR, mother-reared, vs. PR, peer-reared) on ACTH and cortisol responses to stress. Peer-reared T allele carriers exhibited higher plasma levels of ACTH and cortisol (mean percent change + SEM, MR C/C = 100, MR C/T = 4, PR C/C = 42, PR C/T = 3). ***, P < 0.0001; **, P < 0.01.
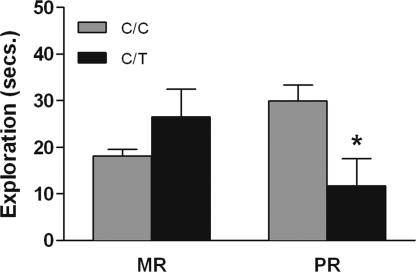
There was an interaction between rearing history and genotype on levels of environmental exploration during stress exposure [F (1, 206) = 3.99, P < 0.04, Fig. 5]. Peer-reared infants exhibited lower levels of environmental exploration if they were carriers of the T allele (Tukey-Kramer, P < 0.05). The same result was observed when the percent decrease in environmental exploration from baseline was calculated [F (1, 206) = 5.8, P < 0.02; Fig. S2]. Among peer-reared monkeys, CRH −248 C/T genotype accounted for 3% of the observed variance.
Fig. 5.
Interaction between rhCRH genotype (C/C vs. C/T) and early rearing history (MR, mother-reared, vs. PR, peer-reared) on levels of environmental exploration during periods of social separation stress. Peer-reared T allele carriers exhibited lower levels of environmental exploration during stress than did other groups of study (mean + SEM, MR C/C = 135, MR C/T = 7, PR C/C = 64, PR C/T = 4). *, P < 0.05.
Alcohol Consumption.
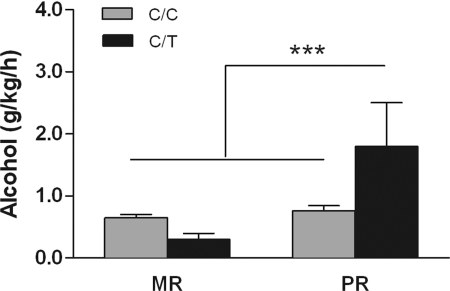
When animals were tested for individual differences in alcohol consumption, we observed an interaction between rearing condition and genotype [F (1, 181) = 11.059, P = 0.001, Fig. 6]. Peer-reared C/T subjects consumed higher levels of alcohol than the other groups of study (1.8 ± 0.7 vs. 0.65 ± 0.047 g/kg/h, Tukey-Kramer, P < 0.05). A significant effect remained after log-transformation of the data [F (1, 175) = 4.9, P = 0.03]. In PR monkeys, genotype accounted for 9% of the observed variance in alcohol consumption.
Fig. 6.
Interaction between rhCRH genotype (C/C vs. C/T) and rearing condition (MR, mother-reared, vs. PR, peer-reared) on levels of voluntary alcohol consumption. When given simultaneous access to alcohol (8.4% vol/vol) and sweetened vehicle in a limited access paradigm, PR animals with the C/T genotype consumed higher levels of alcohol (mean + SEM, MR C/C = 101, MR C/T = 5, PR C/C = 74, PR C/T = 5). ***, P < 0.001.
Discussion
Studies performed in rodents have shown that CRF system up-regulation, driven either by genetic variation or environmental factors, leads to escalated alcohol drinking and, as such, CRF1 antagonists are being developed for the treatment of alcohol dependence (21, 23). However, the relative levels of expression and distributions of key mediators of stress responses differ between rodents and catarrhine primates (36). Demonstrating a link between increased CRF system activity and individual differences in alcohol consumption in a primate species could provide critical support for the notion that rodent findings may translate to humans.
Using a multitiered approach, we have characterized the functional effects of a macaque SNP that is located in a region critical to regulating CRH expression. The functional importance of this region has been empirically verified in numerous in vitro and in vivo studies and is reiterated by the high degrees of intra- and inter-specific conservation. We showed that the T allele not only increases forskolin-stimulated CRH promoter activity, but also disrupts dexamethasone-mediated repression. These findings suggest that this SNP would augment CRH expression following stress.
Amygdaloid nuclei and the BNST drive anxious responding as well as HPA responses to key categories of stressors (37), while positive feedback of corticosteroids onto the amygdala and BNST drives CRH expression within these structures (7). This loop has been postulated as a major mechanism through which behavioral sensitization to stress occurs (7, 8), and it is for this reason that genetic variants that influence endocrine stress responses are of particular interest for gene x environment interactions (33). Although decreased corticosteroid sensitivity conferred by the T allele could theoretically diminish the impact of elevated glucocorticoids on CRH expression in brain regions in which glucocorticoids induce, rather than restrain, CRH expression (i.e., amygdala), the loss of corticosteroid-sensitivity would likely render the system inherently unstable, allowing even small differences in other factors impacting CRH expression to promote allostatic drift. Consistent with this, we find effects of −248 C → T on endocrine and behavioral stress reactivity and alcohol consumption in monkeys with a history of prior stress exposures. Together, our data support the hypothesis that variation within the CRH promoter increases stress responding and alcohol consumption in catarrhine primates, particularly among those with high cumulative stress exposures (i.e., peer-reared macaques).
The major limitation of the present study is that this SNP is rare (MAF <0.05). This is not surprising, given the functional consequences of this SNP and its location within a region under strong purifying selection. Of note, recent studies suggest that purifying selection at the CRH locus has been relaxed in humans (38). Instead, it appears that allelic mutations are being selected, possibly in response to different environmental demands (39, 40). The fact that there is a SNP in the corresponding human region (−201 C → T) suggests that CRH promoter variation could interact with environmental stressors to increase stress responding and alcohol consumption in humans, adding to the growing list of genetic variants that are being studied in macaques to model how genetic and environmental factors interact to increase risk for stress-related disorders.
In a prior report (40), we demonstrated the presence of alternative CRH haplotypes in rhesus macaques. On one of the major haplotypes, there was a SNP (−2232 C/G) that diminished sensitivity to low corticosteroid concentrations, which we predicted would tonically influence CRH expression under basal, non-stressed conditions. The current study reports the effects of a SNP that is present on a derived haplotype in the other major haplotype clade which confers functional effects that would drive increased phasic CRH expression, specifically in response to “stress.” Whereas the −2232 C → G SNP predicted low baseline CSF CRF, high baseline ACTH, bold behavior, and high risk drinking, we find that rhesus carrying the −248 T allele exhibit enhanced stress reactivity and consume higher levels of alcohol, but only in response to a stressful life history (peer rearing). We had previously suggested the −2232 G allele to be a good candidate for modeling risk for alcohol abuse or early onset alcoholism (driven by reward drinking). The human functional equivalent to −248 C → T would be predicted to impart risk for late onset alcoholism (driven by relief drinking), a subtype that is more common among stress-exposed or anxious individuals. This series of studies demonstrates how functional alleles at a single gene can potentially give rise to the same disorder through varied molecular mechanisms and via distinct or even opposing pathogenic pathways.
Alcohol and stress recruit a negative affective state through their influences on overlapping physiologic systems. We have shown that rhesus macaques with functional genetic variation in the CRH promoter exhibit heightened stress reactivity and increased alcohol consumption in response to early life stress exposure. Given the role of the placental CRF system in fetal development in primates, there may be a role for CRH promoter variants in conferring differential sensitivities to prenatal stress or alcohol exposure in humans (7, 41). It is also plausible that since the CRF system is involved in ethanol-induced neuroplasticity (25, 42), functional CRH variation could contribute to risk for transitioning from casual alcohol use to dependence and may be one factor that determines response to treatment with CRF1 antagonists.
Methods
Functional Characterization of −248 C → T.
Comparative genomic analyses were performed using the UCSC Genome Browser (http://genome.ucsc.edu/cgi-bin/hgGateway). We performed EMSA and in vitro reporter assays to determine functional consequences of the −248 T allele. The −248 C → T SNP is located in a region important to CRH transcriptional activity. We used an in vitro system to test whether CRH transcriptional activity was altered as a function the T allele following treatment with agents known to influence CRH promoter activity (TPA, forskolin, and dexamethasone). We used a mouse HT22 hippocampal cell line (43) and were able to demonstrate treatment effects on CRH promoter activity that are consistent with observations made in studies examining CRF expression levels in hypothalamic (IVB) and placental cell lines (Fig. S1). Full methods are available online (SI Text).
Rearing Conditions.
Mother-reared (MR) animals were reared in social groups composed of 8–14 females (about half of whom had same-aged infants) and two adult males. Peer-reared (PR) animals were separated from their mothers at birth and hand-reared in a neonatal nursery for the first 37 days of life. For the first 14 days, they were kept in an incubator and hand-fed. From day 15 until day 37, they were placed alone in a nursery cage and provided a blanket and a terrycloth-covered, rocking surrogate. A bottle from which the infants would feed was fixed to the surrogate. At 37 days of age, they were either placed in a cage with three other age-mates with whom they had continuous contact or were reared in a single cage, with daily periods (2 h) of playtime with age-matched infants. At approximately 8 months of age, animals (MR and PR) were placed into age-matched social groups and housed in large indoor-outdoor runs through late adolescence/adulthood (3.5–5 years), at which point the cohorts were divided into same-sex groups.
Social Separation Stress.
At 6–7 months of age, animals were subjected to four sequential, 4-day-long separations with 3 days of reunion in between (33). Two weeks before the first separation, a blood sample was obtained from the femoral vein under ketamine anesthesia (15 mg/kg, i.m.). During each separation, subjects were separated from their attachment sources (mother or peers). For peer-reared infants, each animal was placed alone into another cage in the nursery in which it could hear but not see or physically contact other animals. For mother-reared monkeys, the mother was removed from the social group. On day 1 of each of the four separations, blood samples were obtained under restraint both before and 1 h following removal from the group cage. All blood samples were obtained between 1,300 and 1,530 h, within 15 min of investigators' entrance into the housing facility for capture and sampling. Plasma ACTH and cortisol RIA used commercially available kits (ICN and DPC, respectively) and were performed according to the instructions of the manufacturers. Assays were performed in duplicate, and the inter- and intra-assay coefficients of variation were all less than 12%.
To examine rates of environmental exploration, focal behavioral scoring was performed in 5-min sessions. Infants were scored two times a week in the social group for the 2 weeks preceding the first mother-infant separation (baseline). During each separation week, levels of environmental exploration were determined by making behavioral observations immediately following separation and at hour 1 (two sessions) (35). Data were collected by multiple observers, and inter-observer reliability was greater than 85% (Pearson's correlation).
Alcohol Consumption.
Adolescent/young adult (age 3.5–5, n = 185) macaques were allowed to freely consume an aspartame-sweetened 8.4% (vol/vol) alcohol solution for 1 h per day, 5 days a week for 4 weeks in the home cage. This method consisted of three phases, which have previously been reported (32): (1) Spout Training; (2) Initial Alcohol Exposure; and (3) Experimental Period. During the experimental phase, alcohol and vehicle (both aspartame-sweetened) were dispensed 5 days a week (Monday–Friday) from 1,300 to 1,400 while the animals were in their home-cage environment. All procedures described were approved by the National Institutes on Alcohol Abuse and Alcoholism and National Institute of Child Health and Human Development Animal Care and Use Committees.
Genotyping for CRH −248 C → T.
DNA was isolated from whole blood using standard extraction methods. Genotyping was performed by the 5′ exonuclease method using fluorescent, allele-specific MGB probes (SI Text). Oligonucleotide primers (Forward: 5′-GGC CTT TCA TAG TAA GAG GTC AAT ATG T-3′; Reverse: 5′-CGC CTC TTG GTG ACG TCA A-3′) and probe sets (−248C, 6FAM-TCA TAA GAA GCC CTT CCA TT and −248T, VIC-GTC ATA AGA AGC TCT TCC ATT) were designed based on the rhesus macaque sequence. The overall error rate was 0.5%, and genotype completion rate was 93%.
Statistical Analyses.
We used a two-way ANOVA to examine the effects of CRH −248 C/T genotype (C/C vs. C/T) and rearing condition (PR vs. MR) on ACTH and cortisol levels responses to stress. Measures were averaged for each time point across the 4 weeks of testing. Because there were differences in baseline levels of ACTH and cortisol among the four groups, percent changes from baseline were calculated, and the percent change for ACTH and cortisol were used as dependent variables for our analyses. The standard in primate studies is to take samples under ketamine anesthesia, which is thought to put a brake on the HPA axis. As levels of ACTH and cortisol in the prestress sample were strongly correlated with those taken several weeks prior under ketamine anesthesia and because results did not differ when the changes in ACTH and cortisol were calculated relative to the sample taken under ketamine several weeks prior, the prestress samples were considered baseline measures.
To examine whether genotype and rearing interacted to influence stress-induced decreases in environmental exploration during social separation experiment, the levels of exploration scored during the 2 weeks before separation stress were averaged, and the exploration scores obtained during the first hour of social separation were averaged across the 4 weeks of the study performed during late infancy. Two-way ANOVAs were performed to assess effects of rearing condition and genotype on environmental exploration (both the levels and percent change) and on voluntary alcohol consumption.
The frequency of the T allele in the National Institutes of Health Animal Center was 3% (298 animals genotyped), and there were no T homozygotes, and an insufficient number of subjects for examining potential interactive effects with the previously reported −2232 C → G SNP. In some instances, ACTH or cortisol values were not obtained due to the difficulty of collecting samples from infants, sample degradation, or inadequate sample volume. In instances in which there was non-normality or non-homogeneity of variance, analyses were repeated on log-transformed data but yielded very similar results on both approaches.
Although this is an outbred colony of macaques, to verify that our effects were attributable to rhCRH variation, and not to general heritability of stress responsivity, we repeated our analyses using a set of six bi-allelic genetic markers used for genotyping in our colony (44, 45). There were no effects of the other markers tested on our phenotypes of interest, even after controlling for rearing condition, suggesting our results to be attributable to effects of rhCRH variation. We also excluded individuals carrying alleles known to predict our phenotypes of interest, but, as results were unchanged, these individuals were included in the final analyses. In the rare instances in which siblings were present, they were also removed from all analyses. Prior analyses indicated there to be two major ancestral rhCRH haplotype clades (H1 and H2). The −248 C → T SNP is on a derivation of the H1 haplotype, and the H2 haplotype appears to decrease stress reactivity (40). Because we were concerned that the individuals with the H2 haplotype, who would be predicted to be less stress reactive, would skew our current result, we repeated our analyses, excluding subjects carrying the H2 haplotype. As results did not differ when the H2 haplotype was excluded, the entire sample was included in the final analyses. Analyses were performed using Statistica (Statsoft). All in vivo analyses had an observed power → 0.80 at alpha = 0.05, except for the analysis of environmental exploration and cortisol where power was approximately 0.50. Where deviation from homogeneity of variance was detected, robustness of the analysis was confirmed by analysis of rank-transformed data. Because we examined the role of a rare minor allele, the analysis is inherently unbalanced. Effective hypothesis decomposition, a method for estimating sums of squares which is robust in unbalanced designs, was therefore used. Criterion for significance was set at P less than or equal to 0.05.
Supplementary Material
Acknowledgments.
We thank Karen Smith, MLS, for assistance in the preparation of this manuscript and the research and animal care staff at the Institutes on Alcohol Abuse and Alcoholism (NIAAA), National Institute of Child Health and Human Development (NICHD), and National Institutes of Health Animal Center for their assistance in data collection. This work was funded by National Alliance for Research on Schizophrenia and Depression and the NIAAA and NICHD intramural programs.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902863106/DCSupplemental.
References
- 1.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43:425–473. [PubMed] [Google Scholar]
- 2.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 3.Sapolsky RM. In: Behavioral Endocrinology. Becker J, Breedlove S, Crews D, McCarthy M, editors. Cambridge, MA: MIT Press; 2002. pp. 409–450. [Google Scholar]
- 4.Vale W, et al. Chemical and biological characterization of corticotropin releasing factor. Recent Prog Horm Res. 1983;39:245–270. doi: 10.1016/b978-0-12-571139-5.50010-0. [DOI] [PubMed] [Google Scholar]
- 5.Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: A role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 6.Merlo PE, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulkin J, Morgan MA, Rosen JB. A neuroendocrine mechanism for sustaining fear. Trends Neurosci. 2005;28:629–635. doi: 10.1016/j.tins.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 9.McEwen BS. Protective and damaging effects of stress mediators: Central role of the brain. Dialogues Clin Neurosci. 2006;8:367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sapolsky RM. In: Coping with the Environment: Neural and Endocrine Mechanisms. McEwen BS, Goodman HM, editors. New York: Oxford Univ Press; 2001. pp. 517–532. [Google Scholar]
- 11.de Kloet CS, et al. Elevated plasma corticotrophin-releasing hormone levels in veterans with posttraumatic stress disorder. Prog Brain Res. 2008;167:287–291. doi: 10.1016/S0079-6123(07)67025-3. [DOI] [PubMed] [Google Scholar]
- 12.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 13.Hundt W, Zimmermann U, Pottig M, Spring K, Holsboer F. The combined dexamethasone-suppression/CRH-stimulation test in alcoholics during and after acute withdrawal. Alcohol Clin Exp Res. 2001;25:687–691. [PubMed] [Google Scholar]
- 14.Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: Implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- 15.Jaferi A, Bhatnagar S. Corticotropin-releasing hormone receptors in the medial prefrontal cortex regulate hypothalamic-pituitary-adrenal activity and anxiety-related behavior regardless of prior stress experience. Brain Res. 2007;1186:212–223. doi: 10.1016/j.brainres.2007.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalin NH, Shelton SE, Davidson RJ. Cerebrospinal fluid corticotropin-releasing hormone levels are elevated in monkeys with patterns of brain activity associated with fearful temperament. Biol Psychiatry. 2000;47:579–585. doi: 10.1016/s0006-3223(99)00256-5. [DOI] [PubMed] [Google Scholar]
- 17.Servatius RJ, et al. A stress-induced anxious state in male rats: Corticotropin-releasing hormone induces persistent changes in associative learning and startle reactivity. Biol Psychiatry. 2005;57:865–872. doi: 10.1016/j.biopsych.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Strome EM, et al. Intracerebroventricular corticotropin-releasing factor increases limbic glucose metabolism and has social context-dependent behavioral effects in nonhuman primates. Proc Natl Acad Sci USA. 2002;99:15749–15754. doi: 10.1073/pnas.232480899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: Inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansson AC, et al. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci USA. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nie Z, et al. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- 23.Sommer WH, et al. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala Crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Egli M. Can experimental paradigms and animal models be used to discover clinically effective medications for alcoholism? Addict Biol. 2005;10:309–319. doi: 10.1080/13556210500314550. [DOI] [PubMed] [Google Scholar]
- 25.Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson RC, Seasholtz AF, Herbert E. Rat corticotropin-releasing hormone gene: Sequence and tissue-specific expression. Mol Endocrinol. 1987;1:363–370. doi: 10.1210/mend-1-5-363. [DOI] [PubMed] [Google Scholar]
- 27.Yao M, Schulkin J, Denver RJ. Evolutionarily conserved glucocorticoid regulation of corticotropin-releasing factor expression. Endocrinology. 2008;149:2352–2360. doi: 10.1210/en.2007-1551. [DOI] [PubMed] [Google Scholar]
- 28.King BR, Nicholson RC. Advances in understanding corticotrophin-releasing hormone gene expression. Front Biosci. 2007;12:581–590. doi: 10.2741/2084. [DOI] [PubMed] [Google Scholar]
- 29.Suomi SJ. In: Plasticity of Development. Brauth SE, Hall MS, Dooling RJ, editors. Cambridge: MIT Press; 1991. pp. 27–56. [Google Scholar]
- 30.Ayala AR, et al. Behavioral, adrenal, and sympathetic responses to long-term administration of an oral corticotropin-releasing hormone receptor antagonist in a primate stress paradigm. J Clin Endocrinol Metab. 2004;89:5729–5737. doi: 10.1210/jc.2003-032170. [DOI] [PubMed] [Google Scholar]
- 31.Habib KE, et al. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci USA 2000 May 23. 2000;97:6079–6084. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barr CS, et al. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry. 2004;61:1146–1152. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- 33.Barr CS, et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Newman TK, et al. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Spinelli S, et al. Association between the recombinant human serotonin transporter linked promoter region polymorphism and behavior in rhesus macaques during a separation paradigm. Dev Psychopathol. 2007;19:977–987. doi: 10.1017/S095457940700048X. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- 37.Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 38.Shimmin LC. Corticotropin releasing hormone (CRH) gene variation: Comprehensive resequencing for variant and molecular haplotype discovery in monosomic hybrid cell lines. DNA Seq. 2007;18:432–442. doi: 10.1080/10425170701388719. [DOI] [PubMed] [Google Scholar]
- 39.Baerwald CG, et al. Distribution of corticotropin-releasing hormone promoter polymorphism in different ethnic groups: Evidence for natural selection in human populations. Immunogenetics. 1999;49:894–899. doi: 10.1007/s002510050570. [DOI] [PubMed] [Google Scholar]
- 40.Barr CS, et al. CRH haplotype as a factor influencing cerebrospinal fluid levels of corticotropin-releasing hormone, hypothalamic-pituitary-adrenal axis activity, temperament, and alcohol consumption in Rhesus macaques. Arch Gen Psychiatry. 2008;65:934–944. doi: 10.1001/archpsyc.65.8.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vitoratos N, Papatheodorou DC, Kalantaridou SN, Mastorakos G. “Reproductive” corticotropin-releasing hormone. Ann N Y Acad Sci. 2006;1092:310–318. doi: 10.1196/annals.1365.029. [DOI] [PubMed] [Google Scholar]
- 42.Pastor R, et al. Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: A urocortin1-independent mechanism. Proc Natl Acad Sci USA. 2008;105:9070–9075. doi: 10.1073/pnas.0710181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maher P, Schubert D. Signaling by reactive oxygen species in the nervous system. Cell Mol Life Sci. 2000;57:1287–1305. doi: 10.1007/PL00000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barr CS, et al. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci USA. 2004;101:12358–12363. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barr CS, et al. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc Natl Acad Sci USA. 2008;105:5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicholson RC, King BR, Smith R. Complex regulatory interactions control CRH gene expression. Front Biosci. 2004;9:32–39. doi: 10.2741/1204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.