Abstract
AIMS
This study evaluated the associations of physical performance and functional status measures with the Drug Burden Index in older Australian men. The Drug Burden Index is a measure of total exposure to anticholinergic and sedative medications that incorporates the principles of dose–response and maximal effect.
METHODS
A cross-sectional survey was performed on community-dwelling older men enrolled in The Concord Health and Ageing in Men Project, Sydney, Australia. Outcomes included chair stands, walking speed over 6 m, 20-cm narrow walk speed, balance, grip strength and Instrumental Activities of Daily Living score (IADLs).
RESULTS
The study population consisted of 1705 men (age 76.9 ± 5.5 years). Of the 1527 (90%) participants who reported taking medications, 21% were exposed to anticholinergic and 13% to sedative drugs. The average Drug Burden Index in the study population was 0.18 ± 0.35. After adjusting for confounders (sociodemographics, comorbidities, cognitive impairment, depression), Drug Burden Index was associated with slower walking speed (P < 0.05), slower narrow walk speed (P < 0.05), balance difficulty (P < 0.01), grip weakness (P < 0.01) and poorer performance on IADLs (P < 0.05). Associations with physical performance and function were stronger for the sedative than for the anticholinergic component of the Drug Burden Index.
CONCLUSIONS
Higher Drug Burden Index is associated with poorer physical performance and functional status in community-dwelling older Australian men. The Drug Burden Index has broad applicability as a tool for assessing the impact of medications on functions that determine independence in older people.
Keywords: elderly, function, medications
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Inappropriate medication use is common among the elderly.
Use of medications with anticholinergic and sedative properties is associated with functional impairments in older people.
Exposure to anticholinergic and sedative medications, measured with Drug Burden Index that includes the principles of dose–response and maximal effect, was associated with impairment in physical and cognitive function in two studies of older people in the USA.
WHAT THIS STUDY ADDS
We evaluated Drug Burden Index in an Australian population of community-dwelling older men, The Concord Health and Ageing in Men Project that enrolled a random sample of community-dwelling men aged ≥70 years living in Sydney, Australia.
In this population, increasing Drug Burden Index was associated with objective impairments of physical performance and functional status.
The Drug Burden Index has broad applicability regardless of healthcare system, prescribing practices, gender or country.
Introduction
Age-associated disability and functional impairment limit independent living and quality of life of older adults [1]. Although clinical trials show that many medications are effective for the management of age-related illnesses in clinical trial subjects, they may also adversely affect function of real-life older people with multiple comorbidities and polypharmacy. Age-related changes in pharmacokinetics and pharmacodynamics, the presence of multiple medical conditions and the increased prevalence of polypharmacy contribute to an increased likelihood of drug interactions and adverse drug reactions in older people [2–4]. Inappropriate medication use in older people is commonly determined by expert consensus such as the modified Beers' Criteria, which include many medications with anticholinergic and sedative properties [5].
Exposure to medications defined by Beers' criteria has been associated with adverse healthcare outcomes in the community-dwelling elderly [6], but not with health outcomes in hospitalised older people [7] or with deficits in functional status in community-dwelling older people [8]. A number of studies have found associations between medications and the risk of falling in older people [9]. Benzodiazepine use is associated with increased risk of hip fracture [10] and impaired physical performance measures [11]. Exposure to drugs with anticholinergic effects measured with a variety of methods is associated with impaired measures of cognitive performance [12, 13], physical performance and functional status [14–16]. Objective measures of physical functioning are important outcomes for older people, and predict subsequent disability, nursing home admission, death and hospitalisation [17, 18].
The Drug Burden Index is a recently developed tool that calculates exposure to medications with anticholinergic or sedative effects. It is based on the principles of cumulative exposure and dose–response [19]. The Drug Burden Index is a linear additive model that incorporates principles of pharmacokinetics and pharmacodynamics to describe total exposure to medications with anticholinergic and sedative effects across drug classes. The Drug Burden Index has been independently associated with poorer performance in tests of physical and cognitive function in a population of well-functioning community-dwelling older people in the USA, aged 70–79 years [19]. Similar associations between the burden of anticholinergic or sedative drugs and poorer physical and cognitive function were observed in a population of frail older women enrolled in the Women's Health and Aging Study (WHAS). These associations were independent of sociodemographic factors and physical and cognitive comorbidities [20].
An evidence-based and easy to use prescribing tool could improve the quality use of medicines in the older population. Investigating the association of medication exposure with functional outcomes in older adults in different countries with different prescribing practices and different access to healthcare and medications is essential to test the robustness of such a tool. The aim of this study was to evaluate the association between the Drug Burden Index and physical performance and functional status measures in a random sample of community-dwelling older men, aged ≥70 years, enrolled in the Concord Health and Ageing in Men Project (CHAMP), Sydney, Australia [21].
Methods
Study population
CHAMP is a longitudinal study of health and ageing in community-dwelling men, aged ≥70 years, living in a defined geographical region (the Local Government Areas of Burwood, Canada Bay and Strathfield surrounding Concord RG Hospital) in Sydney, Australia [21]. The study was approved by the Sydney South West Area Health Service Human Research Ethics Committee Concord Repatriation General Hospital. All participants gave written informed consent. The sampling frame was the New South Wales Electoral Roll. The only exclusion criterion was living in a residential aged care facility. Eligible men in the study area were sent a letter describing the study and, if they had a listed telephone number, were telephoned about 1 week later. Men without listed telephone numbers who did not respond to the first letter were sent a second invitation letter. Baseline data were collected between January 2005 and June 2007. Invitation letters were sent to 3627 men and contact was made with 3005. One hundred and ninety of the contacted men were not eligible for the study because they had moved out of the study area, moved into a nursing home, or had died. Of the 2815 eligible men with whom contact was made, 1511 participated (54%). An additional 194 men aged ≥70 years who lived in the study area volunteered to be in the study independently of the invitation letter system. All participants completed a questionnaire at home before coming to the study clinic. The study questionnaire included questions on sociodemographic factors, medical history, anxiety, depression and physical activity. During the clinic visit objective measures of cognition, depression, muscle strength, gait and balance were obtained.
Medication exposure
Information about medication use was collected during the clinic visit. Participants were instructed to bring all prescription and over-the-counter medications with them to the clinic visit. They were asked whether they had taken any medication either prescribed by their doctor or obtained over the counter during the past month. Details of all medications, including the name, dose, frequency, duration and prescription pattern (regular or as required), were gathered. The Iowa Drug Information Service drug code numbers were used to code and classify the medication data.
Use of sedative and anticholinergic medications was quantified using the Drug Burden Index, a measure of a person's exposure to medications with anticholinergic and sedative properties that incorporates the principles of dose–response and maximal effect [19]. Drug burden (DB) was calculated using the equation:
where BAC indicates the burden from drugs with anticholinergic effects and BS that from drugs with sedative effects. The Drug Burden Index for each drug with anticholinergic or sedative effect was calculated using the following equation:
 |
where D is the daily dose taken by the subject and δ is the recommended minimum dose registered by the Therapeutic Goods Administration of Australia. The minimum recommended daily dose is used as an estimate of the dose required to achieve 50% of the maximum of anticholinergic or sedative effect (DR50). The registered product information, obtained from Medicare Information Management System, was used to define medications with clinically significant anticholinergic and/or sedating effects [22]. Medications with both anticholinergic and sedative effects were classified as anticholinergic. Complementary medications were excluded from the Drug Burden Index calculations.
Outcome measures
Physical performance was assessed by administering the performance battery, a modification of the Established Populations for Epidemiological Studies of the Elderly summary performance score. This is similar to the Health Aging and Body Composition (ABC) score [23]. The Established Populations for Epidemiological Studies of the Elderly summary performance scale is a useful marker for predicting disability in older people over time as well as nursing home admission and mortality [24]. The battery incorporated four tasks: (i) chair stands test – time (seconds) needed to complete five chair stands; (ii) walk speed (m/s) over a 6-m course; (iii) 20-cm narrow walk speed (m/s) over a 6-m course; and (iv) balance tests. Balance was assessed by administering the balance sway meter test, which involves performing the following for 30 s each: floor sway test, foam sway test and race track test [25]. Participants who were unable to complete the balance sway meter test were given a score of 0. Participants scored 1 if they were able to hold the floor sway test but not the foam and the race track test; a score of 2 was given if they held the floor and foam sway positions and a score of 3 if they held all three positions.
Muscle strength was assessed by hand grip strength using a dynamometer. The score was calculated as the grip strength (kg) of the dominant hand (best of two trials) with higher number indicating better function. Hand grip strength has been associated with functional disabilities in older people [26] and as a component of the frailty phenotype [27]. Functional status was measured with the Instrumental Activities of Daily Living (IADLs) scale [28]. For IADLs scale, the score ranges from 0 to 7, with higher scores indicating poorer functional status.
Covariates
Age (continuous), secondary education (completed or not), body mass index (BMI, continuous), history of falls during the previous year (yes or no), cognitive impairment, depression and comorbidities were treated as covariates. The Mini-Mental State Examination (MMSE) score ≤26 was used to screen for cognitive impairment [29]. The presence of depression was defined by a Geriatric Depression Scale (GDS) score of ≥5 [30]. The comorbidity score (continuous) was calculated by summing the presence of the following self-reported conditions: diabetes mellitus, osteoporosis, Parkinson's disease, epilepsy, arthritis, hypertension, stroke, lung disease, cardiac disease, peripheral arterial disease and cancer (excluding skin cancer). Diseases included in this comorbidity score were based on those in the Functional Comorbidity Index, which is associated with physical function in older people [31].
Statistical analysis
Descriptive analyses were generated. Analyses of covariance were used to compare functional outcomes between subjects with Drug Burden Index of zero and subjects with Drug Burden Index greater than zero, adjusting for the effects of age, education, BMI, history of falls, medical conditions, cognitive impairment and depression. Analyses of covariance were also performed to compare the adjusted means (adjusted for the effects of age, education, BMI, history of falls, medical conditions, cognitive impairment and depression) of each of the functional outcomes between subjects exposed to three different levels of Drug Burden Index ranges: 0, 0–1, ≥1. In order to test the Drug Burden Index as a continuous variable, multiple linear regression analyses on subjects with the Drug Burden Index greater than zero (n= 447) were used to evaluate the association between the Drug Burden Index (continuous) and functional outcomes controlling for the effects of age, education, BMI, history of falls, presence of self-reported medical conditions, cognitive impairment and depression. All analyses were performed with SAS statistical software (version 9.1; SAS Institute, Cary, NC, USA). All tests were two tailed. Statistical significance was set at P < 0.05.
Results
The CHAMP study consisted of 1705 men. The mean age (±SD) of participants was 76.9 ± 5.5 years (range 70–97), 50% of whom were born in Australia and 56% had completed secondary school (Table 1). Of the 1705 study participants, 1527 (90%) reported taking medications, 130 reported no medication use and 39 reported taking over-the-counter medications only. Information on medication exposure was missing for nine subjects. Of the 1527 subjects known to be taking medications, 21% were exposed to anticholinergic and 13% to sedative drugs. Of study subjects who reported taking an anticholinergic and/or sedative drug, 46 (3%) did not have daily dose and/or frequency recorded. For Drug Burden Index calculations, these individuals were included using the median dose for the study population in calculations.
Table 1.
Baseline characteristics of CHAMP study population (n= 1705)
| Characteristics | Value ± SD* |
|---|---|
| Age (years) | 76.9 ± 5.5 |
| Marital status, % | |
| Married | 76.8 |
| Widowed | 12.9 |
| Never married | 5.0 |
| Country of birth, % | |
| Australia | 49.8 |
| Italy | 19.5 |
| Great Britain | 4.6 |
| Completed secondary school, % | 55.5 |
| Comorbidity score | 1.0 ± 0.9 |
| BMI (kg m−2) | 27.8 ± 4.0 |
| Depression, % | 12.8 |
| Cognitive impairment, % | 31.5 |
| History of falls, % | 43.1 |
| 6 m walking speed, m/s | 0.9 ± 0.2 |
| 20 cm narrow 6 m walking speed, m s−1 | 0.8 ± 0.2 |
| Chair stands, s | 16.7 ± 6.0 |
| Balance score | 2.8 ± 0.7 |
| Grip strength, kg | 34.3 ± 7.6 |
| IADL score | 0.8 ± 1.5 |
| Number of medications | 4.4 ± 2.7 |
| Number of medications (excluding those with anticholinergic and sedative effects) | 4.0 ± 2.4 |
| Medication exposure, (% subjects) | 89.6 |
| Exposed to anticholinergic medications, % | 20.7 |
| Exposed to sedative medications, % | 13.4 |
| As needed (prn) exposure to anticholinergic and sedative medications, % | 3.1 |
| DBI | 0.18 ± 0.35 |
| DBA | 0.11 ± 0.24 |
| DBS | 0.07 ± 0.22 |
Data are given as means ± SD or percentages. CHAMP, Concord Health and Ageing in Men Project; BMI, body mass index; DBI, Drug Burden Index; DBA, anticholinergic component of Drug Burden Index; DBS, sedative component of Drug Burden Index; IADL, Instrumental Activities of Daily Living.
The average Drug Burden Index in the study population was 0.18 ± 0.35. The mean anticholinergic and sedative components of the Drug Burden Index were 0.11 ± 0.24 and 0.07 ± 0.22, respectively. With respect to anticholinergic drug exposure, the most frequently used drugs were antidepressants [excluding selective serotonin reuptake inhibitors (SSRIs)], sympathomimetics and parasympathomimetics (Table 2). The most frequently reported drugs with sedative effects included SSRIs and anxiolytic drugs. On univariate analysis exposure to all anticholinergic and sedative drug groups except the antihistamines and sympathomimetics were significantly associated with at least one functional outcome (Table 3). The association with functional outcomes was the strongest for anticonvulsant, dopaminergic, spasmolytic and opioid drugs.
Table 2.
Exposure of study participants to medications with anticholinergic and sedating effects
| Drug group | Number exposed (%) |
|---|---|
| Anticholinergics | |
| Antiemetics | 7 (0.4) |
| Antipsychotics | 10 (0.6) |
| Antihistamines | 22 (1.3) |
| Spasmolytics | 26 (1.5) |
| Cardiovascular | 32 (1.9) |
| Parasympathomimetics | 85 (5.0) |
| Antidepressants other than SSRIs | 90 (5.3) |
| Sympathomimetics | 90 (5.3) |
| Sedatives | |
| Dopaminergics | 32 (1.9) |
| Anticonvulsants | 36 (2.1) |
| Opioids | 39 (2.3) |
| Antidepressants-SSRIs | 43 (2.5) |
| Anxiolytics | 77 (4.5) |
n represents the number of study participants taking at least one of the anticholinergic and/or sedative medications. SSRIs, selective serotonin reuptake inhibitors.
Table 3.
Univariate analysis for the association of physical performance, grip strength and functional status with the burden of medications with anticholinergic and/or sedating effects
| Parameter estimate (B) | ||||||
|---|---|---|---|---|---|---|
| Chair Stands | Walking speed | Narrow walk | Balance | Grip strength | IADL score | |
| n= 1548 | n= 1616 | n= 1589 | n= 1613 | n= 1595 | n= 1678 | |
| Anticholinergics | ||||||
| Antiemetics | 0.36 | −0.23 | −0.38 | 0.64 | −1.26 | 5.12** |
| Antipsychotics | −0.02 | −0.08 | −0.04 | −0.54 | −8.72 | 2.90** |
| Antihistamines | 2.06 | −0.03 | −0.01 | 0.01 | −3.82 | 0.25 |
| Spasmolytics | 3.16 | −0.20*** | −0.15 | −1.34** | −9.75** | 1.56*** |
| Cardiovascular | 4.30*** | −0.19** | −0.11 | −0.29 | −5.37*** | 0.39 |
| Parasympathomimetics | 1.17 | −0.10*** | −0.09 | −0.28 | −3.72*** | 0.93** |
| Antidepressants other than SSRIs | 1.36 | −0.07 | −0.06 | −0.27 | −2.80 | 0.86** |
| Sympathomimetics | −0.01 | −0.02 | 0.00 | 0.16 | −0.64 | −0.07 |
| Sedatives | ||||||
| Dopaminergics | 2.29 | −0.11*** | −0.16** | −0.83* | −1.04 | 1.62* |
| Anticonvulsants | 6.29*** | −0.36* | −0.36** | −0.91** | −8.22** | 1.67** |
| Opioids | 2.37 | −0.17*** | −0.18*** | −0.89* | −5.29*** | 1.02*** |
| Antidepressants-SSRIs | 3.21 | −0.04 | −0.19** | −0.31 | 0.90 | 1.16** |
| Anxiolytics | 1.68 | −0.10*** | −0.13** | −0.20 | −2.13 | 0.60*** |
IADL, Instrumental Activities of Daily Living; IADL score – higher score indicates worse function, balance test, 0-worst performance, 3-best performance; grip strength – higher number indicates better function; chair stands, 6 m walking speed and 20 cm narrow walking speed – lower number indicates better performance;
<0.05;
<0.01
<0.0001; only significant P-values shown; SD, standard deviation; SSRIs, selective serotonin reuptake inhibitors; n represents number of subjects completing each test.
Unadjusted and adjusted models comparing each of the functional outcomes between subjects with Drug Burden Index of zero and subjects with Drug Burden Index greater than zero are shown in Table 4. These associations are also shown for the anticholinergic and sedative components of drug burden (Table 4). In the unadjusted models, there was a strong relationship between Drug Burden Index (>0) and each of the functional measures. On univariate analysis, Drug Burden Index (>0) was associated with difficulty in rising (P < 0.01), slow walking speed (P < 0.0001), slow narrow walk speed (P < 0.0001), balance difficulty (P < 0.0001), grip weakness (P < 0.0001) and difficulties in IADLs (P < 0.0001). With respect to anticholinergic and sedative components of the Drug Burden Index, association with physical performance measures, grip strength and IADLs was stronger for the sedative than for the anticholinergic component of Drug Burden Index.
Table 4.
Analysis of covariance to compare physical performance, grip strength and functional status measures with the exposure to the anticholinergic and sedative medications
| DBI > 0 | DBA > 0 | DBS > 0 | ||||
|---|---|---|---|---|---|---|
| B (95% CI) | n | B (95% CI) | n | B (95% CI) | n | |
| Chair stands | ||||||
| n= 1548 | 1.25** (0.58, 1.93) | 395 | 0.90*** (0.14, 1.66) | 283 | 1.71** (0.78, 2.65) | 172 |
| n= 1423 | 0.58 (−0.11, 1.27) | 364 | 0.35 (−0.43, 1.12) | 262 | 0.92 (−0.05, 1.88) | 160 |
| Walking speed | ||||||
| n= 1616 | −0.05* (−0.07, −0.03) | 410 | −0.04** (−0.07, −0.02) | 294 | −0.08* (−0.11, −0.05) | 182 |
| n= 1484 | −0.03*** (−0.05, −0.00) | 379 | −0.02 (−0.05, 0.01) | 273 | −0.05** (−0.08, −02) | 170 |
| Narrow walk | ||||||
| n= 1589 | −0.06* (−0.08, −0.03) | 404 | −0.04*** (−0.07, −0.01) | 290 | −0.12* (−0.15, −0.08) | 178 |
| n= 1461 | −0.03*** (−0.05, −0.01) | 374 | −0.01 (−0.04, 0.02) | 269 | −0.09* (−0.12, −0.06) | 168 |
| Balance | ||||||
| n= 1613 | −0.18* (−0.26, −0.10) | 420 | −0.11*** (−0.20, −0.02) | 298 | −0.32* (−0.42, −0.21) | 190 |
| n= 1468 | −0.11** (−0.18, −0.03) | 384 | −0.03 (−0.11, 0.05) | 273 | −0.20* (−0.31, −0.10) | 175 |
| Grip strength | ||||||
| n= 1595 | −2.08* (−2.93, −1.24) | 412 | −1.99* (−2.95, −1.03) | 290 | −2.28** (−3.42, −1.13) | 189 |
| n= 1458 | −1.09** (−1.90, −0.28) | 381 | −1.08*** (−1.99, −0.17) | 270 | −1.10*** (−2.20, −0.00) | 175 |
| IADL | ||||||
| n= 1678 | 0.46* (0.30, 0.62) | 444 | 0.39* (0.21, 0.57) | 315 | 0.71* (0.49, 0.92) | 203 |
| n= 1541 | 0.18*** (0.04, 0.32) | 407 | 0.13 (−0.03, 0.29) | 289 | 0.36** (0.17, 0.56) | 188 |
For each end-point the first row represents unadjusted model and second row adjusted model for age, education, comorbidities, body mass index, history of falls, cognitive impairment and depression; B, parameter estimate; CI, confidence interval;
<0.05;
<0.01;
<0.0001; only significant P-values shown; DBI, Drug Burden Index; DBA, anticholinergic component of Drug Burden Index; DBS, sedative component of Drug Burden Index; n, number exposed to anticholinergic and/or sedative drugs; IADL, Instrumental Activities of Daily Living; IADL score – higher score indicates worse function; balance test, 0-worst performance, 3-best performance; grip strength – higher number indicates better function; chair stands, 6 m walking speed and 20 cm narrow walking speed – lower number indicates better performance; n first row, number of subjects completing each test; n second row represents available data on subjects after controlling for confounders.
After adjusting for confounders (age, education, BMI, history of falls, medical conditions, cognitive impairment and depression) Drug Burden Index (>0) was associated with slower walking speed (P < 0.05), slower narrow walk speed (P < 0.05), balance difficulty (P < 0.01), grip weakness (P < 0.01) and poorer performance on IADLs (P < 0.05) (Table 4) (Figure 1). The association of Drug Burden Index (>0) with the chair stands test was no longer significant after adjusting for confounders.
Figure 1.
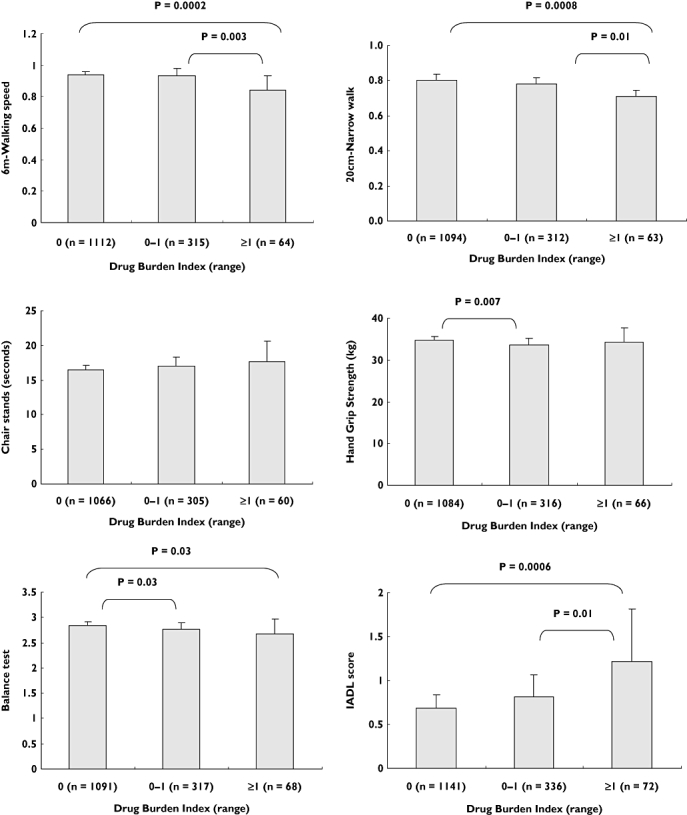
Analysis of covariance for the association of adjusted means of physical performance measures, grip strength and functional status with increasing Drug Burden Index. Means adjusted for age, education, comorbidities, body mass index (BMI), history of falls, cognitive impairment and depression. Drug Burden Index grouped into three intervals (0, 0–1, ≥1). Error bars show 95% confidence interval (CI). IADL, Instrumental activities of daily living; IADL score – higher score indicates worse function, balance test, 0-worst performance, 3-best performance; grip strength – higher number indicates better function; chair stands, 6 m walking speed and 20-cm narrow walking speed, lower number indicates better performance
Sedative drug burden was significantly associated with slow walking speed (P < 0.01), slow narrow walk speed (P < 0.0001), balance difficulty (P < 0.0001), grip weakness (P < 0.05) and poorer performance on IADLs (P < 0.01), after adjusting for confounders. After controlling for confounders, the relationship of sedative drug burden with the chair stands test was no longer significant. After controlling for confounders, anticholinergic drug burden exposure was significantly associated with grip weakness (P < 0.05) only. The associations between anticholinergic drug burden and the chair stands test, walking speed, narrow walk speed, balance and IADLs were not significant after adjusting for the effects of confounding factors.
On subgroup analysis of participants with a Drug Burden Index greater than zero (n= 447), after controlling for confounders, increasing Drug Burden Index was associated with slower walking speed (P < 0.01), slower narrow walk speed (P < 0.01) and difficulties in IADLs (P < 0.01) (data not shown). On this subgroup analysis, increasing Drug Burden Index was not independently associated with difficulty in rising, balance difficulty or grip weakness.
Discussion
Exposure to medications with anticholinergic and sedative effects measured with the Drug Burden Index was independently associated with poorer physical performance measures and functional status in the community-dwelling older Australian men enrolled in the CHAMP study. Furthermore, in this study population, after adjusting for confounders, sedative drug burden was associated with poorer physical performance and impairment of IADLs, whereas increasing anticholinergic drug burden was weakly associated with grip weakness only. The association between higher Drug Burden Index and impairment in function has been observed in older adults in the USA [19, 20]. With respect to different components of the Drug Burden Index, in the Health ABC Study, both anticholinergic and sedative drug burden exposure were associated with decline in physical function [19], whereas in the WHAS of frail older women, association with poorer physical function was stronger for the anticholinergic than for the sedative component [20].
The established pharmacological effects of medications with anticholinergic and sedative effects obviously may explain their effects on function in older people. Adverse effects of anticholinergic drugs include dry mouth, gastrointestinal effects (e.g. constipation, decreased peristalsis), ophthalmological effects (e.g. ocular dryness, inability to accommodate vision) [32] and neuropsychological impairments, such as memory deficits, confusion, disorientation, agitation, hallucinations and delirium. Drugs with sedative effects also cause central nervous adverse effects, including sedation, memory and psychomotor performance impairment, and impairment of neuromuscular processing related to balance control [33].
Differences in the classes of anticholinergic drugs between the CHAMP study and previously reported studies might explain the inconsistent findings with relation to the anticholinergic and sedative components of drug burden. In our study the most frequently used anticholinergic drugs were sympathomimetics (5%) and antidepressants (excluding SSRIs) (5%). In the WHAS population the most common drug classes used were antihistamines, tricyclic antidepressants, benzodiazepines and narcotic analgesics. In comparison, antihistamine use in the CHAMP population was reported by only 22 study participants (1%). Furthermore, the CHAMP study population included older men only, which may explain different results from studies including women, as there are sex differences with respect to the medication exposure [34], the pharmacokinetics and pharmacodynamics of different drugs [35] and function [36].
Observation of a large veteran population in the USA [34] found that older men (23%) are less likely to use inappropriate medications such as analgesic, psychotropic and anticholinergic medications than older women (31%). There is evidence of sex differences in the pharmacokinetics of some anticholinergic [37] and sedative drugs [38]. With regards to functional status into extreme old age, men have been reported to maintain better physical and cognitive function than women [36]. End-points from the CHAMP study such as grip strength, walking speed and IADLs are markers of frailty status in older men [39], and the association of Drug Burden Index with these end-points was not evaluated in the Health ABC population. The Health ABC analysis tested the association between Drug Burden Index and a continuous composite score as a measure of overall physical performance.
Regardless of the observed differences with respect to sedative and anticholinergic components of the drug burden, the relationship between the higher Drug Burden Index and impairment in functional performance was observed across populations of older adults with different healthcare access, prescribing practices and medication exposure. This association is further strengthened as the load of the anticholinergic and sedative medicines was quantified based on the principles of cumulative exposure and dose–response pharmacological principles, rather than the total number of drugs prescribed [40]. In relation to the magnitude of meaningful change, on the subgroup analysis of participants with a Drug Burden Index greater than zero one unit increase in Drug Burden Index was significantly associated with decrease in walking speed by 0.07 m/s, which is a clinically important change [41].
The CHAMP population represents a large random sample of community-dwelling older Australian men. Objective and clinically validated physical performance measures were used in this study. Information on medication exposure was obtained by checking all medications brought to the clinic by the study subjects rather than from interview-based self-reports. There are several limitations to this study. With respect to the drug burden calculations, the minimum recommended daily dose was used as an estimate of the dose needed to achieve 50% of the maximum effect. The accuracy of the estimate of minimum efficacious dose may differ among drugs and study subjects with different pharmacokinetic and pharmacodynamic characteristics. The possibility of misclassification bias should be considered when evaluating the effects of anticholinergic and sedative drugs, as some drug classes have both anticholinergic and sedative effects. Furthermore, the functional impairment associated with these drugs may be due to activity beyond their anticholinergic and sedative effects. This study was conducted in older men only, living in a single geographical region. Hence, these findings should not be generalized to older women. Regarding the functional outcome assessments, information bias in interviewing and reporting IADL difficulties should be taken into account. The possibility of temporal and indication bias should be also considered, as it may compromise the reliability and validity of the outcome data. Each analysis was limited to those participants in whom relevant functional outcomes and cognition data were available. The accuracy of the MMSE as a screening test for dementia may vary between men born in non-English speaking countries (46.5% of this study population) and those born in English speaking countries.
In summary, the use of anticholinergic and sedative drugs was commonly reported by the community-dwelling older Australian men participating in the CHAMP study. The average Drug Burden Index in the CHAMP study population was very similar to that of community-dwelling older people in the USA. Higher Drug Burden Index was associated with impaired objective physical performance measures and IADLs in the CHAMP population. An electronic calculator that feeds into prescribing software could be implemented to inform prescribers about Drug Burden Index and to estimate the impact of medicines on an older patient's function. The Drug Burden Index appears to be a valid tool for predicting medication-related impairment of function in older people, regardless of the healthcare system, prescribing practices, gender or country. Optimising use of medications with anticholinergic and sedative effects using an assessment tool such as the Drug Burden Index may reduce functional decline and disability among older adults. Further interventional studies are required to assess whether the association between increasing Drug Burden Index and impaired function is causative, and whether reductions in Drug Burden Index result in improvements in function in older adults.
Acknowledgments
This study was supported by National Health and Medical Research Council of Australia, Ageing and Alzheimer's Research Foundation, and Geoff and Elaine Penney Ageing Research Unit.
Competing interests
D.R.A. and S.N.H. hold an international patent for the Drug Burden Index with Dr D.E. Mager.
REFERENCES
- 1.Fried TR, Bradley EH, Williams CS, Tinetti ME. Functional disability and health care expenditures for older persons. Arch Intern Med. 2001;161:2602–7. doi: 10.1001/archinte.161.21.2602. [DOI] [PubMed] [Google Scholar]
- 2.Shi S, Morike K, Klotz U. The clinical implications of ageing for rational drug therapy. Eur J Clin Pharmacol. 2008;64:183–99. doi: 10.1007/s00228-007-0422-1. [DOI] [PubMed] [Google Scholar]
- 3.Hilmer SN, McLachlan AJ, Le Couteur DG. Clinical pharmacology in the geriatric patient. Fundam Clin Pharmacol. 2007;21:217–30. doi: 10.1111/j.1472-8206.2007.00473.x. [DOI] [PubMed] [Google Scholar]
- 4.McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev. 2004;56:163–84. doi: 10.1124/pr.56.2.4. [DOI] [PubMed] [Google Scholar]
- 5.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–24. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 6.Laroche ML, Charmes JP, Nouaille Y, Picard N, Merle L. Is inappropriate medication use a major cause of adverse drug reactions in the elderly? Br J Clin Pharmacol. 2007;63:177–86. doi: 10.1111/j.1365-2125.2006.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onder G, Landi F, Liperoti R, Fialova D, Gambassi G, Bernabei R. Impact of inappropriate drug use among hospitalized older adults. Eur J Clin Pharmacol. 2005;61:453–9. doi: 10.1007/s00228-005-0928-3. [DOI] [PubMed] [Google Scholar]
- 8.Hanlon JT, Fillenbaum GG, Kuchibhatla M, Artz MB, Boult C, Gross CR, Garrard J, Schmader KE. Impact of inappropriate drug use on mortality and functional status in representative community dwelling elders. Med Care. 2002;40:166–76. doi: 10.1097/00005650-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc. 1999;47:30–9. doi: 10.1111/j.1532-5415.1999.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 10.Cumming RG, Le Couteur DG. Benzodiazepines and risk of hip fractures in older people: a review of the evidence. CNS Drugs. 2003;17:825–37. doi: 10.2165/00023210-200317110-00004. [DOI] [PubMed] [Google Scholar]
- 11.Gray SL, LaCroix AZ, Hanlon JT, Penninx BW, Blough DK, Leveille SG, Artz MB, Guralnik JM, Buchner DM. Benzodiazepine use and physical disability in community-dwelling older adults. J Am Geriatr Soc. 2006;54:224–30. doi: 10.1111/j.1532-5415.2005.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lechevallier-Michel N, Molimard M, Dartigues JF, Fabrigoule C, Fourrier-Reglat A. Drugs with anticholinergic properties and cognitive performance in the elderly: results from the PAQUID Study. Br J Clin Pharmacol. 2005;59:143–51. doi: 10.1111/j.1365-2125.2004.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulsant BH, Pollock BG, Kirshner M, Shen C, Dodge H, Ganguli M. Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry. 2003;60:198–203. doi: 10.1001/archpsyc.60.2.198. [DOI] [PubMed] [Google Scholar]
- 14.Landi F, Russo A, Liperoti R, Cesari M, Barillaro C, Pahor M, Bernabei R, Onder G. Anticholinergic drugs and physical function among frail elderly population. Clin Pharmacol Ther. 2007;81:235–41. doi: 10.1038/sj.clpt.6100035. [DOI] [PubMed] [Google Scholar]
- 15.Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc. 2008;56:2203–10. doi: 10.1111/j.1532-5415.2008.02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nebes RD, Pollock BG, Halligan EM, Kirshner MA, Houck PR. Serum anticholinergic activity and motor performance in elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62:83–5. doi: 10.1093/gerona/62.1.83. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 18.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–7. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 19.Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham BG, Harris TB, Hanlon JT, Rubin SM, Shorr RI, Bauer DC, Abernethy DR. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167:781–7. doi: 10.1001/archinte.167.8.781. [DOI] [PubMed] [Google Scholar]
- 20.Cao YJ, Mager DE, Simonsick EM, Hilmer SN, Ling SM, Windham BG, Crentsil V, Yasar S, Fried LP, Abernethy DR. Physical and cognitive performance and burden of anticholinergics, sedatives, and ACE inhibitors in older women. Clin Pharmacol Ther. 2008;83:422–9. doi: 10.1038/sj.clpt.6100303. [DOI] [PubMed] [Google Scholar]
- 21.Cumming RG, Handelsman D, Seibel MJ, Creasey H, Sambrook P, Waite L, Naganathan V, Couteur DL, Litchfield M. Cohort profile: the Concord Health and Ageing in Men Project (CHAMP) Int J Epidemiol. 2008;38:374–8. doi: 10.1093/ije/dyn071. [DOI] [PubMed] [Google Scholar]
- 22.Donohoo E. MIMS Annual 2007. Sydney: CMPMedica Australia; 2007. [Google Scholar]
- 23.Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, Harris T. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–9. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lord SR, Tiedemann A, Chapman K, Munro B, Murray SM, Gerontology M, Ther GR, Sherrington C. The effect of an individualized fall prevention program on fall risk and falls in older people: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:1296–304. doi: 10.1111/j.1532-5415.2005.53425.x. [DOI] [PubMed] [Google Scholar]
- 26.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–60. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 27.Walston J, Fried LP. Frailty and the older man. Med Clin North Am. 1999;83:1173–94. doi: 10.1016/s0025-7125(05)70157-7. [DOI] [PubMed] [Google Scholar]
- 28.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 31.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Feinberg M. The problems of anticholinergic adverse effects in older patients. Drugs Aging. 1993;3:335–48. doi: 10.2165/00002512-199303040-00004. [DOI] [PubMed] [Google Scholar]
- 33.Cutson TM, Gray SL, Hughes MA, Carson SW, Hanlon JT. Effect of a single dose of diazepam on balance measures in older people. J Am Geriatr Soc. 1997;45:435–40. doi: 10.1111/j.1532-5415.1997.tb05167.x. [DOI] [PubMed] [Google Scholar]
- 34.Bierman AS, Pugh MJ, Dhalla I, Amuan M, Fincke BG, Rosen A, Berlowitz DR. Sex differences in inappropriate prescribing among elderly veterans. Am J Geriatr Pharmacother. 2007;5:147–61. doi: 10.1016/j.amjopharm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz JB. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther. 2007;82:87–96. doi: 10.1038/sj.clpt.6100226. [DOI] [PubMed] [Google Scholar]
- 36.Terry DF, Sebastiani P, Andersen SL, Perls TT. Disentangling the roles of disability and morbidity in survival to exceptional old age. Arch Intern Med. 2008;168:277–83. doi: 10.1001/archinternmed.2007.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauri MC, Volonteri LS, Colasanti A, Fiorentini A, De Gaspari IF, Bareggi SR. Clinical pharmacokinetics of atypical antipsychotics: a critical review of the relationship between plasma concentrations and clinical response. Clin Pharmacokinet. 2007;46:359–88. doi: 10.2165/00003088-200746050-00001. [DOI] [PubMed] [Google Scholar]
- 38.Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004;8:413–25. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, Barrett-Connor E, Orwoll ES. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–23. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 40.Agostini JV, Han L, Tinetti ME. The relationship between number of medications and weight loss or impaired balance in older adults. J Am Geriatr Soc. 2004;52:1719–23. doi: 10.1111/j.1532-5415.2004.52467.x. [DOI] [PubMed] [Google Scholar]
- 41.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–9. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]


