Abstract
Forty-five strains of ureolytic Escherichia coli of human origin, isolated in the United States between 1956 and 1977, were characterized by geographical distribution, site of infection, serotype, resistance to antibiotics, and biochemical reactions. All strains were studied for the ability to generate clones of nonureolytic E. coli (segregants), and a subset of these were selected for plasmid analysis and a variety of bacterial matings. There did not appear to be a common geographical distribution, serotype, antibiogram, or other aberrant biochemical reactions other than the hydrolysis of urea among these strains. The predominance of urinary tract isolates (46.7% total) may reflect a relationship between urea hydrolysis and pathogenesis at this site. Ten of the strains (22.2%) did segregate nonureolytic E. coli colonies, and all possessed at least one common plasmid species with a molecular weight of about 65 X 10(6). Only strain 1138-77 serotype O16:H6 conjugally transfered the ability to hydrolyze urea, ferment sucrose, and resist inhibition by sulfadiazide simultaneously. The resulting, recombination-deficient E. coli K-12 tranconjugant was found to possess a plasmid with a molecular weight of about 80 X 10(6) to 90 X 10(6).
Full text
PDF

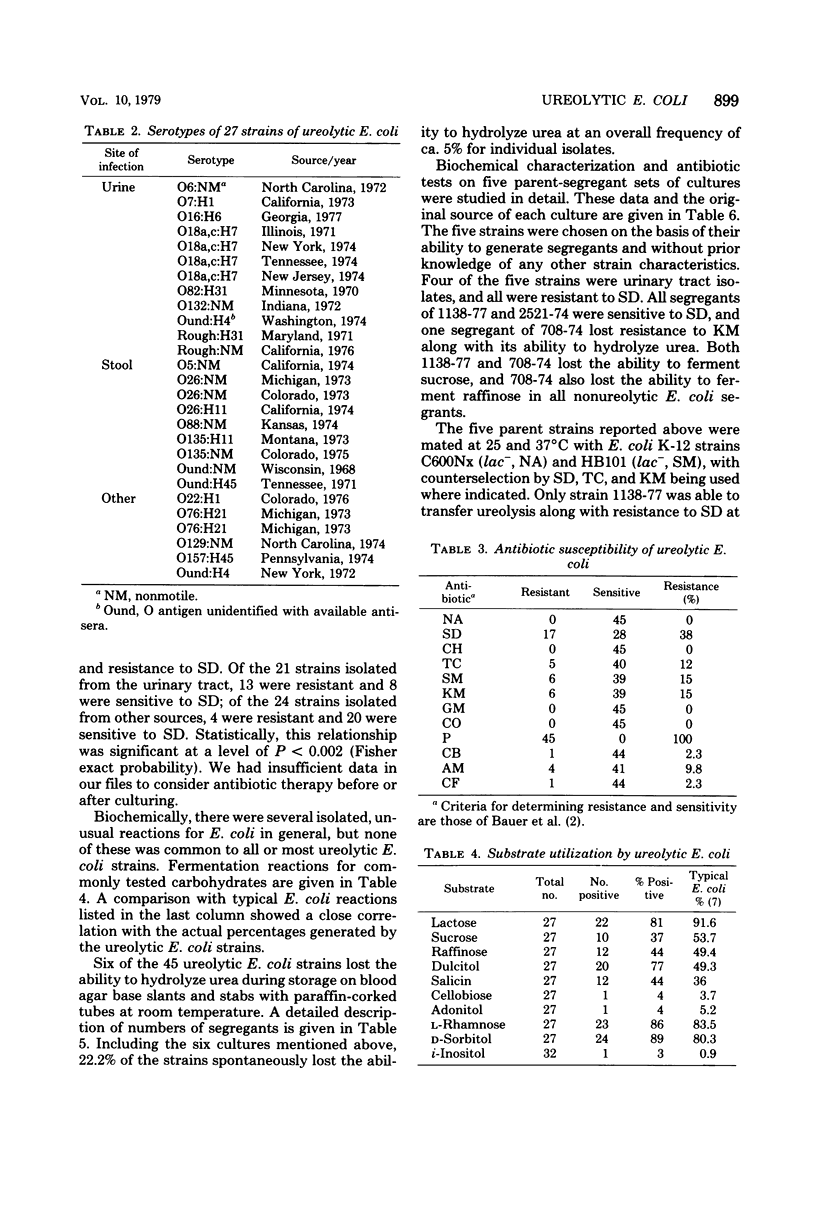
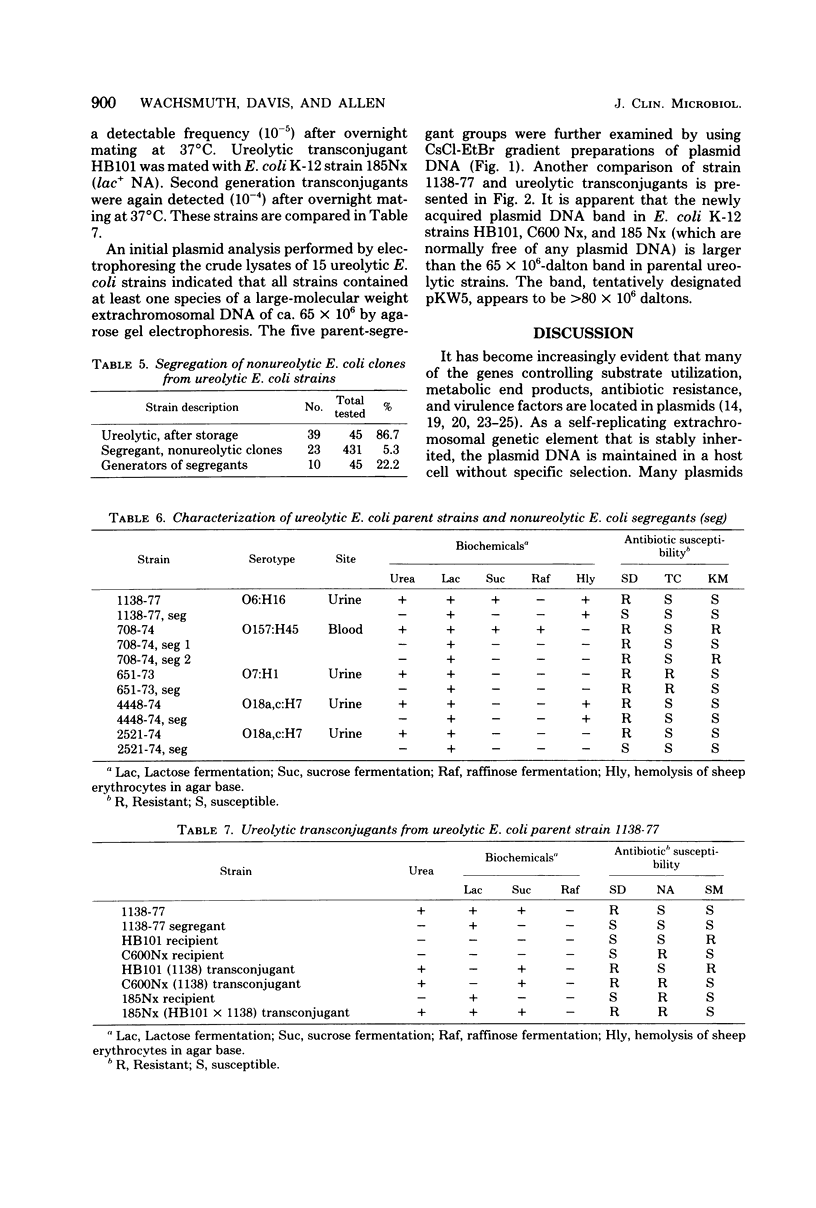

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson M., Medalia O., Griffel B. Prevention of ascending pyelonephritis in mice by urease inhibitors. Nephron. 1974;12(2):94–104. doi: 10.1159/000180271. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Cook A. R. The elimination of urease activity in Streptococcus faecium as evidence for plasmid-coded urease. J Gen Microbiol. 1976 Jan;92(1):49–58. doi: 10.1099/00221287-92-1-49. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Ghosal D., Saedler H. Tn951: a new transposon carrying a lactose operon. Mol Gen Genet. 1978 Apr 6;160(2):215–224. doi: 10.1007/BF00267484. [DOI] [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Moon H. W., Whipp S. C. Detection of heat-labile Escherichia coli enterotoxin with the use of adrenal cells in tissue culture. Science. 1974 Jan 25;183(4122):334–336. doi: 10.1126/science.183.4122.334. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Hickman F. W., Brenner D. J., Schreiber M., Rickenbach D. G. Unusual Enterobacteriaceae. "Proteus rettgeri" that "change" into Providencia stuartii. J Clin Microbiol. 1977 Oct;6(4):373–378. doi: 10.1128/jcm.6.4.373-378.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Campbell J. W. Inhibition of bacterial urease. Invest Urol. 1973 Nov;11(3):234–238. [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976 Mar;13(5):346–350. [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M. Prevention of infected urinary stones by urease inhibition. Invest Urol. 1973 Nov;11(3):228–233. [PubMed] [Google Scholar]
- Kopecko D. J., Brevet J., Cohen S. N. Involvement of multiple translocating DNA segments and recombinational hotspots in the structural evolution of bacterial plasmids. J Mol Biol. 1976 Dec;108(2):333–360. doi: 10.1016/s0022-2836(76)80124-6. [DOI] [PubMed] [Google Scholar]
- Layne P., Hu A. S., Balows A., Davis B. R. Extrachromosomal nature of hydrogen sulfide production in Escherichia coli. J Bacteriol. 1971 Jun;106(3):1029–1030. doi: 10.1128/jb.106.3.1029-1030.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesher R. J., Jones W. H. Urease production from clinical isolates of beta-hemolytic Escherichia coli. J Clin Microbiol. 1978 Sep;8(3):344–345. doi: 10.1128/jcm.8.3.344-345.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveen H. H., Falk G., Borek B., Diaz C., Lynfield Y., Wynkoop B. J., Mabunda G. A., Rubricius J. L., Christoudias G. C. Chemical acidification of wounds. An adjuvant to healing and the unfavorable action of alkalinity and ammonia. Ann Surg. 1973 Dec;178(6):745–753. doi: 10.1097/00000658-197312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclaren D. M. The influence of acetohydroxamic acid on experimental proteus pyelonephritis. Invest Urol. 1974 Sep;12(2):146–149. [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew B. H., Jorgensen J., Counts G. W., Falkow S. Association of hemolysin production, hemagglutination of human erythrocytes, and virulence for chicken embryos of extraintestinal Escherichia coli isolates. Infect Immun. 1978 Apr;20(1):50–54. doi: 10.1128/iai.20.1.50-54.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Highsmith A. K., Wachsmuth I. K. Resistance plasmid transfer by Serratia marcescens in urine. Antimicrob Agents Chemother. 1977 Mar;11(3):449–450. doi: 10.1128/aac.11.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenker S., Breen K. J., Hoyumpa A. M., Jr Hepatic encephalopathy: current status. Gastroenterology. 1974 Jan;66(1):121–151. [PubMed] [Google Scholar]
- Sellin M., Cooke D. I., Gillespie W. A., Sylvester D. G., Anderson J. D. Micrococcal urinary-tract infections in young women. Lancet. 1975 Sep 27;2(7935):570–572. doi: 10.1016/s0140-6736(75)90166-x. [DOI] [PubMed] [Google Scholar]
- Shipley P. L., Gyles C. L., Falkow S. Characterization of plasmids that encode for the K88 colonization antigen. Infect Immun. 1978 May;20(2):559–566. doi: 10.1128/iai.20.2.559-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Parsell Z. Transmissible substrate-utilizing ability in enterobacteria. J Gen Microbiol. 1975 Mar;87(1):129–140. doi: 10.1099/00221287-87-1-129. [DOI] [PubMed] [Google Scholar]
- Vuye A., Pijck J. Urease activity of enterobacteriaceae: which medium to choose. Appl Microbiol. 1973 Dec;26(6):850–854. doi: 10.1128/am.26.6.850-854.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsmuth I. K., Falkow S., Ryder R. W. Plasmid-mediated properties of a heat-stable enterotoxin-producing Escherichia coli associated with infantile diarrhea. Infect Immun. 1976 Aug;14(2):403–407. doi: 10.1128/iai.14.2.403-407.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Maker M. D. Unclassified, lactose-fermenting, urease-producing member of the family Enterobacteriaceae resembling Escherichia coli. J Clin Microbiol. 1975 Jul;2(1):70–71. doi: 10.1128/jcm.2.1.70-71.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]