Abstract
AIMS
Outcomes of patients undergoing percutaneous coronary intervention (PCI) with drug-eluting stents (DES) and bare metal stents (BMS) have not been evaluated separately for specific dual and triple antiplatelet agent use. The purpose of this meta-analysis was to determine whether triple antiplatelet therapy (combination of clopidogrel, aspirin and cilostazol) has any advantage in efficacy compared with standard dual antiplatelet therapy (aspirin and clopidogrel) in patients undergoing PCI.
METHODS
Electronic and printed sources were searched till May 2008 for randomized controlled clinical trials (RCTs) of cilostazol in combination with aspirin and clopidogrel. Pooled weighted mean difference (WMD) and pooled odds ratio (OR) with 95% confidence intervals (CIs) were calculated.
RESULTS
A total of four RCTs including 1457 patients with a median follow-up period of 6–9 months were included in the analysis. The rates of major adverse cardiac and/or cerebrovascular events (MACE/MACCE), stent thrombosis and bleeding were not significantly different between triple and dual antiplatelet therapy groups. Pooled analysis showed that cilostazol was associated with significantly decreased incidence of in segment restenosis (ISR) (OR 0.51, 95% CI 0.38, 0.68; P < 0.00001), increased minimum luminal diameter (MLD) (WMD 0.16, 95% CI 0.10, 0.22; P < 0.00001) for both DES and BMS and also individually. However, the rates of target vessel revascularization (OR 0.45, 95% CI 0.25, 0.83; P = 0.01 and late lumen loss (pooled WMD 0.14, 95% CI 0.2, 0.07; P = 0.001) were decreased significantly only in the DES group receiving triple therapy.
CONCLUSIONS
Cilostazol appears to be effective in reducing the rates of ISR without any significant benefit for MACE/MACCE.
Keywords: antiplatelet, cilostazol, coronary, intervention, percutaneous, triple
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT?
Different strategies have been evaluated for their efficacy in reducing stent thrombosis and restenosis in patients undergoing percutaneous coronary intervention (PCI).
Triple antiplatelet therapy is one such strategy that has been shown to improve the efficacy outcomes associated with PCI.
Cilostazol is an antiplatelet agent that is being prescribed as a component of triple-therapy regimen in various centres in our country, and the beneficial effect of cilostazol addition to other antiplatelet regimens has been observed.
However, the extent of the efficacy is not uniformly in favour of the triple therapy compared with dual therapy.
Moreover, the use of this agent with bare metal stents (commonly used in developing countries) and drug-eluting stents has not been separately looked into.
WHAT THIS STUDY ADDS
Triple antiplatelet therapy, with cilostazol as a component, reduces restenosis rates and repeat revascularizations post PCI without any significant increase in bleeding risk.
The beneficial effect of cilostazol is more evident with drug-eluting stents.
Its use with bare metal stents needs to be explored further.
Introduction
Recently we were faced with a clinical query regarding the usefulness of triple antiplatelet therapy (cilostazol, aspirin and clopidogrel combination) for patients undergoing percutaneous transluminal coronary angioplasty in our hospital. The underlying logic for off-label use of cilostazol in this setting is that cilostazol has been shown to inhibit vascular smooth muscle proliferation and intimal hyperplasia after endothelial injury, in addition to its antiplatelet effect [1, 2]. As against this, currently used antiplatelet drugs such as aspirin and clopidogrel have no effect on neointimal hyperplasia. More importantly, randomized clinical trials have shown the beneficial effect of cilostazol in reducing long-term major adverse cardiovascular and cerebrovascular events (MACCE) and preventing both angiographic and clinical restenosis in patients [3, 4]. However, the extent of the efficacy is not uniformly in favour of triple therapy vs. dual therapy.
The performance of bare metal stents (BMS) is suboptimal in terms of freedom from restenosis and repeat percutaneous coronary intervention (PCI) in lesions at high risk of restenosis. Another aspect of management of these patients is that BMS are the most commonly used stents in our setting due to poor affordability of drug-eluting stents (DES). Thus there is a great need to prevent neointimal hyperplasia and stent restenosis. Some pharmacological agents have been investigated for their usefulness when added to the standard therapeutic regimen in reducing restenosis rates when used in patients undergoing PCI with BMS. However, this has largely been unsuccessful [4].
Ours is a tertiary care centre, and in recent years increasing attempts have been made to incorporate principles of evidence-based medicine in guiding clinical practice. Such evidence is also used in making policy-related decisions. These practices are based on reports that have shown better therapeutic outcomes associated with evidence-based medicine [5, 6]. In order to provide an evidence-based answer to the clinical query, which is pertinent to the practice at our centre, we undertook a literature search. During our preliminary literature search we came across 30 studies and, interestingly, on the day of query we also came across a systematic review on the same topic [7]. The main conclusions of the review were that cilostazol was safe and effective in reducing the risk of restenosis and repeat revascularization without any significant increase in bleeding rates and incidence of stent thrombosis (ST). In the above meta-analysis, 23 studies were analysed, of which only three compared the triple therapy (clopidogrel based) with dual therapy. No subgroup analysis specifically addressed our query related to the use of the triple regimen of aspirin, clopidogrel and cilostazol. With this background we undertook a meta-analysis to answer the query raised in cardiology practice.
Methods
Search strategy and study selection
We systematically searched Medline, Cochrane Central Register of Controlled Trials, and Embsase for all relevant articles up to May 2008. We first entered the medical subjects heading (MeSH) terms and text words, including cilostazol AND percutaneous coronary intervention AND stents. Next, we searched using the MeSH terms and text words with antiplatelet therapy AND stents. Additionally, we entered these terms separately. Boolean logic was used for all searches. Two investigators carried out the search independently. We then combined all the searches and retrieved the relevant articles. Manual search was made by going through the reference lists of the retrieved articles and through Index Medicus and key cardiology journals. Conference abstracts were obtained from conference coverages appearing in journals and other internet-based sources.
Data extraction
Data extraction forms were used to obtain the following information: characteristics of study participants, number of participants, type of intervention (dose, duration), randomization, blinding, study outcomes and duration of follow-up. The data were extracted independently by two investigators and compiled by a third investigator. Differences were removed by consensus.
Randomized controlled trials of cilostazol in combination with aspirin and clopidogrel (triple antiplatelet therapy) in patients undergoing PCI with BMS or DES were included in the study. We planned to include only those randomized controlled clinical trials in which the patients received clopidogrel and aspirin (dual antiplatelet therapy) in the control group. Exclusion criteria were the following: (i) studies that did not meet the inclusion criteria related to antiplatelet therapy, (ii) open label studies, (iii) studies with follow-up of <6 months, (iv) studies in which angiographic end-points were not evaluated, and (v) uncontrolled studies.
Study outcomes
The primary end-point evaluated for the present analysis was major adverse cardiac events (MACE) or MACCE. MACE included myocardial infarction (MI), symptom-driven repeat revascularization and death, and MACCE included the components MACE and stroke. Secondary end-points were (i) target vessel revascularization (TVR); (ii) ST, defined as any of the following: angiographic documentation of stent occlusion with or without the presence of thrombus associated with an acute ischaemic event, unexplained sudden death, and MI not clearly attributable to another coronary lesion; (iii) death; (iv) in segment restenosis (ISR); (v) in segment late lumen loss (LL); (vi) in segment minimum luminal diameter (MLD); and (vii) bleeding rates at 6 or 9 months, major bleeding defined as a need for transfusion, a reduction in haemoglobin of ≥5 g dl−1, need for surgical intervention, or resulting in hypotension requiring inotropic support.
For all the evaluated variables, subgroup analysis was undertaken to compare triple and dual antiplatelet therapy for patients undergoing PCI with BMS or DES.
Pertinent data were also extracted for assessment of quality of the studies as described previously by Khan et al. [8]. Briefly, information related to randomization, baseline comparability, blinding, withdrawal and intention-to-treat analysis. Inverted funnel plot was generated for assessment of publication bias.
Analysis
Continuous data were expressed as mean (SD) and weighted mean difference (WMD) was obtained. The data from various studies were pooled and expressed as pooled WMD with 95% confidence interval (CI). Dichotomous data were expressed as odds ratio (OR) with 95% CI. The data were pooled by random effects model in case significant heterogeneity (detected by χ2 test) was found, otherwise the fixed effects model was used. A P-value <0.05 was considered to be significant. Rosenthal File Drawer's Method was used to evaluate the number of studies with conflicting results that would be required to change the findings of the analysis into statistically nonsignificant results. Revman (Version 4.2) was used for all the analyses.
Results
Thirty hits were obtained when the combined MeSH terms were used. From the initial search, 26 studies were considered as potentially eligible. These were further evaluated for eligibility. Four were found to be eligible for inclusion in this meta-analysis. Twenty-two were excluded because they did not meet the inclusion criteria. The study characteristics of the four included studies are shown in Table 1. The included studies satisfied most of the criteria for quality assessment (Table 2).
Table 1.
Characteristic features of included studies
| Study | CREST (2005)[11] | Chen et al. (2006)[9] | DECLARE-Long (2007)[10] | DECLARE-DIABETES (2008)[12] | ||||
|---|---|---|---|---|---|---|---|---|
| Patient characteristics | Age 18–75 years. Having indication for bare metal stent implantation with lesion length ≤30 mm. Reference vessel diameter 2.0–4.0 mm. Diameter stenosis rate range 50–100% determined by coronary angiography | Patients with stable or unstable angina or silent myocardial ischaemia undergoing coronary stent implantation. De novo target-lesion stenosis of >50% but <100% in a native coronary artery that was successfully treated by placement of a bare-metal intracoronary stent | >18 years of age with angina pectoris and/or positive stress test findings and a native coronary lesion. Angiographic eligibility for inclusion was a target lesion with a diameter stenosis >50%, visual reference diameter ≥2.5 mm and length ≥25 mm, and a planned total stent length ≥32 mm | Diabetic patients >18 years of age with angina pectoris or positive stress test and a native coronary lesion. Diameter stenosis >50% and reference diameter ≥2.5 mm | ||||
| Type of stent | Bare metal stent | Bare metal stent | Drug-eluting stent | Drug-eluting stent | ||||
| Clinical follow-up | 6 months | 6–9 months | 9 months | 9 months | ||||
| No. of patients for angiographic follow-up | 259 | 267 | 52 | 54 | 250 | 250 | 200 | 200 |
| No. of patients for clinical follow-up | 354 | 351 | 60 | 60 | 250 | 250 | 200 | 200 |
| Study groups | Triple | Dual | Triple | Dual | Triple | Dual | Triple | Dual |
| MLD (mean ± SD) | 1.77 ± 0.68 | 1.62 ± 0.75 | 2.14 ± 0.52 | 1.82 ± 0.36 | 2.07 ± 0.55 | 1.93 ± 0.57 | 2.15 ± 0.52 | 2.03 ± 0.58 |
| LL (mean ± SD) | 0.5 ± 0.6 | 0.75 ± 0.66 | 0.81 ± 0.39 | 1.18 ± 0.54 | 0.34 ± 0.49 | 0.51 ± 0.49 | 0.42 ± 0.5 | 0.53 ± 0.49 |
| Restenosis rate (%) | 22.01 | 34.46 | 14 | 32 | 6.7 | 11.2 | 8 | 15.6 |
| MACE (n) | 67 | 65 | – | – | 7 | 19 | 6 | 14 |
| Stent | 1 | 1 | – | – | 1 | 1 | 0 | 1 |
| Thrombosis (n) | 54 | 56 | – | – | 9 | 18 | 7 | 16 |
| TVR (n) | 3 | 2 | – | – | 0 | 2 | 1 | 0 |
| Deaths (n) | 13 | 16 | – | – | 2 | 4 | 3 | 3 |
| Bleeding (n) | 13 | 16 | NM | NM | 2 | 4 | 3 | 3 |
LL, lumen loss; MACE, major adverse cardiac events; MLD, mean lumen diameter; TVR, target vessel revascularization; NM, not mentioned.
Table 2.
Quality assessment of studies analysed
| Randomization | Baseline comparability | Blinding | Withdrawal | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Checklist items | Truly random | Allocation concealment | No. stated | Presented | Achieved | Eligibility criteria specified | Co-interventions identified | Assessors | Administration | Participants | Procedures assessed | >80% in final analysis | Reasons stated | Intention to treat analysis |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| CREST (2005) [11] | √ | √ | √ | √ | √ | √ | √ | √ | NS | NS | NS | √ | √ | √ |
| Chen et al. (2006) [9] | √ | √ | √ | √ | √ | √ | √ | √ | NS | NS | NS | √ | √ | √ |
| DECLARE-long (2007) [10] | √ | √ | √ | √ | √ | √ | √ | √ | NS | NS | NS | √ | √ | √ |
| DECLARE-DIABETES (2008) [12] | √ | √ | √ | √ | √ | √ | √ | √ | NS | NS | NS | √ | √ | √ |
NS, not specified.
MACE/MACCE, TVR, ISR and MLD were reported in all the studies, for a total population of 1725 patients. One study [9] did not report stent thrombosis and deaths, therefore these end-points were evaluated for 1457 patients.
MACE/MACCE
Four studies [9–12] including 1725 patients were included for this analysis. There was no difference in the incidence of MACE/MACCE between the triple therapy and dual therapy groups (pooled OR 0.51, 95% CI 0.25, 1.03; P = 0.06). However, on subgroup analysis there was a significant difference between the two groups favouring the triple drug therapy in patients undergoing PCI with DES (OR 0.38, 95% CI 0.38, 0.72; P = 0.003) (Figure 1).
Figure 1.
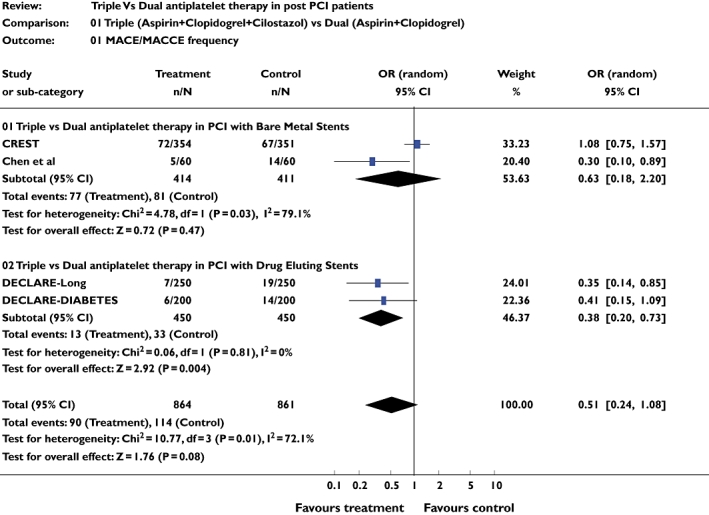
Studies comparing major adverse cardiac event/major adverse cardiac and cerebrovascular event frequencies in triple and dual antiplatelet therapy groups using pooled odds ratio (OR)
Target vessel revascularization
Four studies [9–12] including 1725 patients were included for this analysis. There was no difference in the incidence of TVR between triple and dual antiplatelet therapy for the combined analysis of BMS and DES (pooled OR 0.75, 95% CI 0.53, 1.04; P = 0.08). However, when the DES were considered alone, there were significant differences between the two groups favouring triple drug therapy in patients with DES placement (pooled OR 0.45, 95% CI 0.25, 0.83; P = 0.01) (Figure 2).
Figure 2.
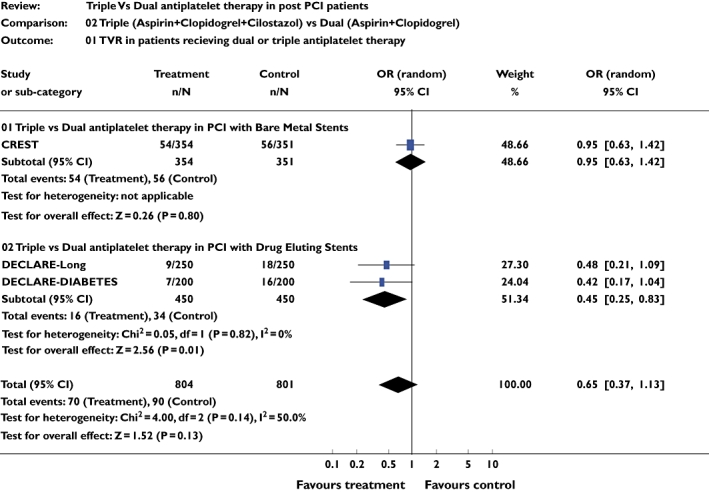
Studies comparing target vessel revascularization frequency in triple and dual antiplatelet therapy groups using pooled odds ratio (OR)
Stent thrombosis and deaths
Three studies [10–12] including 1457 patients were included for this analysis. There was no difference in the incidence of stent thrombosis between triple and dual antiplatelet therapy for the combined analysis (pooled OR 0.75, 95% CI 0.14, 3.62; P = 0.68). The same studies were analysed for mortality. There was no difference in mortality during the follow-up periods between triple and dual antiplatelet therapy (pooled OR 1.00, 95% CI 0.29, 3.45; P = 0.99). Similar results were seen on subgroup analysis (Figure 3a,b).
Figure 3.
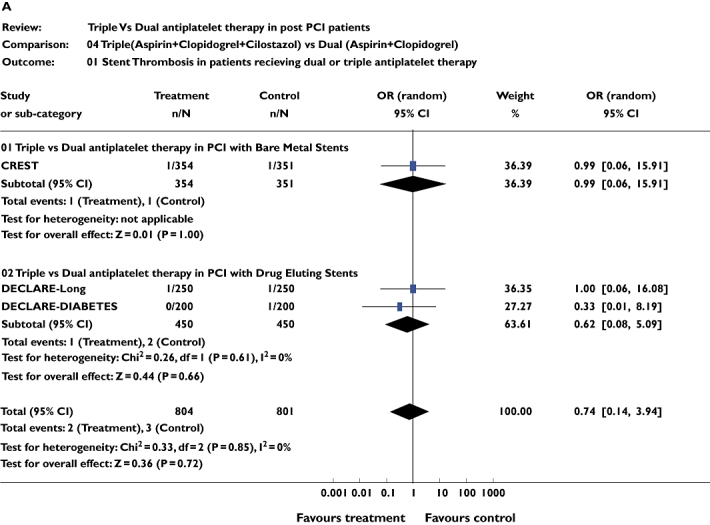
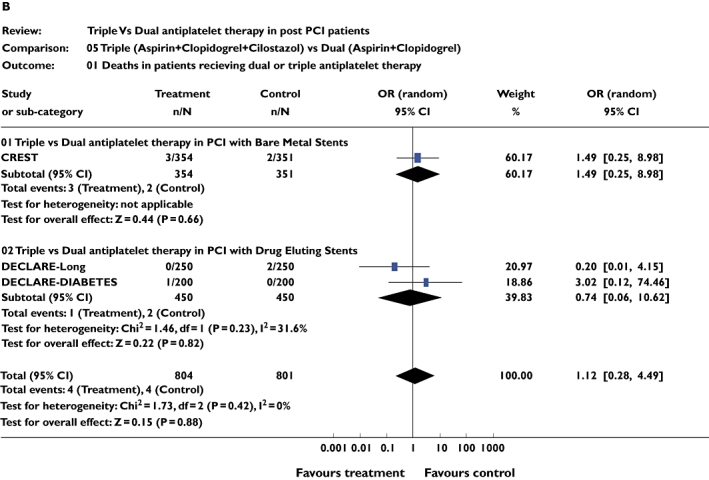
(a) Studies comparing stent thrombosis in triple and dual antiplatelet therapy groups using pooled odds ratio (OR). (b) Studies comparing number of deaths in triple and dual antiplatelet therapy groups using pooled OR
In segment restenosis
Four studies [9–12] including 1725 patients were included for this analysis. The pooled OR of angiographic restenosis at 6 months was 0.51 (95% CI 0.38, 0.68; P = 0.00001), which was statistically significant in favouring triple therapy. Similar results were observed on subgroup analysis (Figure 4).
Figure 4.
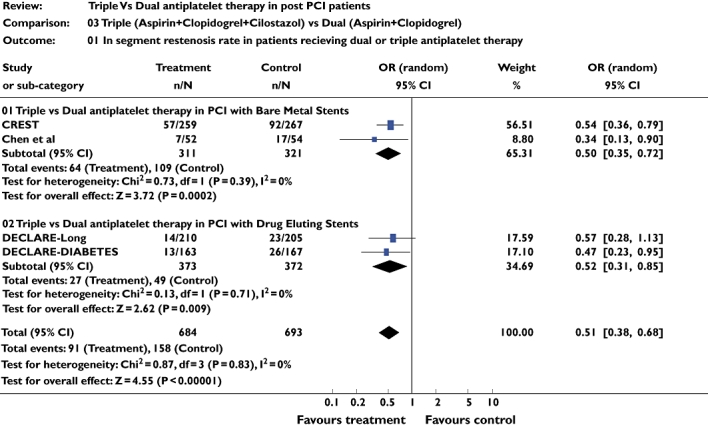
Studies comparing in-segment restenosis rates in triple and dual antiplatelet therapy groups using pooled odds ratio (OR)
Minimum luminal diameter
Four studies [9–12] including 1725 patients were included for this analysis. There was a significant difference in the MLD at the end of 6 months between the triple therapy and dual therapy groups with pooled WMD = 0.16 (95% CI 0.10, 0.22; P < 0.00001). The MLD in the BMS and DES followed a similar trend. For BMS group pooled WMD was 0.21 (95% CI 0.11, 0.31; P < 0.0001) and pooled WMD for DES group was 0.13 (95% CI 0.05, 0.21; P < 0.001).
Late lumen loss
Four studies [9–12] including 1725 patients were included for this analysis. The pooled WMD of late LL was −0.37 (95% CI −0.74, 0.01; P = 0.05), which was not statistically significant in favouring triple therapy. However, for the DES the triple therapy resulted in significantly less late LL with a pooled WMD −0.14 (95% CI −0.21, −0.07; P = 0.001), whereas for the BMS pooled WMD −0.59 (95% CI −1.41, −0.22; P = 0.00001), triple therapy was found to be beneficial in preventing late LL in both DES and BMS stented patients.
Bleeding
Three studies [10–12] including 1457 patients were included for this analysis. There was no significant difference in bleeding episodes between the two groups (pooled OR 0.77, 95% CI 0.41, 1.44; P = 0.42) (Figure 5).
Figure 5.
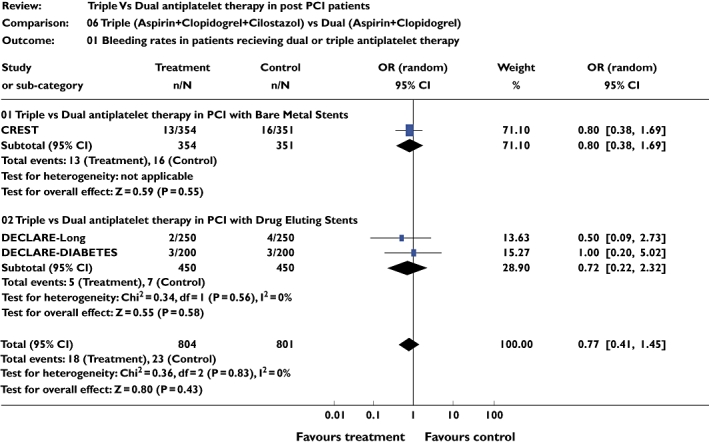
Studies comparing bleeding frequency in triple and dual antiplatelet therapy groups using pooled odds ratio (OR)
Publication bias
Funnel plot was constructed using the OR values obtained from MACE/MACCE. From the funnel plot the possibility of publication bias in the analysis could not be ruled out (Figure 6).
Figure 6.
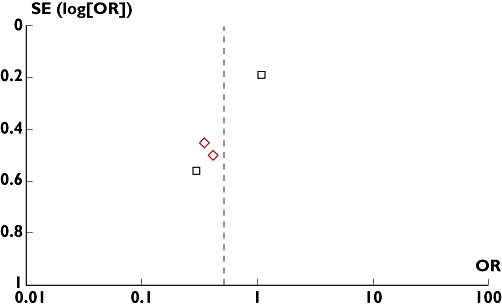
Funnel plot. Assessing publication bias using odds ratio (OR) of major adverse cardiac event/major adverse cardiac and cerebrovascular event frequency of the included studies. Triple vs Dual antiplatelet therapy in PCI with Bare Metal Stents (□); Triple vs Dual antiplatelet therapy in PCI with Drug Eluting Stents ( )
)
Discussion
The results of our study showed that addition of cilostazol does not significantly decrease the incidence of MACE or MACCE for the overall analysis of PCI with either DES or BMS. However, there was a significant reduction in MACE with triple therapy in the subgroup of studies that included patients who had undergone PCI with DES. Whereas MACE was consistently reduced with triple therapy in the two studies done in patients with DES, results were conflicting in case of BMS. In the CREST study [10], the incidence of MACE was more in the triple therapy group. The lesions and patient characteristics in DECLARE DIABETES [11] and DECLARE-Long [12] were different from those of Chen et al. [9] and the CREST study [10]. In the former, patients were either diabetic or had long lesions. It is likely that the benefits of triple therapy are more pronounced in patients with long lesions and in diabetics. Despite a higher event rate (18.3–23.3%) in the control groups in patients who underwent PCI with BMS, triple therapy with cilostazol failed to show any beneficial effect. This is surprising, since the drugs used to coat the DES are aimed to do precisely what cilostazol was used for.
As against the primary end-point, triple antiplatelet therapy significantly reduced restenosis rates overall as well as in subgroups of studies of BMS and DES. The antiproliferative properties of cilostazol may have contributed to this beneficial effect. Restenosis rates have largely been reduced by the DES. Addition of pharmacological agents with the aim of reducing restenosis rates in patients undergoing PCI with BMS has shown conflicting results [4]. As against this, cilostazol has consistently shown reduction in restenosis rates. The results of the previous meta-analysis showed that cilostazol reduced the restenosis rate and repeat revascularization in patients undergoing PCI after acute coronary syndrome. Many of the studies analysed had used BMS and it is known that BMS is associated with an increased incidence of restenosis. Our subgroup results concur with the previous meta-analysis done by Biondi-Zoccai et al. [7] and support that addition of cilostazol in patients with BMS reduces restenosis rates. However, in that meta-analysis 13 out of 23 studies analysed used ticlopidine as a part of an antiplatelet regimen. In our analysis we did not include studies that used ticlopidine.
Stent thrombosis was not shown to be significantly reduced by the use of cilostazol in either of the stent groups. Similar results were seen in the study done by Biondi-Zoccai et al. [7]. Stent thrombosis is of special concern with DES, and cilostazol was not shown to affect the incidence significantly. These data should be interpreted cautiously, since stent thrombosis has an event rate of 1% at 1 year [13] and there is an incremental risk of stent thrombosis of 0.2–0.5% per year thereafter [14, 15]. The maximum follow-up period of the pooled studies was 9 months, hence the results of our meta-analysis do not reflect long-term events. Cilostazol was found to be safe considering the low bleeding risk. In our analysis there was no significant difference between the dual and triple therapy groups. An increased incidence of major and minor bleeding events was observed in patients receiving abciximab and cilostazol [16]. However, cilostazol use was associated with a significantly increased incidence of gastrointestinal disturbance and rash when used in combination with aspirin and clopidogrel [12].
Keeping in tune with the standard practice of our Drug Information Unit, which received the query, we framed our evidence-based answer as follows: ‘though triple therapy significantly reduces restenosis rates compared to dual therapy when given to patients with DES and/or BMS, there is no significant reduction in MACE/MACCE and stent thrombosis rate. However, for patients with long lesions and patients receiving DES, triple antiplatelet therapy significantly reduces incidence of MACE. There is no significant difference in bleeding rates, target vessel revascularization and mortality in the two groups’.
It is well known that systematic reviews are associated with limitations, and the results obtained with these methods should be analysed accordingly. In our study publication bias could not be ruled out. The numbers of patients analysed were too low to reflect the data on the whole population. Clinical events such as stent thrombosis, bleeding and death were low in all the studies.
In conclusion, evidence needs to be generated for the use of the triple therapy regimen in patients undergoing PCI with BMS who have long lesions or who are diabetic. More evidence is needed for the effect of triple therapy on long-term follow-up.
Competing interests
None declared.
REFERENCES
- 1.Pan X, Arauz E, Krzanowski JJ, Fitzpatrick DF, Polson JB. Synergistic interactions between selective pharmacological inhibitors of phosphodiesterase isozyme families PDE III and PDE IV to attenuate proliferation rate of vascular smooth muscle cells. Biochem Pharmacol. 1994;48:827–35. doi: 10.1016/0006-2952(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 2.Kubota Y, Kichikawa K, Uchida H, Maeda M, Nishimine K, Makutani S, Sakaguchi S, Yoshioka T, Ohishi H, Kimura Y, Yoshikawa T. Pharmacologic treatment of intimal hyperplasia after metallic stent placement in peripheral arteries: an experimental study. Invest Radiol. 1995;30:532–37. doi: 10.1097/00004424-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Park SW, Lee CW, Kim HS, Lee NH, Nah DY, Hong MK, Kim JJ, Park SJ. Effect of cilostazol on angiographic restenosis after coronary stent placement. Am J Cardiol. 2000;86:499–503. doi: 10.1016/s0002-9149(00)01001-8. [DOI] [PubMed] [Google Scholar]
- 4.Tsuchikane E, Fukuhara A, Kobayashi T, Kirino M, Yamasaki K, Kobayashi T, Izumi M, Otsuji S, Tateyama H, Sakurai M, Awata N. Impact of cilostazol on restenosis after percutaneous coronary balloon angioplasty. Circulation. 1999;100:21–6. doi: 10.1161/01.cir.100.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Mostaza-Prieto JM, Martín-Jadraque L, López I, Tranche S, Lahoz C, Taboada M, Mantilla T, Soler B, Monteiro B, Sanchez-Zamorano MA. Evidence-based cardiovascular therapies and achievement of therapeutic goals in diabetic patients with coronary heart disease attended in primary care. Am Heart J. 2006;15:1064–70. doi: 10.1016/j.ahj.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Vasaiwala S, Nolan E, Ramanath VS, Fang J, Kearly G, Van Riper S, Kline-Rogers E, Otten R, Cody RA, Eagle KA. A quality guarantee in acute coronary syndromes: the American College of Cardiology's Guidelines Applied in Practice program taken real-time. Am Heart J. 2007;153:16–21. doi: 10.1016/j.ahj.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Biondi-Zoccai GG, Lotrionte M, Anselmino M, Moretti C, Agostoni P, Testa L, Abbate A, Cosgrave J, Laudito A, Trevi GP, Sheiban I. Systematic review and meta-analysis of randomized clinical trials appraising the impact of cilostazol after percutaneous coronary intervention. Am Heart J. 2008;155:1081–9. doi: 10.1016/j.ahj.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Khan K, Ter Riet G, Glanville J, Sowden AJ, Kleijnen J. Undertaking Systematic Reviews of Research on Effectiveness—CRD Guidance for Carrying Out or Commissioning Reviews. 2nd edn. York: NHS Center for Reviews and Dissemination (CRD), University of York; 2001. CRD Report 4. [Google Scholar]
- 9.Chen YD, Lu YL, Jin ZN, Yuan F, Lü SZ. A prospective randomized antiplatelet trial of cilostazol versus clopidogrel in patients with bare metal stent. Chin Med J (Engl) 2006;119:360–6. [PubMed] [Google Scholar]
- 10.Lee SW, Park SW, Kim YH, Yun SC, Park DW, Lee CW, Hong MK, Kim HS, Ko JK, Park JH, Lee JH, Choi SW, Seong IW, Cho YH, Lee NH, Kim JH, Chun KJ, Park SJ, DECLARE-Long Study Investigators Comparison of triple versus dual antiplatelet therapy after drug-eluting stent implantation (from the DECLARE-Long trial) Am J Cardiol. 2007;100:1103–8. doi: 10.1016/j.amjcard.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Douglas JS, Jr, Holmes DR, Jr, Kereiakes DJ, Grines CL, Block E, Ghazzal ZM, Morris DC, Liberman H, Parker K, Jurkovitz C, Murrah N, Foster J, Hyde P, Mancini GB, Weintraub WS, Cilostazol for Restenosis Trial (CREST) Investigators Coronary stent restenosis in patients treated with cilostazol. Circulation. 2005;112:2826–32. doi: 10.1161/CIRCULATIONAHA.104.530097. [DOI] [PubMed] [Google Scholar]
- 12.Lee SW, Park SW, Kim YH, Yun SC, Park DW, Lee CW, Hong MK, Kim HS, Ko JK, Park JH, Lee JH, Choi SW, Seong IW, Cho YH, Lee NH, Kim JH, Chun KJ, Park SJ. Drug eluting stenting followed by cilostazol treatment reduces late restenosis in patients with diabetes mellitus (Declare Diabetes trial) J Am Coll Cardiol. 2008;51:1181–7. doi: 10.1016/j.jacc.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 13.Cutlip DE, Baim DS, Ho KK, Popma JJ, Lansky AJ, Cohen DJ, Carrozza JP, Jr, Chauhan MS, Rodriguez O, Kuntz RE. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001;103:1967–71. doi: 10.1161/01.cir.103.15.1967. [DOI] [PubMed] [Google Scholar]
- 14.Ong AT, McFadden EP, Regar E, de Jaegere PP, van Domburg RT, Serruys PW. Late angiographic stent thrombosis (LAST) events with drug eluting stent. J Am Coll Cardiol. 2005;45:2088–92. doi: 10.1016/j.jacc.2005.02.086. [DOI] [PubMed] [Google Scholar]
- 15.Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug eluting stents. A meta analysis of randomized clinical trials. Am J Med. 2006;119:1056–61. doi: 10.1016/j.amjmed.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Hong EH, Kim MY, Park JE, Lee MH, Oh JM, Shin WG. Efficacy and safety of abciximab in combination with cilostazol in patients undergoing stenting. Int J Clin Pharmacol Ther. 2007;45:355–65. doi: 10.5414/cpp45355. [DOI] [PubMed] [Google Scholar]


