Abstract
Aims: The present study sought to investigate the relationship between the HPA axis reactivity to stress, the endogenous opioid system and stress-induced drinking behavior. Methods: In the present study, 74 non-treatment-seeking alcohol-dependent subjects were tested under two mood conditions, neutral and stress, in separate testing sessions. Salivary cortisol measurements were obtained following stress induction and during the neutral control condition. Multiple measurements of alcohol intake, latency to access the alcohol cue and craving for alcohol were obtained during cue-availability testing. In addition, 52 of the study subjects were genotyped for the μ-opioid receptor. Results: A blunted cortisol response to stress was significantly correlated with increased alcohol intake following stress exposure compared to alcohol intake during the neutral session. There was not a clear correlation between the change in cortisol in response to stress and the change in latency to access alcohol or alcohol craving in response to stress. Carriers of the Asp40 variant of the μ-opioid receptor exhibited a dampened cortisol response to stress, higher alcohol intake and greater craving in response to stress compared to Asn40 homozygotes, although these differences were not statistically significant. Conclusions: The results of the present study indicate that a blunted biological stress response was correlated with increased drinking in response to stress. The Asp40 variant of the μ-opioid receptor may be associated with this HPA axis hyporeactivity although the small sample size used in the present study did not permit adequate evaluation of this association.
INTRODUCTION
Stress relief is thought to be a major motivation for excessive alcohol consumption. The physiologic mechanism of stress relief following alcohol consumption is thought to occur at least in part via the body's main stress-response pathway, the hypothalamic pituitary (HPA) axis. Acute alcohol administration has been shown to enhance levels of HPA axis hormones in humans and animal models (Rivier et al., 1990; Piazza and Le Moal, 1996, 1997; Culpepper-Morgan and Kreek, 1997; Koob and Le Moal, 1997). As dependence on alcohol develops, HPA axis activity appears to become dysregulated, and over time, chronic exposure to alcohol may actually decrease the responsiveness of the HPA axis to external stimuli (Dave et al., 1986; Rivier et al., 1990; Inder et al., 1995; Le et al., 2000; Rasmussen et al., 2000; Zorrilla et al., 2001). Moreover, self-reported alcohol craving has been shown to be inversely correlated with cortisol levels in alcohol-dependent individuals (O’Malley et al., 2002), suggesting that decreased HPA axis activity in this population may be associated with heightened alcohol craving. Studies show that chronic alcohol consumption may be associated with decreased responsiveness of the HPA axis to external stressors (Adinoff et al., 1990; Errico et al., 1993; Costa et al., 1996; Lovallo et al., 2000; Errico et al., 2002; Sorocco et al., 2006; Dai et al., 2007), and a greater risk of relapse has been associated with a blunted cortisol response to both alcohol cues and psychological stress (Junghanns et al., 2003, 2005). Individuals who experience an attenuated HPA response to stress may also experience a blunted HPA response to alcohol and may therefore consume excessive amounts of alcohol to increase cortisol secretion and achieve the desired biological response.
The blunted physiologic stress response observed in alcoholics may be due to irregular activity of stimulatory and inhibitory neurotransmitter circuits that impact the HPA axis, such as the endogenous opioid system (Gianoulakis, 1998). Importantly, there is considerable evidence linking the endogenous opioid system to the development and maintenance of alcoholism (for review, see Gianoulakis, 2004). Evidence from previous research indicates that there may be an inherited, genetic basis for the altered HPA axis and endogenous opioid system functioning observed in some alcoholics. Abnormal HPA activity has been observed in non-dependent persons with a positive family history of alcoholism (Schuckit et al., 1987; Wand and Dobs, 1991; Waltman et al., 1994; Costa et al., 1996). One recent study found that β-endorphin release from the pituitary following stress was increased to a greater degree in individuals with no family history of alcoholism than in individuals with a positive family history (Dai et al., 2005). Wand and colleagues (1998, 1999a, 1999b) have demonstrated that family history of alcoholism may also influence the cortisol response to opioid receptor blockade. The fact that the altered HPA axis activity in response to opioid receptor blockade is similar in non-dependent family history positive individuals and alcohol-dependent persons suggests that these differences represent an inborn, inherited variation in the normal stress response involving the endogenous opioid system.
The A118G (Asn40Asp) variant of the μ-opioid receptor is a potential mediator for the altered HPA axis activity observed in alcoholics. The A118G polymorphism, which produces the Asp40 variant of the μ-opioid receptor, binds β-endorphin three times more strongly than the Asn40 allele (Bond et al., 1998). It has been hypothesized that individuals carrying the Asn40 allele may exhibit altered drinking behavior that is associated with this heightened interaction between β-endorphins and μ-opioid receptors. Results of the two treatment studies indicate that alcoholic subjects with at least one copy of the variant allele who were treated with naltrexone experienced improved clinical outcomes compared to those treated with placebo and those who did not carry the variant allele (Oslin et al., 2003; Anton et al., 2008). However, a separate study examining the effects of naltrexone found no significant difference in drinking behavior between carriers and non-carriers of the variant allele (Tidey et al., 2008), and in a laboratory study, McGeary et al. (2006) found that naltrexone increased alcohol craving in response to alcohol cues in carriers of the variant allele. Thus, the relationship between the A118G polymorphism of the μ-opioid receptor and a potential benefit of naltrexone treatment remains unclear.
Interestingly, the A118G variant may also be associated with an abnormal stress response. Individuals with at least one copy of the A118G allele demonstrated heightened ACTH and cortisol responses to naloxone than those who were not carriers of the allele (Wand et al., 2002; Hernandez-Avila et al., 2003). Individuals with the A118G polymorphism also exhibit a blunted cortisol response to psychological stress (Chong et al., 2006). This evidence suggests that the higher affinity binding observed for the receptor variant confers higher inhibitory opioidergic tone directed at CRH-producing cells leading to increased HPA axis response to opioid receptor blockade but decreased response to stress.
The present study seeks to test the hypothesis that a blunted HPA axis response to stress may represent increased vulnerability to stress-induced drinking and relapse, and that this increased vulnerability may be mediated by the A118G polymorphism in the μ-opioid receptor.
METHODS
All recruitment, screening and testing procedures used in this study adhered to the guidelines set forth by the National Advisory Council of the NIAAA for administering alcohol to humans (NIAAA, 2005), specifically those which address administering alcohol to alcoholics. All procedures were also approved by the Institutional Review Board at Indiana University and met requirements for informed consent and confidentiality. Power and sample size calculations were estimated using preliminary data for this study taken from 19 pilot subjects.
Subjects
Non-treatment-seeking alcoholics whose preferred beverage was beer were recruited through newspaper advertisements. Prospective subjects underwent initial screening over the phone, and eligible subjects were scheduled to undergo additional screening in the laboratory. Subjects were tested individually between 1:00 and 7:00 pm. They were instructed to abstain from alcohol starting at midnight on the day of testing to ensure a BAC of zero upon presentation at the laboratory. They were also asked to refrain from consuming food or caffeinated beverages after 9:00 am on the day of testing so that all subjects presented in approximately the same prandial state. Upon arrival at the laboratory, subjects provided informed consent and were given a breathalyzer test using an Alco-Sensor IV Intoximeter (St. Louis, MO, USA). Qualifying subjects were served a standardized light snack prior to starting the experiment, followed by a restroom break and, for smokers, a cigarette break. Subjects were encouraged to arrive at their sessions via public transportation or to have a friend transport them, and they were not released from the study until their BAC fell to 0.00 g/dL. Following the second testing session, each subject received information concerning the potential risks of their high level of consumption. Referrals for treatment were available at the subject's request; however, none of the subjects requested this information. Subjects were paid $50 after each test session was completed.
Inclusion criteria
Study subjects were non-treatment-seeking alcohol-dependent men and women between the ages of 21 and 70. Alcohol dependence was determined by conducting the Structured Clinical Interview for DSM-IV (First et al., 1996). None of the subjects had received treatment for their alcohol problems within the past year, nor were they interested in receiving treatment.
Exclusion criteria
Potential subjects were excluded if they had any medical condition that would have precluded them from consuming alcohol, if they had recently used any drug other than alcohol, nicotine or caffeine as determined by a urine drug screen, or if they scored ≥8 on the Clinical Institute Withdrawal Assessment for Alcohol Scale (CIWA, Sullivan et al., 1989), indicating they were at risk for significant withdrawal symptoms. Other exclusionary criteria included current depression, use within the past month of any mood-altering drug that might affect the study's outcome (e.g. anti-depressants), being under court order not to consume alcohol, inability to be reached by phone or inability to understand the study questionnaires and procedures in English. Female subjects took a urine pregnancy test and pregnant or breast-feeding women were also excluded.
Of the 377 potential participants who were screened over the phone, 80 qualified to participate in the study and attended at least one testing session. Six subjects did not return for their second session and their data were omitted from all analyses. The data from these subjects were also excluded from analyses. Of the 74 subjects who completed both study sessions, 22 were not genotyped for the μ-opioid receptor gene (OPRM1) as they were recruited and underwent laboratory testing prior to the addition of the genotyping component of the study. Therefore, data from 74 total subjects were used to analyze the correlations between changes in cortisol and drinking behavior, and data from 52 total subjects were used to analyze the relationships between OPRM1 genotype and drinking behavior. Characteristics for all 74 subjects who were tested are summarized in Table 1.
Table 1.
Subject demographics
| Age | 36.6 ± 9.2 |
| Sex | 52 M, 22 F |
| % Minority | 60.8 |
| % Unmarried | 91.9 |
| % Employed | 33.8 |
| % Smokers | 78.4 |
| % FH+ | 92 |
| Education (years) | 12.6 ± 1.4 |
| % DA | 16.8 ± 13.5 |
| D/DD | 9.8 ± 4.6 |
| % HDD | 68.6 ± 21.1 |
| ADS score | 12.7 ± 6.9 |
Mean values given with standard deviations.
% FH+ = percent with positive family history of alcoholism; % DA = percent days abstinent; D/DD = mean drinks per drinking days; % HDD = percent heavy drinking days (≥4 drinks for women, ≥5 drinks for men).
Intake measures
The Alcohol Dependence Scale (ADS; Skinner and Horn, 1984) was used to quantify the level of dependence in alcohol-dependent subjects. The Time Line Follow Back Interview (TLFB) (Sobell and Sobell, 1996) was used to assess alcohol consumption for the preceding 30 days. The Family Tree Questionnaire (Mann et al., 1985) was used to determine the inherited risk for alcoholism in each individual. The Fagerstrom Test for Nicotine Dependence (Heatherton et al., 1991) was used to collect smoking history and degree of dependence.
Stress induction
The Paced Auditory Serial Addition Test (PASAT; Gronwall, 1977) was used to induce stress in the subjects. Researchers have found that brain injury patients as well as healthy individuals report a negative affective response to the PASAT procedures (Roman et al., 1991; Spreen and Strauss, 1991; Lezak, 1995). Holdwick and Wingenfeld (1999) demonstrated the ability of the PASAT to significantly elevate subjective stress levels and evoke associated negative feelings. The PASAT has since been used in the laboratory as a psychological stressor by researchers investigating the effects of mood on behavior (Feldner et al., 2006; Vanderkaay and Patterson, 2006).
Subjects were randomly assigned to the order of the stress condition. They were told that the purpose of the study was to investigate the cognitive abilities of individuals who consume alcohol on a regular basis. The stress induction procedure took ∼15 min. Subjects were tested at two different stimulus presentation rates and were given an unpaced practice trial. The subjects’ performance was not scored and no feedback was provided, but the experimenter gave the impression of scoring and taking notes on the subject's performance in order to increase the subject's anxiety. The neutral control condition consisted of having the subjects sit quietly for the same length of time as the PASAT task.
Salivary cortisol concentration measurement
Saliva samples were collected 5 min after the stress and neutral mood induction procedures to determine salivary cortisol concentrations and assess the ability of the stress manipulation to elicit a biological response through the HPA axis. The measurement taken after the neutral mood induction served as a within-subjects control. Sample collection and assay procedures are based on those published by Klimes-Dougan and colleagues (2001). Assays were performed using salivary cortisol EIA kits (Research Kit, 10-67100, Diagnostic Systems Laboratories, Webster, TX, USA). This kit has demonstrated excellent accuracy in determining salivary cortisol levels in other studies (Bodani and Edwards, 1999; Raff et al., 2002).
Cue-availability procedure
Following the stress induction or neutral mood procedures, drinking behavior was assessed using the cue-availability paradigm. A computer monitor guided the subjects through the trials by displaying instructions and study questions. All subjects completed eight practice trials with a water cue. Subjects were tested individually and were exposed to 40 trials at each of two sessions completed on separate days. Each subject's preferred beer of the three ‘light’ brands available served as the alcohol cue and was poured into a pilsner beer glass. A sliding glass window that could be locked provided access to the cue box. When the cue was available for consumption (window unlocked), the subjects were told that they could take a drink of the beverage. Tones then prompted the subject to look at the monitor and respond, using the keypad, to the craving questions that were presented in random order.
The craving scale consisted of four items derived from the analyses of the 47-item Alcohol Craving Questionnaire (Singleton et al., 1995). The four items that were selected correlate most highly with the overall score. The items were (1) I want to use alcohol right now; (2) I have an urge to drink right now; (3) It would be great to use alcohol right now; (4) Nothing would be better than drinking right now. Reliability estimates for this item set indicate an internal consistency of 0.89. All questions were answered on a scale of 1–7 representing the degree to which the subject agreed with the given statement. The questions were presented at baseline and intermittently during the cue-availability procedure for a total of nine assessments. Motivation to drink alcohol was defined as the latency in milliseconds to attempt to open the window of the cue box to access the cue. Latency was measured on all trials. Alcohol intake was measured on all trials when the alcohol cue was available for consumption. Measurements were obtained by weighing the serving glass before and after each trial that the alcohol cue is available for consumption. Measurements were performed outside of the view of the subjects using a Navigator scale by OHAUS (model no. N1 B110).
Genotyping the OPRM1 at Asn40Asp (A118G); AAC to GAC
Following participation in the second testing session, a finger stick was performed on the subjects to obtain a spot of blood on filter paper. This blood spot was used as a DNA sample for PCR-based genotyping. Genotyping was performed by researchers at the Alcohol Research Center at the Indiana University School of Medicine. The ‘Generation Kit’ from Gentra Systems (Minneapolis, MN, USA) was used for DNA isolation. Genotyping was performed using a custom designed TaqMan SNP Genotyping Assay obtained from Applied Biosystems (Foster City, CA, USA). The Assays-by-Design service custom-designed, synthesized, formulated and tested the TaqMan primer and fluorescent probe assays. Genotyping was carried out according to the protocol outlined in the literature provided. In a single tube, the template DNA was added to the TaqMan Universal PCR Master Mix, which contained the primers, probes and buffer. This step was followed by thermal cycling and detection. Genotypes were detected using the ABI Prism 7700 Sequence Detection System.
Data analysis
All analyses were performed with the SPSS mainframe statistical package (SPSS Inc., Chicago, IL, USA). Prior to carrying out the analyses, all data were tested to ensure a normal distribution. The latency data did not follow a normal distribution and were therefore transformed using a conservative square-root calculation. To determine if salivary cortisol levels were increased following the stress induction compared to the neutral mood condition, a two-tailed paired samples t-test was performed on the cortisol data.
In order to examine whether cortisol response to stress was correlated with changes in alcohol intake, latency to access the cue or alcohol craving in response to stress, correlational analyses were carried out on these measures. Percent change in salivary cortisol, alcohol intake, craving and latency from the neutral to stress sessions were calculated and used in the analyses to allow a normalized comparison of stress response between individuals. To test the hypothesis that genotype at the OPRM1 gene would be associated with changes in cortisol concentration, alcohol intake, craving or latency to access the cue in response to stress, independent samples t-tests were performed for each outcome measure using the genotype as a grouping variable.
RESULTS
Genotyping for the A118G polymorphism
The subject sample included 42 asparagine (Asn) homozygotes (81%) and 10 aspartate (Asp) carriers (19%). There were no Asp homozygotes.
Salivary cortisol concentration
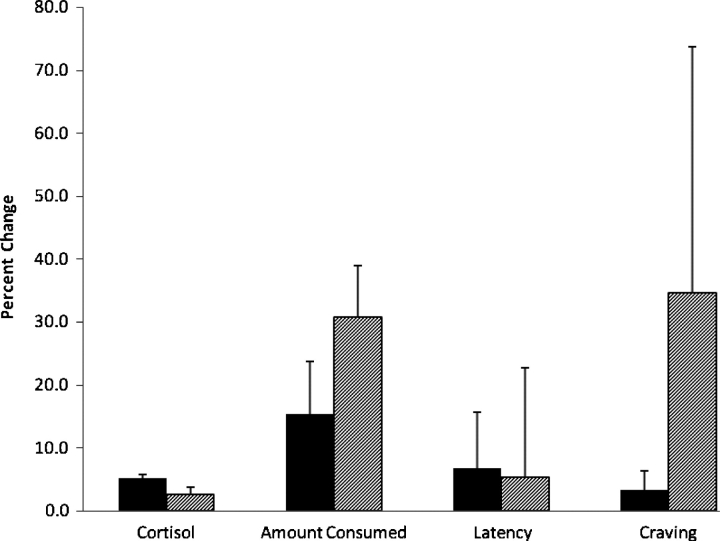
The mean salivary cortisol concentration measured following the PASAT task (mean = 5.87 nmol/L, SEM = 0.052) was significantly greater than that measured following the neutral control condition (mean = 5.58 nmol/L, SEM = 0.051) [t(73) = 10.80, P = 0.000]. Overall, the mean percent increase in salivary cortisol from the neutral baseline level to the post-stressor level was 5.3% (SEM = 0.5%). For individuals who were Asn homozygotes for the OPRM1 gene, the mean percent change in salivary cortisol concentration between the neutral and stress conditions was 5.2% (SEM = 0.6%). For carriers of the A118G polymorphism, the mean percent change in salivary cortisol concentration between the neutral and stress conditions was 2.7% (SEM = 1.1%). These results are displayed in fig. 1. An independent samples t-test revealed a trend for Asn homozygotes to have a greater mean increase in cortisol concentration in response to stress than Asp carriers, although this difference was not statistically significant [t(50) = 1.864, P = 0.068]. No significant correlations were found between any of the subject characteristics listed in Table 1 and the change in cortisol in response to stress (all P > 0.05).
Fig. 1.
Percent change from the neutral to the stress condition for salivary cortisol concentration, alcohol intake, latency to access the alcohol cue and alcohol craving. Solid bars represent the means for subjects who were homozygous for the Asn40 allele of the μ-opioid receptor; hatched bars represent the means for carriers of the Asp40 allele of the receptor. Error bars represent the standard error of the mean.
Alcohol intake
The mean alcohol intake measured for all 74 subjects under the neutral and stress conditions is reported in Table 2. As reported in Table 3, a significant negative correlation was observed between the change in salivary cortisol in response to stress and the change in alcohol intake in response to stress (r = −0.252, P = 0.015). A greater change in cortisol from the neutral to the stress condition was correlated with a smaller change in alcohol intake between the neutral and stress conditions. Figure 1 displays the mean percent change in alcohol intake from the neutral to the stress condition for the 52 genotyped subjects. Although the Asn homozygotes had a smaller mean increase in alcohol intake in response to stress (mean = 15.3%, SEM = 9.0%) than the Asp carriers (mean = 30.7%, SEM = 17.4%), an independent samples t-test revealed no statistical significance between the groups [t(50) = −0.757, P = 0.453].
Table 2.
Mean alcohol intake, latency to access the alcohol cue and alcohol craving under the neutral and stress conditions
| Neutral | Stress | |
|---|---|---|
| Alcohol intake (oz) | 2.08 ± 0.13 | 2.19 ± 0.14 |
| Latency to access the alcohol cue (ms) | 2148 ± 62 | 2162 ± 67 |
| Alcohol craving | 4.21 ± 0.20 | 4.02 ± 0.21 |
Mean values with standard errors of the means for all 74 subjects tested in the study.
Alcohol craving measured on a 1–7 scale (see the text): 1 = low craving, 7 = high craving.
Table 3.
Correlations between changes in salivary cortisol concentration from the neutral to the stress condition and changes in drinking behavior in response to stress
| R | P | |
|---|---|---|
| Alcohol intake | −0.252 | 0.015 |
| Latency to access the alcohol cue | −0.147 | 0.107 |
| Alcohol craving | −0.076 | 0.259 |
Latency to access the alcohol cue
The mean latency to access the alcohol cue measured for all 74 subjects under the neutral and stress conditions is reported in Table 2. As reported in Table 3, there was no significant correlation observed between change in salivary cortisol in response to stress and change in latency to access the cue in response to stress (r = −0.147, P = 0.107). An independent samples t-test revealed no statistical significance between the mean percent change in latency from the neutral to the stress condition for the Asn homozygotes (mean = 6.7%, SEM = 3.1%) and the Asp carriers [mean = 5.3%, SEM = 8.2%; t(50) = 0.188, P = 0.851]. These results are displayed in Fig. 1.
Alcohol craving
The mean alcohol craving reported for all 74 subjects in the neutral and stress conditions is reported in Table 2. As reported in Table 3, there was no significant correlation observed between change in salivary cortisol in response to stress and change in craving for alcohol in response to stress (r = −0.076, P = 0.259). Figure 1 displays the mean percent change in craving from the neutral to the stress condition for the 52 genotyped subjects. Although the Asn homozygotes had a smaller mean increase in craving in response to stress (mean = 3.2%, SEM = 8.4%) than the Asp carriers (mean = 34.6%, SEM = 39.1%), an independent samples t-test revealed no statistical significance between the groups [t(50) = −1.237, P = 0.222]. In addition, the baseline craving score was used as a covariate in the analyses. However, no significant association was found between baseline craving and stress-induced changes in craving (r = −0.203, P = 0.082).
DISCUSSION
Psychological stress is thought to be an important trigger for uncontrolled alcohol consumption or relapse to drinking following a period of abstinence. However, the biological link between stress and drinking has not been well characterized. The present study examined interactions between the HPA axis and the endogenous opioid system as a potential pathway mediating stress-induced alcohol consumption.
The PASAT task utilized as a stressor in this study successfully induced a biological response to stress in the subjects. Salivary cortisol concentrations were significantly greater following the stress task than in the neutral control condition, indicating activation of the HPA axis in response to the stressor. The findings from the present study indicate that a blunted biological response to stress is associated with increased alcohol consumption following exposure to stress, as the change in the cortisol level between the neutral and stressed conditions was negatively correlated with the change in consumption between the two conditions. This finding is consistent with the previous research that demonstrated an association between an attenuated hormonal response to alcohol cues or stress and an increased risk for relapse (Junghanns et al., 2003, 2005). It is possible that individuals with blunted HPA axis reactivity to external stimuli may seek to consume alcohol in response to stress in order to compensate for their hormonal insufficiency. Thus, HPA axis function could serve as a predictor of risk for alcohol dependence in alcohol-naïve or social drinkers, or as a predictor for risk of relapse in abstinent alcohol-dependent individuals. Using HPA axis reactivity as a predictive marker could help to identify individuals at risk for dependence or relapse prior to development of those conditions, which would allow the individuals and their treatment providers to take preventive action.
Change in cortisol in response to stress and change in latency in response to stress were not significantly correlated although there was a trend for these measures to be negatively correlated. This trend suggests that a dampened biological response to stress may also be associated with an increased motivation to consume alcohol, which corroborates the correlation observed between cortisol response and alcohol intake. It is possible that the relatively short latency times observed during the study may have limited variability in the latency measure and prevented observation of statistical significance. The modification of the experimental paradigm to increase the amount of movement required to access the cue could improve the sensitivity of the latency measure, thereby creating a reasonable proxy for motivation to drink that may predict actual consumption.
There was no significant correlation between changes in cortisol and craving for alcohol in response to stress in the present study. This finding deviates from previous research demonstrating that the hormonal response to stress may influence craving for alcohol in dependent individuals (O’Malley et al., 2002). In the present study, the mean craving scores at baseline as well as in both the neutral and stress conditions were relatively high, and craving ratings showed very little variability throughout the trials regardless of stressed or neutral condition. It is possible that no effect of stress on craving was observed because of a ‘ceiling’ effect that prevented accurate measurement of changes in craving in response to stress. Alternatively, failure to find a correlation between craving and cortisol responses to stress despite the above reported findings for intake and latency may indicate that subjective craving is not a reliable predictor of actual alcohol use in this experimental paradigm. The concept of craving and its role in addictive behavior has been long debated in the fields of alcohol and drug research, and many studies have failed to find a consistent correlation between measures of craving and measures of drug use. Future studies should seek alternative means of assessing subjective urge to drink and should continue to include multiple measures of propensity to drink such as alcohol intake and latency to drink in order to accurately assess the various aspects of drinking behavior.
The investigation into whether the Asp40 allele of the μ-opioid receptor is associated with altered HPA axis reactivity to stress indicated a trend for carriers of the variant receptor allele to exhibit a blunted cortisol response to stress compared to those who were not carriers, although this trend was not statistically significant. This result is consistent with previous research demonstrating that carriers of the Asp40 allele exhibit an abnormal cortisol response to naloxone and stress (Wand et al., 2002; Hernandez-Avila et al., 2003; Chong et al., 2006).
In light of the evidence indicating that a blunted hormonal response to stress increases risk of relapse (Junghanns et al., 2003, 2005), it was hypothesized that individuals with the A118G polymorphism would also exhibit greater increases in drinking behavior in response to stress secondary to hyporeactivity of the HPA axis. The present study demonstrated that subjects with the receptor variant did in fact have a greater percent change in alcohol intake in reaction to stress than did those who were not carriers. Although the percent change for the heterozygotes was twice that of the Asn40 homozygotes, the difference was not statistically significant. Similarly, craving for alcohol increased to a greater degree in response to stress for the Asp40 carriers than the Asn40 homozygotes. Craving for alcohol increased 34.6% in response to stress for carriers of the variant allele but only 3.2% for the non-carriers. However, as with the alcohol intake data, this difference was not found to be statistically significant. A comparison of the percent change in latency to access the cue in response to stress between the Asn40 homozygote and Asp heterozygote groups did not mirror the findings for alcohol intake. The change in motivation to consume alcohol in reaction to a stressor as measured by latency was similar in the two groups.
It was surprising that the differences in the cortisol, alcohol intake and alcohol craving data between the Asn40 homozygotes and the Asp40 carriers were not significant despite sizable differences between the groups. The high degree of variability within both groups observed for these measures provides a possible explanation for why these differences were not significant. The standard error calculated for the data was likely too large to permit an accurate determination of potential differences between the groups. It is possible that a larger sample size would decrease the standard error in the data and permit observation of significant differences. The small sample size for Asp40 carriers therefore represents a substantial limitation of the present investigation.
Due to the fact that genotyping for the A118G polymorphism was not included in the initial study protocol, a large percentage of subjects (30%) were not tested for the receptor variant. The lack of genotype information for all 74 subjects represents a significant limitation of this study, as the inclusion of such data from these subjects may have altered the reported findings regarding the relationships between drinking behavior and OPRM1 genotype. Of the 52 subjects who were genotyped, 42 were homozygous for the Asn40 allele while 10 were heterozygous carriers of the Asp40 allele; no subjects were homozygous for the Asp40 allele. At least one copy of the A118G polymorphism is present in 24–36% of individuals of European descent (Bergen et al., 1997; Bond et al., 1998; Crowley et al., 2003), but in less than 1% of African Americans (Gelernter et al., 1999). Because African Americans made up 60% of the subjects in the present study, the number of Asp40 carriers was quite low. Attempts were made to specifically recruit individuals with the polymorphism, but these efforts failed to substantially increase the number of carriers due to the fact that the majority of respondents to study advertisements were minorities. Obtaining a sufficiently large sample size to permit an accurate assessment of differences between individuals with the polymorphism and those without would have required many more months of subject recruitment, and this avenue was not pursued to avoid compromising the ability to complete the other aims of the study. Future investigations should take into account the difficulty of recruiting carriers and should obtain a larger sample size in order to achieve sufficient power to more accurately assess the effects of the A118G polymorphism on drinking behavior. It is also possible that population stratification, for example differences in allele frequencies between groups arising from systematic differences in ethnic background, could have confounded the results. There is little research to date indicating whether population stratification may influence observations associated with the A118G genotype, but future studies should consider this possibility.
Overall, the results of the current study indicate intriguing associations among stress-induced alcohol drinking behavior, the HPA axis and the endogenous opioid system that necessitate further investigation. These findings represent an important addition to the body of research investigating determinants of hazardous alcohol drinking behavior and future studies should aim to further elucidate the nature of these associations. Biological measurement of stress responsiveness may provide a method for identifying individuals who are most at risk for serious stress-related alcohol problems. Alcohol-dependent persons who exhibit blunted HPA axis reactivity to stress could therefore be targeted with behavioral or pharmacological therapies that counteract the impact of stress or normalize HPA axis activity in an effort to reduce increased drinking that is provoked by stress exposure. Additionally, identification of carriers of the A118G polymorphism may be a useful tool for identifying individuals most at risk for stress-induced relapse to drinking. Alcohol-dependent carriers of the variant receptor may be more responsive to treatment with opioid receptor antagonists or pharmacotherapies targeting the HPA axis, and screening alcoholics in treatment for the polymorphism could provide a means for targeting specific treatments to those who are most likely to demonstrate response. Future research should also investigate whether certain patient characteristics other than genotype, such as degree of alcohol dependence or family history of alcohol dependence, may be predictably associated with an altered hormonal response to stress and stress-induced drinking. Identification and characterization of the factors that contribute to chronic alcohol consumption and relapse will facilitate the development of new treatments for alcohol dependence and thereby improve patient care and quality of life.
Acknowledgments
We would like to thank the laboratory of Dr Lucy Carr for genotyping the blood samples for this study. This study was supported by NIH grant #P60 AA07611-19 Pilot Project 43.
References
- Adinoff B, Martin PR, Bone GH, et al. Hypothalamic–pituitary–adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Arch Gen Psychiatry. 1990;47:325–30. doi: 10.1001/archpsyc.1990.01810160025004. [DOI] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O’Malley S, et al. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65:135–44. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Kokoszka J, Peterson R, et al. Mu-opioid receptor gene variants: lack of association with alcohol dependence. Mol Psychiatry. 1997;2:490–4. doi: 10.1038/sj.mp.4000331. [DOI] [PubMed] [Google Scholar]
- Bodani UC, Edwards S. A rapid and sensitive enzyme immunoassay for the determination of salivary cortisol. Clin Chem. 1999;45:A79. [Google Scholar]
- Bond C, LaForge KS, Tian M, et al. Single-nucleotide polymorphism in the human mu-opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA. 1998;95:9608–13. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong RY, Oswald L, Yang X, et al. The micro-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31:204–11. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- Costa A, Bono G, Martignoni E, et al. An assessment of hypothalamo–pituitary–adrenal axis functioning in non-depressed, early abstinent alcoholics. Psychoneuroendocrinology. 1996;21:263–75. doi: 10.1016/0306-4530(96)00001-7. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Oslin DW, Patkar AA, et al. A genetic association study of the mu-opioid receptor and severe opioid dependence. Psychiatr Genet. 2003;13:169–73. doi: 10.1097/00041444-200309000-00006. [DOI] [PubMed] [Google Scholar]
- Culpepper-Morgan JA, Kreek MJ. Hypothalamic–pituitary–adrenal axis hypersensitivity to naloxone in opioid dependence: a case of naloxone-induced withdrawal. Metabolism. 1997;46:130–4. doi: 10.1016/s0026-0495(97)90289-4. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C. Differences in the peripheral levels of beta-endorphin in response to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Alcohol Clin Exp Res. 2005;29:1965–75. doi: 10.1097/01.alc.0000187599.17786.4a. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Santella S, et al. Response of the HPA-axis to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Psychoneuroendocrinology. 2007;32:293–305. doi: 10.1016/j.psyneuen.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Dave JR, Eiden LE, Karanian JW, et al. Ethanol exposure decreases pituitary corticotropin-releasing factor binding, adenylate cyclase activity, proopiomelanocortin biosynthesis, and plasma beta-endorphin levels in the rat. Endocrinology. 1986;118:280–6. doi: 10.1210/endo-118-1-280. [DOI] [PubMed] [Google Scholar]
- Errico AL, King AC, Lovallo WR, et al. Cortisol dysregulation and cognitive impairment in abstinent male alcoholics. Alcohol Clin Exp Res. 2002;26:1198–204. doi: 10.1097/01.ALC.0000025885.23192.FF. [DOI] [PubMed] [Google Scholar]
- Errico AL, Parsons OA, King AC, et al. Attenuated cortisol response to biobehavioral stressors in sober alcoholics. J Stud Alcohol. 1993;54:393–8. doi: 10.15288/jsa.1993.54.393. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Leen-Feldner EW, Zvolensky MJ, et al. Examining the association between rumination, negative affectivity, and negative affect induced by a paced auditory serial addition task. J Behav Ther Exp Psychiatry. 2006;37:171–87. doi: 10.1016/j.jbtep.2005.06.002. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P), Version 2.0. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Gelernter J, Kranzler H, Cubells J. Genetics of two mu-opioid receptor gene (OPRM1) exon I polymorphisms: population studies, and allele frequencies in alcohol- and drug-dependent subjects. Mol Psychiatry. 1999;4:476–83. doi: 10.1038/sj.mp.4000556. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Alcohol-seeking behavior: the roles of the hypothalamic–pituitary–adrenal axis and the endogenous opioid system. Alcohol Health Res World. 1998;22:202–10. [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2004;4:39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44:367–73. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Wand G, Luo X, et al. Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the mu-opioid receptor locus (OPRM1) Am J Med Genet B Neuropsychiatr Genet. 2003;118:60–5. doi: 10.1002/ajmg.b.10054. [DOI] [PubMed] [Google Scholar]
- Holdwick DJ, Jr, Wingenfeld SA. The subjective experience of PASAT testing. Does the PASAT induce negative mood? Arch Clin Neuropsychol. 1999;14:273–84. doi: 10.1093/arclin/14.3.273. [DOI] [PubMed] [Google Scholar]
- Inder WJ, Joyce PR, Ellis MJ, et al. The effects of alcoholism on the hypothalamic–pituitary–adrenal axis: interaction with endogenous opioid peptides. Clin Endocrinol. 1995;43:283–90. doi: 10.1111/j.1365-2265.1995.tb02033.x. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, et al. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 2003;38:189–93. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Tietz U, Dibbelt L, et al. Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol. 2005;40:80–5. doi: 10.1093/alcalc/agh107. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, et al. Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev Psychopathol. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, et al. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–24. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. 3rd edn. New York: Oxford University Press; 1995. [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, et al. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–8. [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, et al. Family Tree Questionnaire for assessing family history of drinking problems. In: Lettieri JND, Sayers M, editors. Alcoholism Treatment Assessment Instruments. Rockville, MD: NIAAA; 1985. [Google Scholar]
- McGeary JE, Monti PM, Rohsenow DJ, et al. Genetic moderators of naltrexone's effects on alcohol cue reactivity. Alcohol Clin Exp Res. 2006;30:1288–96. doi: 10.1111/j.1530-0277.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism . Recommended Council Guidelines on Ethyl Alcohol Administration in Human Experimentation. Rockville, MD: United States Department of Health and Human Services; 2005. [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, et al. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo–pituitary–adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–52. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–78. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Brain Res Rev. 1997;25:359–72. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Raff H, Homar PJ, Burns EA. Comparison of two methods for measuring salivary cortisol. Clin Chem. 2002;48:207–8. [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, et al. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo–pituitary–adrenal axis. Alcohol Clin Exp Res. 2000;24:1836–49. [PubMed] [Google Scholar]
- Rivier C, Imaki T, Vale W. Prolonged exposure to alcohol: effect on CRF mRNA levels, and CRF- and stress-induced ACTH secretion in the rat. Brain Res. 1990;520:1–5. doi: 10.1016/0006-8993(90)91685-a. [DOI] [PubMed] [Google Scholar]
- Roman DD, Edwall GE, Buchanan RJ, et al. Extended norms for the Paced Auditory Serial Addition Task. Clin Neuropsychol. 1991;5:33–40. [Google Scholar]
- Schuckit MA, Gold E, Risch C. Plasma cortisol levels following ethanol in sons of alcoholics and controls. Arch Gen Psychiatry. 1987;44:942–5. doi: 10.1001/archpsyc.1987.01800230022005. [DOI] [PubMed] [Google Scholar]
- Singleton EG, Tiffany ST, Henningfield JE. Development and validation of a new questionnaire to assess craving for alcohol. In: Harris L S, editor. Problems of Drug Dependence, 1994: Proceedings of the 56th Annual Scientific Meeting, The College on Problems of Drug Dependence, Inc. Vol. 153. Rockville, MD: The Institute; 1995. p. 289. [Google Scholar]
- Skinner HA, Horn JL. Alcohol Dependence Scale: Users Guide. Toronto, ON: Addiction Research Foundation; 1984. [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Timeline Followback (TLFB) User's Manual. Toronto, ON: Addiction Research Foundation; 1996. [Google Scholar]
- Sorocco KH, Lovallo WR, Vincent AS, et al. Blunted hypothalamic–pituitary–adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int J Psychophysiol. 2006;59:210–7. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. New York: Oxford University Press; 1991. [Google Scholar]
- SPSS Base 14.0 for Windows 2005. SPSS Inc., Chicago, IL.
- Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Monti PM, Rohsenow DJ, et al. Moderators of naltrexone's effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alcohol Clin Exp Res. 2008;32:58–66. doi: 10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkaay MM, Patterson SM. Nicotine and acute stress: effects of nicotine versus nicotine withdrawal on stress-induced hemoconcentration and cardiovascular reactivity. Biol Psychol. 2006;71:191–201. doi: 10.1016/j.biopsycho.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Waltman C, McCaul ME, Wand GS. Adrenocorticotropin responses following administration of ethanol and ovine corticotropin-releasing hormone in the sons of alcoholics and control subjects. Alcohol Clin Exp Res. 1994;18:826–30. doi: 10.1111/j.1530-0277.1994.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic–pituitary–adrenal axis in actively drinking alcoholics. J Clin Endocrinol Metab. 1991;72:1290–5. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, Ali M. Adrenocorticotropin responses to naloxone in sons of alcohol-dependent men. J Clin Endocrinol Metab. 1999a;84:64–8. doi: 10.1210/jcem.84.1.5373. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, Ali M, et al. Adrenocortical responses and family history of alcoholism. Alcohol Clin Exp Res. 1999b;23:1185–90. [PubMed] [Google Scholar]
- Wand GS, Mangold D, El Deiry S, et al. Family history of alcoholism and hypothalamic opioidergic activity. Arch Gen Psychiatry. 1998;55:1114–9. doi: 10.1001/archpsyc.55.12.1114. [DOI] [PubMed] [Google Scholar]
- Wand GS, McCaul M, Yang X, et al. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26:106–14. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology. 2001;158:374–81. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]