Abstract
Primary intraocular lymphoma, recently suggested to be renamed primary retinal lymphoma, is a subset of primary central nervous system lymphoma and is usually an aggressive diffuse large B-cell lymphoma. Between 56% and 85% of patients who initially present with primary intraocular lymphoma alone will develop cerebral lesions. Patients typically complain of decreased vision and floaters, most likely secondary to the chronic vitritis and subretinal lesions. The diagnosis of primary intraocular lymphoma can be difficult to make and requires tissue for diagnosis. The atypical lymphoid cells are large and display a high nuclear to cytoplasmic ratio, prominent nucleoli, and basophilic cytoplasm. Flow cytometry, immunohistochemistry, cytokine analysis, and gene rearrangements also aid in the diagnosis. Local and systemic treatments, such as chemotherapy and radiation, are employed, although the relapse rate remains high.
Primary intraocular lymphomas (PIOLs) arise from the retina and rarely from the uvea. Those arising from the retina, which were recently suggested be renamed primary retinal lymphoma (PRL) and which are formally known as ocular reticulum cell sarcomas, comprise a subset of primary central nervous system lymphoma (PCNSL).1,2 It involves the retina, vitreous, and optic nerve head—with or without central nervous system (CNS) involvement. 1,3 Most PIOL are diffuse large B-cell lymphomas; rarely, these lymphomas are T cell in origin.4 Although rare, the incidence of PIOL has increased during the past 20 years in both immunocompetent and immunocompromised individuals alike.3,5 Ocular disease is bilateral in 80% of cases.1 Local and systemic treatments, such as chemotherapy and radiation, are employed, although the relapse rate remains high.3
CLINICAL FEATURES
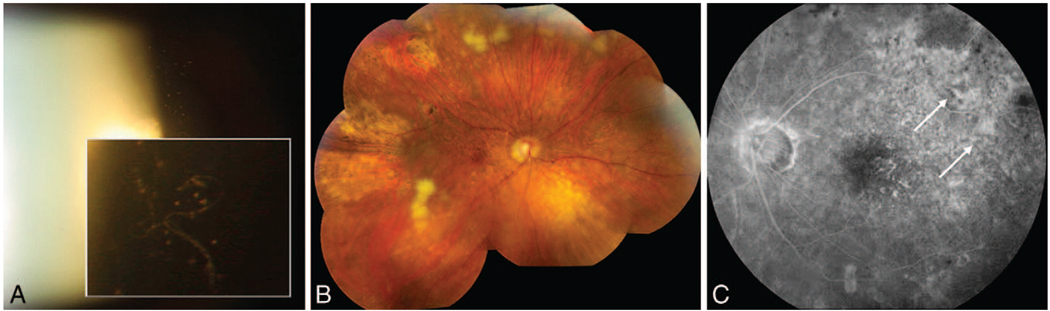
Primary intraocular lymphoma generally masquerades as a chronic intermediate uveitis that is unresponsive to corticosteroids in older individuals (median age in the 60s).2,3,5 Common symptoms are blurred vision and floaters and, less commonly, photophobia and ocular pain.3,4,6–9 Typical signs include clumps or sheets of cells in the vitreous (Figure 1, A), as the “inflammation” seen on clinical examination is secondary to the primary disorder (lymphoma cells) and to the reactive inflammatory cells in the vitreous.3,9–12
Figure 1.
Clinical presentations of primary retinal lymphoma. A, Slit lamp photography: sheets of vitreous cells seen in a patient later diagnosed with primary intraocular lymphoma (PIOL) via a diagnostic pars plana vitrectomy. B, Fundoscopy: active and inactive subretinal lesions throughout the fundus with areas of retinal necrosis in a patient diagnosed with PIOL. C, Fluorescein angiogram: areas of blockage (white arrows) of the retinal pigment epithelium and no signs consistent with inflammation in a patient with PIOL.
Multifocal, cream-colored, subretinal lesions can be seen in the fundus (Figure 1, B).3 A study of 17 patients with PIOL, at the National Eye Institute (Bethesda, Maryland), demonstrated that the most common fluorescent angiographic findings were granularity, late staining, and small foci of blockage at the level of the retinal pigment epithelium (RPE), without the typical signs of inflammation, such as perivascular staining or leakage or macular edema (Figure 1, C).3,8
TISSUE DIAGNOSIS
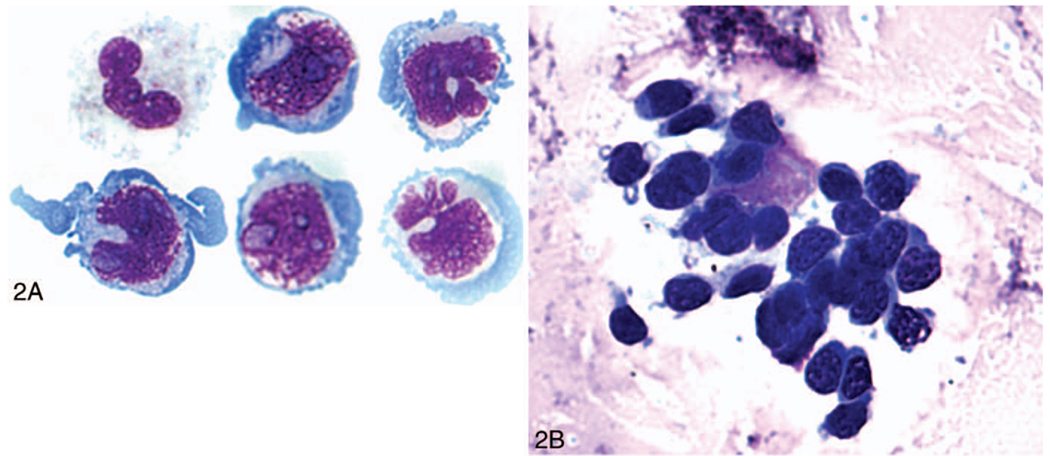
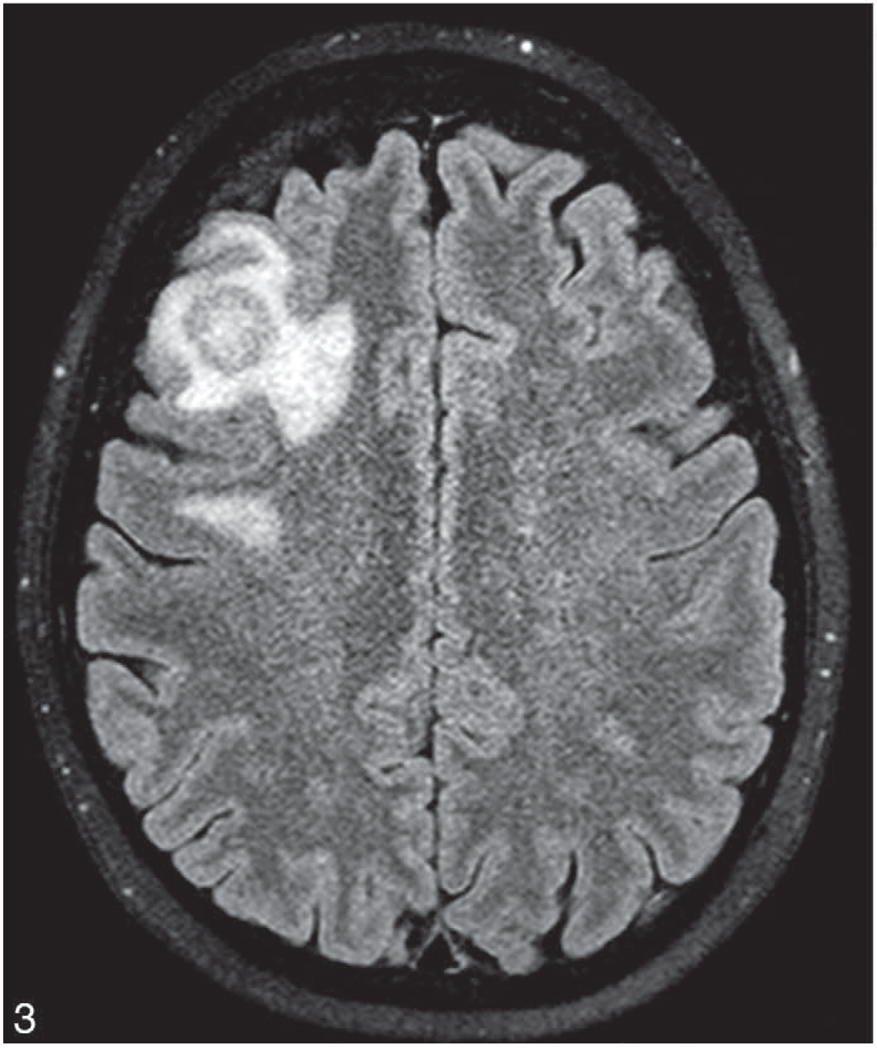
The diagnosis of PIOL is based on the identification of atypical lymphoid cells in the eye. However, the diagnosis can be made if the lymphoma cells are found in the cerebrospinal fluid (CSF) because PIOL is a subset of PCNSL (Figure 2, A). Indeed, it is possible to find CNS lesions through neuroimaging, such as with a computed-tomography scan or magnetic-resonance imaging (Figure 3). Recommendations from the National Eye Institute are to perform a lumbar puncture for CSF evaluation before a diagnostic vitrectomy or a vitreous or aqueous aspiration because it is less invasive.3,12 The processing and examination of CSF and vitreous are the same and include cytology, flow cytometry, cytokine, and molecular analyses.
Figure 2.
Cytology. A, Large and atypical lymphoid cells with large nuclei, prominent nucleoli, and basophilic cytoplasm found in the lumbar puncture specimen of a patient later diagnosed with primary intraocular lymphoma. B, Vitreous sample containing large lymphoid cells with large nuclei, prominent nucleoli, and basophilic cytoplasm (Giemsa, original magnifications ×1000 [A] and ×640 [B]).
Figure 3.
Brain magnetic resonance imaging. Axial T2 imaging showing a 2-cm, heterogeneous lesion with surrounding inflammation in a patient diagnosed with primary intraocular lymphoma via a lumbar puncture specimen.
Although PIOL cells are first located between the RPE and Bruch membrane, these malignant cells are usually found in the vitreous (Figure 2, B) on the first clinical presentation. A diagnostic vitrectomy, therefore, is the preferred sampling method, although analysis can be obtained via vitreous or aqueous aspiration, external chorioretinal biopsy, and transvitreal retinal, subretinal, or chorioretinal biopsy.6,12,13 However, chorioretinal biopsies are associated with more complications than vitrectomy.12 Mishandling of specimens and prior treatment with corticosteroids may lower the diagnostic yield.6 A study at the National Eye Institute of 12 patients demonstrated that 30% of cases of PIOL had a previous false-negative sampling. 3,6
The protocol for obtaining an adequate sample via a pars plana vitrectomy starts with the standard 3-port pars plana approach. Before onset, it is vital that the pathologist who will receive the specimen is aware of the diagnosis and when the specimen is to be delivered. Because the sample is processed through cytology, flow cytometry, cytokine, and molecular analysis, a complete core vitrectomy is recommended.12 Undiluted vitreous is first obtained for cytologic analysis. A dilute specimen is then obtained with the infusion on and cutting of the remaining vitreous. Vitreous wash fluid that has been collected in the cassette should also be used for microbiologic cultures. Because the cells degenerate rapidly, it is recommended that the vitreous specimen be placed in a specimen container with Roswell Park Memorial Institute (Buffalo, New York) culture medium and be taken immediately to an awaiting cytologist or ocular pathologist who will perform the appropriate steps to obtain the best yield.12 Collection and analysis of the supernatant may be used via enzyme-linked immunosorbent assay or polymerase chain reaction to rule out possible infectious etiologies, such as viruses or tuberculosus.14,15
Cytology
The atypical lymphoid cells are usually large and pleomorphic, with scant basophilic cytoplasm and large nuclei (Figure 2).3,5 Other findings include hypersegmented, round, or clover-shaped nuclei with prominent nucleoli and rare mitoses.3,5 Although these cells can be identified using the hematoxylin-eosin or Papanicolau test stain, the characteristics of the malignant B cells may be better revealed using either Giemsa or Diff-Quick (IMEB, SanMarcos, California) staining.12 The specimens may often be paucicellular and mixed with debris, reactive inflammatory cells (lymphocytes and macrophages), and necrotic lymphoma cells.3,12 The immunophenotype of monoclonality supports the cytologic diagnosis of lymphoma. Most PIOL are monoclonal B-cell lymphomas that stain positively for B-cell markers, such as CD19, CD20, and CD22 and show restricted expression of either κ or λ chain.1 Concomitant expression of BCL6 and MUM1 has also been reported in 5 patients with PIOL.3
Flow Cytometry
Although a cytologic diagnosis is still the gold standard, immunophenotyping obtained from flow cytometry can be very helpful in making the diagnosis. This technique has been used in detection of newly diagnosed, aggressive B-cell lymphoma at risk for CNS involvement.16 Flow cytometry can analyze several different markers simultaneously and has been used to confirm monoclonality in both B-cell and T-cell PIOLs.2
Cytokine Analysis
Inflammatory conditions are associated with high levels of the proinflammatory cytokine interleukin (IL) 6, whereas B-lymphoma cells secrete high levels of IL-10, a TH2-cytokine.17 B-cell PRL can exhibit high IL-10 levels and IL-10:IL-6 ratios greater than 1.0, which is suggestive of this malignacy.14 At the National Eye Institute, determination of the IL-10:IL-6 ratio in the vitreous of the suspected cases of PRL correctly identified 74.7% of the cases, with a sensitivity of 74.3% and a specificity of 75.0%.18 Interleukin-10 levels in the aqueous have also been found to be significantly elevated in patients with PIOL.13
Molecular Analysis
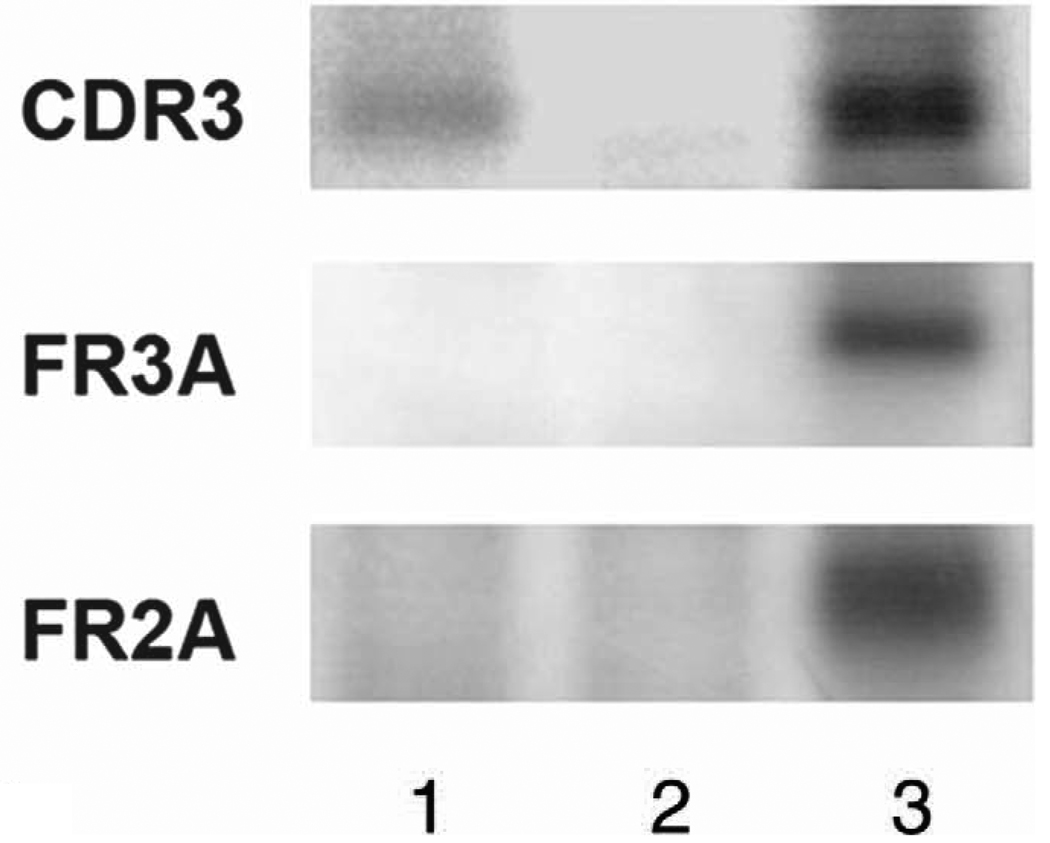
Molecular analysis of PIOL is a useful adjunct to diagnosing PIOL.19 Microdissection and polymerase chain reaction allow for selection of a relatively pure cell population from cytologic or histopathologic slides, which will improve the diagnosis for those samples that are composed of a few PIOL cells admixed with many reactive inflammatory cells.20 Similar to studies of systemic non-Hodgkin lymphomas, ocular specimens from patients with B-cell PIOL have revealed similar IGH rearrangements, particularly in the third complementarity-determining region (CDR3) of the IGH variable region (Figure 4).20 Monoclonality of B-cell populations can be detected using FR2, FR3, and/or CDR3 primers.21
Figure 4.
Gel electrophoresis of the polymerase chain reaction product of a patient with primary intraocular lymphoma (PIOL) showing IGH gene rearrangement in the complementarity-determining region 3, consistent with PIOL. Lane 1, patient with PIOL; lane 2, negative control; lane 3, positive control.
DIFFERENTIAL DIAGNOSIS
Because “vitritis” is one of the most common clinical presentations for PIOL, it is important to consider the different diagnoses that may present in a similar manner because treatment options will obviously differ. The differential diagnosis includes infectious, inflammatory, and other neoplastic processes.22 Infectious etiologies include endophthalmitis secondary to bacterial, viral, fungal, or parasitic infections.22 A vitreous sample is obtained and should be sent for bacterial or fungal cultures. Polymerase chain reaction analysis is used for the detection of microorganisms and viruses.14,15 Although vitreous samples can be used for the detection of parasitic infections, such as toxocariasis, clinical findings and serum antibody titers are used more often for a diagnosis.23
Inflammatory etiologies include uveitis-glaucoma-hyphema syndrome, sympathetic ophthalmia, sarcoidosis, pars planitis, multiple sclerosis, rheumatoid arthritis, birdshot chorioretinopathy, Behcet disease, Vogt-Koyanagi-Harada disease, sterile endophthalmitis, multifocal choroiditis, and acute posterior multifocal placoid pigment epitheliopathy.3 The presence of changes consistent with inflammation on fluorescein angiograms, such as vessel leakage, optic disc leakage, and cystoid macular edema, would go against a PIOL diagnosis.2,8 A vitreous, aqueous, or CSF sample would lack atypical lymphoid cells, but inflammatory cells, such as macrophages, lymphocytes and neutrophils, and the IL-10:IL-6 ratio would be less than 1.3,22
Other neoplastic processes may include metastasis of systemic non-Hodgkin lymphoma, which is usually located in the choroid (uvea).24 Patients with PIOL have vitreous, retinal, subretinal, or retinal-pigment epithelium involvement and may reveal areas of retinal necrosis. Patients with advanced systemic disease could likely present with overlapping signs of choroidal, retinal, and vitreal involvement.3
PATHOGENESIS
The exact lymphomagenesis is unknown. Several theories of development and mechanisms currently exist. One theory is that neoplastic transformation occurs in one of the lymphocyte subpopulations that may situate in the eye, such as the choroid, even though these locations are not the most common areas for PIOL to be found.9 Conversely, neoplastic transformations may occur in a population of systemic lymphocytes possessing receptors with a tropism for ocular ligands. Another theory pertains to immunologically privileged sites and their inherent susceptibility to allow cellular aberrancy to occur as compared with systemic sites with more immune surveillance. 1 Polyclonal inflammatory proliferation may then select for an aberrant, monoclonal, malignant cell population. At this time, there have been no markers of genetic susceptibility identified for PIOL.1
Chemokines with their receptors on lymphocytes are involved in both immune cell surveillance and accumulation. Studies looking at breast cancer, for example, have implicated chemokines in the processes of tumor growth, localization, and metastasis.25 Recent evidence suggests that chemokines may be involved in PCNSL and PIOL. At the National Eye Institute, examination of retinal tissues from 2 enucleated eyes, an eye found to be normal on autopsy, and a chorioretinal biopsy from 3 patients with PIOL found that RPE cells infiltrated by PIOL had a lower expression of CXCL12 and CXCL13 compared with those adjacent RPE cells not yet invaded.26 Thus, RPE cells that express CXCL12 and CXCL13 may serve as a guide for malignant PIOL cells.
Larocca et al27 have demonstrated that most PCNSLs seem to be derived from the germinal center as evidence by the frequent expression of BCL6 in non–acquired immune deficiency syndrome PCNSL. Coupland and coworkers28 showed, in 50 patients, that most had immunohistochemical evidence of a germinal center derivation. However, the exact derivation of the PIOL cells remains an enigma. Most likely, there is heterogeneity in PIOL.
In immunocompromise patients, PIOL has been associated with reactivation of latent Epstein-Barr virus.21 Epstein-Barr virus preferentially infects B cells and can lead to proliferation. Human herpes virus 8 has also been detected and associated with PRL, as well as with Toxoplasmosis gondii.21
TREATMENT AND PROGNOSIS
Treatment of PIOL is aimed at eradicating ocular lymphoma cells and at preventing spread to the CNS. The optimal treatment protocol of PRL is yet to be determined. Systemic and intrathecal therapies have been employed with and without the use of radiation and have achieved remission, although relapse is common.29 Conventional therapies, such as CHOP (cyclophosphamide, adriamycin, vincristine, and prednisone) and methotrexate, have been employed. High-dose methotrexate is used and can cause delayed neurotoxic side effects. Orbital radiation can lead to prolonged remission, but the adverse effects are many: radiation retinopathy, optic neuropathy, and dry eye; furthermore, radiation does not necessarily prolong survival.29 Rituximab, a monoclonal antibody against the CD20 antigen present on virtually all PCNSL tumors, has been employed both systemically and intravitreally.29,30 Intravitreal methotrexate has been used successfully in some patients to treat local recurrences, but disease relapse is common when therapy is discontinued.29 Hematopoietic stem-cell rescue has also been employed after intense chemotherapy for refractory disease.31
Recently, in a retrospective study of 83 human immunodeficiency virus–negative, immunocompetent patients with PRL was assembled from 16 centers in 7 countries.5 Median time to diagnosis was 6 months. Diagnosis was made by vitrectomy (n = 74; 89%), choroidal/retinal biopsy (n = 6; 7%), and ophthalmic exam (n = 3; 4%). A total of 11% of patients (9 of 83) had positive CSF cytology. Initial treatment was categorized as focal in 23 patients (28%; intraocular methotrexate, ocular radiotherapy) or extensive in 53 patients (64%; systemic chemotherapy, whole brain radiotherapy); 6 (7%) received no therapy, and details are unknown in 1 case (1%). Forty-seven patients (57%) relapsed at the following sites: brain 47% (n = 22), eyes 30% (n = 14), brain and eyes 15% (n = 7), and systemic 8% (n = 4). The median time to relapse was 19 months. Focal therapy alone did not increase the risk of brain relapse. Median progression-free and overall survival were 29.6 and 58 months, respectively, and were unaffected by treatment type.5
At the National Eye Institute in collaboration with the National Cancer Institute, an immunotoxin was developed and employed in the murine PIOL model.32 HA22 is a hybrid protein consisting of Pseudomonas exotoxin A covalently linked to an anti-CD22 monoclonal antibody. This immunotoxin interacts with the CD22 molecule on the surface of lymphoma cells, leading to internalization of the exotoxin and cell death.32 The ability to eradicate PIOL in the murine model is an intriguing finding that may lead to future human treatments.
As PIOL is associated with a poor prognosis, with most patients dying of CNS disease, a definitive diagnosis is important for appropriate treatment. To decrease intraocular surgery and minimize intraocular surgical complications, we recommend CNS evaluation, including brain scan and CSF cytology, before examining ocular fluids and tissues. Primary retinal lymphoma can be a difficult diagnosis to make and may be easily missed. If suspected, all possible diagnostic techniques should be exhausted to fully rule out its presence.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Chan CC, Gonzalez JA. Hackensack, NJ: World Scientific Publishing Co Pte Ltd; Primary Intraocular Lymphoma. 2007
- 2.Coupland SE, Damato B. Understanding intraocular lymphoma. Clin Experiment Ophthalmol. 2008;36(6):564–578. doi: 10.1111/j.1442-9071.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- 3.Chan CC, Wallace DJ. Intraocular lymphoma: update on diagnosis and management. Cancer Control. 2004;11(5):285–295. doi: 10.1177/107327480401100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman PM, McKelvie P, Hall AJ, et al. Intraocular lymphoma: a series of 14 patients with clinicopathological features and treatment outcomes. Eye. 2003;17:513–521. doi: 10.1038/sj.eye.6700378. [DOI] [PubMed] [Google Scholar]
- 5.Grimm SA, Pulido JS, Jahnke K, et al. Primary intraocular lymphoma: an international primary central nervous system lymphoma collaborative group report. Ann Oncol. 2007;18(11):1851–1855. doi: 10.1093/annonc/mdm340. [DOI] [PubMed] [Google Scholar]
- 6.Whitcup SM, de Smet MD, Rubin BI, et al. Intraocular lymphoma: clinical and histopathologic diagnosis. Ophthalmology. 1993;100(9):1399–1406. doi: 10.1016/s0161-6420(93)31469-7. [DOI] [PubMed] [Google Scholar]
- 7.Read RW, Zamir E, Rao NA. Neoplastic masquerade syndromes. Surv Ophthalmol. 2002;47(2):81–124. doi: 10.1016/s0039-6257(01)00305-8. [DOI] [PubMed] [Google Scholar]
- 8.Velez G, Chan CC, Csaky KG. Fluorescein angiographic findings in primary intraocular lymphoma. Retina. 2002;22(1):37–43. doi: 10.1097/00006982-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Chan CC, Buggage RR, Nussenblatt RB. Intraocular lymphoma. Curr Opin Ophthalmol. 2002;13(6):411–418. doi: 10.1097/00055735-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Akpek EK, Ahmed I, Hochberg FH, et al. Intraocular-central nervous system lymphoma: clinical features, diagnosis, and outcomes. Ophthalmology. 1999;106(9):1805–1810. doi: 10.1016/S0161-6420(99)90341-X. [DOI] [PubMed] [Google Scholar]
- 11.Nussenblatt RB, Whitcup SM, Palestine AG. Uveitis: Fundamentals and Clinical Practice. 2nd ed. St. Louis, MO: Mosby; 1996. [Google Scholar]
- 12.Gonzales JA, Chan CC. Biopsy techniques and yields in diagnosing primary intraocular lymphoma. Int Ophthalmol. 2007;27(4):241–250. doi: 10.1007/s10792-007-9065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassoux N, Giron A, Bodaghi B, et al. IL-10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2007;48(7):3253–3259. doi: 10.1167/iovs.06-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dworkin LL, Gibler TN, Van Gelder RN. Real-time quantitative polymerase chain reaction diagnosis of infectious posterior uveitis. Arch Ophthalmol. 2002;120(11):1534–1539. doi: 10.1001/archopht.120.11.1534. [DOI] [PubMed] [Google Scholar]
- 15.Gupta V, Gupta A, Rao NA. Intraocular tuberculosis—an update. Surv Ophthalmol. 2007;52(6):561–587. doi: 10.1016/j.survophthal.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Hegde U, Filie A, Little RF, et al. High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: the role of flow cytometry versus cytology. Blood. 2005;105(2):496–502. doi: 10.1182/blood-2004-05-1982. [DOI] [PubMed] [Google Scholar]
- 17.Benjamin D, Park CD, Sharma V. Human B cell interleukin 10. Leuk Lymphoma. 1994;12(3–4):205–210. doi: 10.3109/10428199409059591. [DOI] [PubMed] [Google Scholar]
- 18.Wolf LA, Reed GF, Buggage RR, et al. Vitreous cytokine levels. Ophthalmology. 2003;110(8):1671–1672. doi: 10.1016/S0161-6420(03)00811-X. [DOI] [PubMed] [Google Scholar]
- 19.White VA, Gascoyne RD, Paton KE. Use of the polymerase chain reaction to detect B- and T-cell gene rearrangements in vitreous specimens from patients with intraocular lymphoma. Arch Ophthalmol. 1999;117(6):761–765. doi: 10.1001/archopht.117.6.761. [DOI] [PubMed] [Google Scholar]
- 20.Shen DF, Zhuang Z, LeHoang P, et al. Utility of microdissection and polymerase chain reaction for the detection of immunoglobulin gene rearrangement and translocation in primary intraocular lymphoma. Ophthalmology. 1998;105(9):1664–1669. doi: 10.1016/S0161-6420(98)99036-4. [DOI] [PubMed] [Google Scholar]
- 21.Chan CC. Molecular pathology of primary intraocular lymphoma. Trans Am Ophthalmol Soc. 2003;101(0):275–292. [PMC free article] [PubMed] [Google Scholar]
- 22.Wittenberg LA, Maberley DA, Ma PE, et al. Contribution of vitreous cytology to final clinical diagnosis fifteen-year review of vitreous cytology specimens from one institution. Ophthalmology. 2008;115(11):1944–1950. doi: 10.1016/j.ophtha.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 23.Good B, Holland CV, Taylor MR, et al. Ocular toxocariasis in schoolchildren. Clin Infect Dis. 2004;39(2):173–178. doi: 10.1086/421492. [DOI] [PubMed] [Google Scholar]
- 24.Parikh AH, Khan SH, Wright JD, Jr, Oh KT. Systemic non-Hodgkin’s lymphoma simulating primary intraocular lymphoma. Am J Ophthalmol. 2005;139(3):573–574. doi: 10.1016/j.ajo.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 25.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 26.Chan CC, Shen D, Hackett JJ, et al. Expression of chemokine receptor, CXCR4 and CXCR5, and chemokines, BLC and SDF-1, in the eyes of patients with primary intraocular lymphoma. Ophthalmology. 2003;110(2):421–426. doi: 10.1016/S0161-6420(02)01737-2. [DOI] [PubMed] [Google Scholar]
- 27.Larocca LM, Capello D, Rinelli A, et al. The molecular and phenotypic profile of primary central nervous system lymphoma identifies distinct categories of the disease and is consistent with histogenetic derivation from germinal center-related B cells. Blood. 1998;92(3):1011–1019. [PubMed] [Google Scholar]
- 28.Coupland SE, Loddenkemper C, Smith JR, et al. Expression of immunoglobulin transcription factors in primary intraocular lymphoma and primary central nervous system lymphoma. Invest Ophthalmol Vis Sci. 46(11):3957–3964. doi: 10.1167/iovs.05-0318. [DOI] [PubMed] [Google Scholar]
- 29.Sou R, Ohguro N, Maeda T, Saishin Y, Tano Y. Treatment of primary intraocular lymphoma with intravitreal methotrexate. Jpn J Ophthalmol. 2008;52(3):167–174. doi: 10.1007/s10384-008-0519-9. [DOI] [PubMed] [Google Scholar]
- 30.Ohguro N, Hashida N, Tano Y. Effect of intravitreous rituximab injections in patients with recurrent ocular lesions associated with central nervous system lymphoma. Arch Ophthalmol. 2008;126(7):1002–1003. doi: 10.1001/archopht.126.7.1002. [DOI] [PubMed] [Google Scholar]
- 31.Soussain C, Suzan F, Hoang-Xuan K, et al. Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. J Clin Oncol. 2001;19(3):742–749. doi: 10.1200/JCO.2001.19.3.742. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Mahesh SP, Shen D, et al. Eradication of tumor colonization and invasion by a B cell specific immunotoxin in a murine model for human primary intraocular lymphoma. Cancer Res. 2006;66(21):10586–10593. doi: 10.1158/0008-5472.CAN-06-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]