Abstract
AIM
To investigate the pharmacokinetic interaction between darunavir/ritonavir (DRV/r) and nevirapine (NVP) in 19 HIV-infected patients.
METHODS
An open-label, randomized, crossover study. Patients received Treatment A [NVP 200 mg b.i.d. plus ≥2 nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs)] and Treatment B [A plus DRV/r 300/100 mg b.i.d. (DRV oral solution)] or Treatment B2 [A plus DRV/r 400/100 mg b.i.d. (DRV tablet)] in two 14-day sessions.
RESULTS
Mean NVP AUC12h increased by 27% [least square means ratio 1.27 (95% confidence interval 1.02, 1.58)]. Mean DRV and ritonavir exposures were similar to historical data. Co-administration was well tolerated.
CONCLUSIONS
DRV/r and NVP have no clinically relevant interaction. No dose adjustments are required.
Keywords: darunavir, nevirapine, pharmacokinetic interactions, ritonavir
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The protease inhibitor (PI) darunavir with low-dose ritonavir (DRV/r) and the non-nucleoside reverse transcriptase inhibitor (NNRTI) nevirapine (NVP) are used in combination with other antiretroviral agents and they may be co-administered for the treatment of HIV-1 infection.
There is the potential for a pharmacokinetic interaction between NVP and DRV/r, since these drugs use similar metabolic pathways.
WHAT THIS STUDY ADDS
This study assesses for the first time the extent of the drug–drug interaction between NVP and DRV/r in the relevant population of HIV-1-infected patients.
Darunavir (DRV, TMC114) co-administered with low-dose ritonavir (RTV) as DRV/r 600/100 mg b.i.d. is indicated for the treatment of HIV-1-infected, treatment-experienced adult patients [1]; once-daily DRV/r 800/100 mg has recently received approval in the USA in treatment-naive patients [1]. Because DRV/r may be co-administered with nevirapine (NVP) in HIV-infected patients and NVP, DRV and RTV are substrates of CYP3A and use similar metabolic pathways [2–4], the potential pharmacokinetic interaction of NVP and DRV/r was investigated in the present study.
Methods
This open-label, randomized, crossover (two 14-day sessions) study was conducted in HIV-1-infected patients who were on a stable therapy of NVP 200 mg b.i.d. and ≥2 nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) for ≥16 weeks. Patients were randomized to receive Treatment A (NVP 200 mg b.i.d. and ≥2 NRTIs) in one session and Treatment B (NVP 200 mg b.i.d., ≥2 NRTIs and DRV/r 300/100 mg b.i.d. oral solution) or Treatment B2 (NVP 200 mg b.i.d., ≥2 NRTIs and DRV/r 400/100 mg b.i.d. tablets) in the other session. All treatments were administered for 13 days plus an additional morning dose on day 14. There was no wash-out period between the two sessions. Since an oral tablet formulation of DRV that was to replace the oral solution for further clinical development became available during the study, the interaction between NVP and DRV/r was studied using the DRV solution and tablet. Furthermore, a 300-mg tablet was not available at that time—there were 200-mg and 400-mg DRV tablets only. To allow comparison of the study results with historical pharmacokinetic data, the DRV/r dose regimen and formulation in Treatments B and B2 were the same as those used previously [5, 6].
All study medication was taken with food. On day 14, NVP and DRV/r had to be taken within 15 min after completing a standard breakfast, and full pharmacokinetic profiles of NVP, DRV and RTV were determined. Plasma samples were taken predose on days 1, 7, 12, 13 and 14, and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 9 and 12 h postdose on day 14. Drug plasma concentrations were determined by validated methods.
Adverse events (AEs) and laboratory abnormalities were assessed and graded according to the AIDS Clinical Trials Group severity scale.
Descriptive statistics were calculated for NVP, DRV and RTV plasma concentrations. The primary pharmacokinetic parameters were minimum (Cmin) and maximum (Cmax) plasma concentrations and area under the curve from time of administration to 12 h postdosing (AUC12h) on the logarithmic scale.
Statistical analyses compared Treatments B (test) and B2 (test) vs. A (reference) for NVP (cross-over comparison); Treatment B (test) for DRV and RTV vs. historical data [5] (reference); and Treatment B2 (test) for DRV and RTV vs. historical data [6] (reference). Only paired observations for the compared treatments were included in a statistical analysis of NVP. The least square (LS) means of the primary pharmacokinetic parameters for each treatment group were calculated using a linear mixed effects model. A 95% confidence interval (CI) was constructed around the difference between the LSmeans of test and reference treatment.
Written informed consent was obtained from all patients.
Results
Nineteen HIV-1-infected patients (74% male, 74% White) were randomized to four panels. Panel 1 (n = 7) received Treatment A followed by Treatment B; panel 2 (n = 4) received Treatment B followed by A; panel 3 (n = 4) received Treatment A followed by B2; and panel 4 (n = 4) received Treatment B2 followed by A. Patients had a median plasma viral load of 1.8 (1.7–3.0) log10 copies ml−1, median CD4+ cell count of 450 (105–974) × 106 cells l−1 and median age of 43 (33–56) years.
Sixteen patients received both treatment schedules; one discontinued during follow-up, resulting in 15 patients completing the study. One patient discontinued due to an unrelated serious adverse event (SAE), two withdrew consent and one discontinued due to noncompliance. All available samples were used.
Mean plasma concentration–time curves of NVP (Figure 1) were higher after administration of DRV/r (Treatment B or B2) and NVP plus NRTIs compared with NVP plus NRTIs alone (Treatment A). Mean pharmacokinetic parameters for NVP during the different treatments are shown in Table 1. Based on the LSmeans ratio, the mean exposure (AUC12h) of NVP was 27% higher when DRV/r (as tablet and as solution) was co-administered with NVP plus NRTIs. The mean NVP Cmin increased by 18% when co-administered with DRV/r 300/100 mg b.i.d. and by 47% when co-administered with DRV/r 400/100 mg b.i.d. The mean NVP Cmax increased slightly with DRV/r co-administration (14% and 18% with DRV solution and tablet, respectively).
Figure 1.
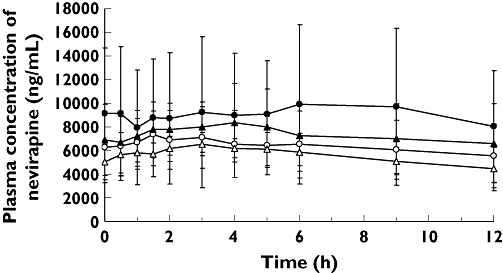
Mean plasma concentration–time curves of nevirapine after oral administration of nevirapine and nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) alone (Treatment A; per Panel) and in combination with darunavir with low-dose ritonavir (DRV/r) (Treatment B: 300/100 mg b.i.d. oral solution; Treatment B2: 400/100 mg b.i.d. tablets). Treatment A, P1/2 (—○—); Treatment A, P3/4 (—▵—); Treatment B, P1/2 (—•—); Treatment B2, P3/4 (—▴—)
Table 1.
Pharmacokinetic results of nevirapine after administration of NVP and NRTIs alone (Treatment A) and in combination with DRV/r (Treatments B and B2)
| Panel 1 + 2 | Panel 3 + 4 | |||
|---|---|---|---|---|
| Pharmacokinetic parameter Mean ± SD [tmax: median (range)] | NVP + NRTIs (Treatment A) | NVP + NRTIs + DRV/r 300/100 mg b.i.d. oral solution (Treatment B) | NVP + NRTIs (Treatment A) | NVP + NRTIs + DRV/r 400/100 mg b.i.d. tablets (Treatment B2) |
| n | 9 | 8 | 8 | 8 |
| AUC12h, ng h−1 ml−1 | 76 570 ± 29 948 | 109 749 ± 68 732 | 66 933 ± 14 441 | 88 148 ± 33 335 |
| Cmax, ng ml−1 | 8 064 ± 2 531 | 10 670 ± 6 579 | 7 005 ± 1 348 | 8 560 ± 3 178 |
| C0h, ng ml−1 | 6 273 ± 3 005 | 9 106 ± 5 557 | 5 031 ± 1 520 | 6 895 ± 3 020 |
| Cmin, ng ml−1 | 5 192 ± 2 137 | 7 031 ± 4 804 | 4 273 ± 1 614 | 6 220 ± 2 589 |
| tmax, h | 1.5 (0.0–12.0) | 4.5 (0.5–9.0) | 3.0 (0.5–6.0) | 4.0 (2.0–5.0) |
| LSmeans ratio (95% CI) | Treatment B vs. A | Treatment B2 vs. A | ||
| AUC12h, ng h ml−1 | – | 1.27 (1.02, 1.58) | – | 1.27 (1.09, 1.49) |
| Cmax, ng ml−1 | – | 1.14 (0.87, 1.50) | – | 1.18 (0.99, 1.42) |
| Cmin, ng ml−1 | – | 1.18 (0.87, 1.60) | – | 1.47 (1.14, 1.92) |
SD, standard deviation; tmax, time to maximum plasma concentration; NVP, nevirapine; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; DRV/r, darunavir with low-dose ritonavir; n, number of subjects; AUC12h, area under plasma concentration–time curve from time of intake to 12 h after dosing; Cmax, maximum plasma concentration; C0h, predose plasma concentration; Cmin, minimum plasma concentration; LS, least square; CI, confidence interval.
Mean DRV and RTV exposures were generally similar to those observed in previous DRV/r trials [5, 6]. Regardless of DRV formulation, the mean DRV AUC12h and Cmin were higher when DRV/r was co-administered with NVP plus NRTIs: 9% higher AUC12 and 23% higher Cmin with DRV solution; 24% higher AUC12 and 2% higher Cmin with DRV tablet. Furthermore, the mean DRV Cmax was 16% lower with DRV solution and 40% higher with DRV tablet.
Small changes were observed in the mean RTV AUC12h (16% decrease with Treatment B, 10% increase with Treatment B2), Cmax (14% decrease with Treatment B, 23% increase with Treatment B2) and Cmin (17% decrease with Treatment B, 2% decrease with Treatment B2) when DRV/r was co-administered with NVP and NRTIs.
Overall, co-administration of DRV/r and NVP plus NRTIs was generally well tolerated. One patient discontinued the study due to grade 3 subarachnoid haemorrhage, which was reported as an SAE but considered unrelated to treatment. No other AEs leading to discontinuation were reported. Fourteen patients (74%) reported ≥1 AE during the trial. AEs were more frequently reported during co-administration of NVP and DRV/r than with stable therapy with NVP plus NRTIs alone: 18% (three of 17 patients) during Treatment A, 64% (seven of 11 patients) during Treatment B, and 88% (seven of eight patients) during Treatment B2. Most AEs were grade 1 or 2. The most commonly reported AEs were diarrhoea (seven patients; 37%) and headache (five patients; 26%). Gastrointestinal disorders were only reported during treatment including DRV/r. Only one AE (diarrhoea during Treatment B) was considered as ‘very likely’ related to treatment. No liver-related AEs or clinically relevant changes in laboratory parameters were observed.
Discussion
The findings showed that, regardless of DRV formulation, the addition of DRV/r to a regimen of NVP and NRTIs in HIV-1-infected patients can increase NVP steady-state exposure (AUC12h). However, the magnitude of this change is not considered clinically relevant based on these findings coupled with data on the safety and efficacy of NVP plus DRV/r-containing regimens in HIV-infected patients in long-term controlled clinical trials [7, 8].
The presence of NVP did not seem to have a clinically relevant influence on the pharmacokinetics of DRV or RTV, because the exposures of both compounds were generally comparable to those observed in previous trials with DRV/r [5, 6].
Consistent with observations in the present study, available pharmacokinetic interaction data of NVP and protease inhibitors (PIs) show that, in most cases, no clinically relevant interactions occur when NVP and PIs are co-administered. For example, co-administration of NVP and unboosted saquinavir (SQV) in HIV-infected patients resulted in the statistically significant reduction of SQV Cmax by 29% (P = 0.049) and AUC by 27% (P = 0.03), which were less than the 30% change used to define a clinically significant interaction [9]. In the same study, SQV caused a statistically insignificant decrease (3%) in NVP AUC [9]. The addition of NVP to a regimen containing lopinavir with low-dose RTV (LPV/r) can decrease LPV exposure. Although the clinical relevance of this observation has not been fully established, a higher dose of LPV/r is recommended when combined with NVP in treatment-experienced patients [10, 11].
Safety assessments showed that co-administration of DRV/r and NVP plus ≥2 NRTIs was generally well tolerated.
The interaction between DRV/r and NVP has been studied at a dose lower than the recommended dosage for treatment-experienced adults (i.e. DRV/r 600/100 mg b.i.d.). However, based on the small increases in NVP exposure during co-administration with DRV/r 300/100 mg (oral solution) and 400/100 mg b.i.d. (tablet), and the lack of dose-proportionality in DRV pharmacokinetics between DRV/r 400/100 and 600/100 mg b.i.d. [12], a comparable small increase in NVP exposure is expected when NVP is combined with DRV/r 600/100 mg b.i.d. Therefore, the combination of NVP and DRV/r can be used without dose adjustments in HIV-1-infected patients.
Acknowledgments
This study was sponsored by Tibotec Pharmaceuticals Inc. The authors thank Iris Weimar for medical writing support.
Competing interests
V.S., E.L., M. DP, T.V. and R.M.W.H. are employees of Tibotec. A.P. has attended advisory boards, been reimbursed for symposia and consulted for Tibotec and Boehringer Ingelheim.
REFERENCES
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-infected Adults and Adolescents. Department of Health and Human Services; 2008. pp. 1–139. November 3, Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf (last accessed 17 April 2008. [Google Scholar]
- 2.Back D, Sekar V, Hoetelmans RM. Darunavir: pharmacokinetics and drug interactions. Antivir Ther. 2008;13:1–13. [PubMed] [Google Scholar]
- 3.Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother. 2008;42:1048–59. doi: 10.1345/aph.1K615. [DOI] [PubMed] [Google Scholar]
- 4.Erickson DA, Mather G, Trager WF, Levy RH, Keirns JJ. Characterization of the in vitro biotransformation of the HIV-1 reverse transcriptase inhibitor nevirapine by human hepatic cytochromes P-450. Drug Metab Dispos. 1999;27:1488–95. [PubMed] [Google Scholar]
- 5.Arastéh K, Clumeck N, Pozniak A, Lazzarin A, De Meyer S, Muller H, Peeters M, Rinehart A, Lefebvre E. TMC114-C207 Study Team. TMC114/ritonavir substitution for protease inhibitor(s) in a non-suppressive antiretroviral regimen: a 14-day proof-of-principle trial. AIDS. 2005;19:943–7. doi: 10.1097/01.aids.0000171408.38490.01. [DOI] [PubMed] [Google Scholar]
- 6.Jackson A, Boffitto M, Moyle G, Hill A, Sekar V, Lefebvre E, De Pauw M, DeMasi R, Hoetelmans R. The pharmacokinetic (PK) profile of darunavir with low-dose ritonavir (DRV/r) in various multiple-dose regimens over 120 hours. HIV Med. 2008;9:P31. [Google Scholar]
- 7.Clotet B, Bellos N, Molina JM, Cooper D, Goffard JC, Lazzarin A, Wöhrmann A, Katlama C, Wilkin T, Haubrich R, Cohen C, Farthing C, Jayaweera D, Markowitz M, Ruane P, Spinosa-Guzman S, Lefebvre E, POWER 1 and 2 Study Groups Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369:1169–78. doi: 10.1016/S0140-6736(07)60497-8. [DOI] [PubMed] [Google Scholar]
- 8.Madruga JV, Berger D, McMurchie M, Suter F, Banhegyi D, Ruxrungtham K, Norris D, Lefebvre E, de Béthune MP, Tomaka F, De Pauw M, Vangeneugden T, Spinosa-Guzman S, TITAN Study Group Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet. 2007;370:49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- 9.Sahai J, Cameron W, Salgo M, Stewart F, Myers M, Lamson M, Gagnier P. Drug interaction study between saquinavir (SQV) and nevirapine (NVP) 4th Conference on Retroviruses and Opportunistic Infections (CROI), Washington DC, USA, 22–26 January 1997; Abstract 614.
- 10.Boehringer Ingelheim Pharmaceuticals Inc. Viramune® (nevirapine) US Prescribing Information. revised June 2008. Available at http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Viramune/Viramune.pdf (last accessed 23 October 2008.
- 11.Abbott Laboratories Limited. Kaletra® tablets (lopinavir/ritonavir 200/50 mg) Summary of Product Characteristics. revised 7 October 2008. Available at http://emc.medicines.org.uk/emc/assets/c/html/displaydoc.asp?documented=18442 (last accessed 23 October 2008.
- 12.Sekar V, De Meyer S, Vangeneugden T, Lefebvre E, De Pauw M, Van Baelen B, De Paepe E, de Béthune M-P, Hoetelmans R, Parys W. Pharmacokinetic/pharmacodynamic (PK/PD) analyses of TMC114 in the POWER 1 and POWER 2 trials in treatment-experienced HIV-infected patients. 13th Conference on Retroviruses and Opportunistic Infections (CROI), Denver, CO, USA, 5–9 February 2006; Abstract J-121.


