Abstract
AIMS
Azathioprine, mercaptopurine and thioguanine are commonly used to treat autoimmune disorders, leukaemia and solid organ transplantation. However, azathiopurine and its metabolites can also cause adverse reactions such as myelosuppression. These manifestations may be attributed to polymorphisms or mutations in the thiopurine methyltransferase (TPMT) gene that might result in low TPMT enzyme activity. Our aim was to investigate if azathioprine-related myelosuppression is associated with TPMT polymorphism, which in turn affects its enzyme activity.
METHODS
A 61-year-old Chinese man with severe atopic eczema developed moderate myelosuppression with standard doses of azathioprine. His TPMT activity was measured using radiochemical assay. Genotyping of TPMT *3C, *3A and *6 were screened using polymerase chain reaction-restriction fragment length polymorphism. Novel mutation was detected by sequencing. Family studies of his three other siblings were performed.
RESULTS
After 4 weeks of azathioprine treatment, the patient's white blood cells and absolute neutrophil count dropped by 40–45%. He was then taken off azathioprine, and blood counts returned to normal. TPMT activity test showed intermediate levels of 9.1 nmol h−1 ml−1 peripheral red blood cells (pRBC). Resequencing of the TPMT gene revealed a missense mutation Phe→Leu at 208 aa position in exon 9 (ss105107120). Two of his three siblings were heterozygous for 208F→L, which accounts for the decreased enzyme activity (brother 8.9 nmol h−1 ml−1 pRBC, sister 8.8 nmol h−1 ml−1 pRBC). The remaining sibling had wild-type allele with normal enzyme activity. Screening of 100 normal healthy Chinese subjects did not reveal any individual with this mutation.
CONCLUSION
We report a novel mutation TPMT*26 (208F→L) associated with a decrease in TPMT enzyme activity.
Keywords: azathiopurine-related toxicity, novel mutation, thiopurine S-methyltransferase
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
More than 27 mutations in the TPMT gene causing decreased TPMT activity have been reported in various races.
Decreased TPMT activity is associated with increased myelosuppression from azathioprine or thioguanine treatment.
TPMT*3C is the most common genetic variant found in Asian populations.
WHAT THIS STUDY ADDS
TPMT*26 (208F→L) is a novel mutation, previously not reported, which is associated with reduced TPMT activity in three siblings in an Asian Chinese family.
This mutation is associated with increased sensitivity to azathioprine clinically.
Introduction
Azathiopurine, a prodrug is converted to 6-mercaptopurine (6-MP) in vivo, plays an important role in the treatment of autoimmune diseases such as rheumatoid arthritis, dermatitis and systemic lupus erythematosus [1, 2]. Azathiopurine is converted to 6-MP in vivo where it is subsequently metabolized to either active 6-thioguanine nucleotide metabolites (6-TGNs) by the enzyme inosine monophosphate dehydrogenase, or to inactive 6-methylmercaptopurine ribonucleotide metabolites by the enzyme thiopurine methyltransferase (TPMT) [3, 4]. Genetic polymorphisms of TPMT have been well documented to cause myelosuppression when patients are treated on azathiopurine [5]. This is due to the inability of TPMT enzyme to metabolize 6-MP, resulting in the conversion of 6-TGNs and, when incorporated into DNA, exerting cytotoxicity that triggers cell cycle arrest and apoptosis [6, 7]. Genotyping the common TPMT alleles prior to the start of thiopurine therapy is now recommended in clinical practice. To define accurately those at risk, identification of novel variants is critical, especially for populations that have not been well studied like the Asians. Here, we report a novel mutation detected in a patient with severe atopic eczema who displayed azathiopurine sensitivity while on treatment.
Methods
A 61-year-old man diagnosed with severe atopic eczema developed myelosuppression on azathiopurine. Blood was initially taken from the patient and subsequently from his three siblings for TPMT activity and genotyping. Measurement of TPMT activity was carried out using radiochemical assay [8] genotyping on the three common alleles present in the Asian population (*3C, *3A and *6), which were screened using polymerase chain reaction (PCR)-restriction fragment length polymorphism [9–11]. Sequencing of the open reading frame of the TPMT gene was performed to detect novel mutations after TPMT activity was low [11]. We have also developed Amplified Refractory Mutation System (ARMS)-PCR based on allele-specific discrimination at the 3′ end for the detected novel mutation (see below).
Informed consent was obtained from the patient and siblings prior to taking blood for study. This study was approved by the DSRB-B/04/275 (Domain-specific Review Board under the National Healthcare Group).
Detection of the TPMT 208F→L Novel Mutation
ARMS-PCR was performed using a pair of mutation-specific primers that harboured a single nucleotide mismatch at the 3′ end. Briefly, PCR was performed simultaneously in two separate reactions, one with the wild-type primer set and the other with the mutant primer set. Forward primer was 5′-GTCCACAAACTTACCAAA-3′ or 5′-GTCCACAAACTTACCAAG-3′ and reverse primer 5′-GCCACATCATCACCTAT-3′. The β-globin was used as housekeeping gene, forward pimer 5′-CAACTTCATCCACGTTCACC-3′ and reverse primer 5′-GAAGAG CCAAGGACAGGTAC-3′.
PCR reaction was carried out in a 20-µl reaction mixture containing 20 mmol l−1 Tris–HCl (pH 8.0), 50 mmol l−1 KCl, 25 µmol l−1 MgCl2, 0.2 mmol l−1 of each deoxyribonucleotide triphosphate, 0.5 µmol l−1 of each primer, 0.5 U of Platinum®Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA) and 200 ng genomic DNA. The expected PCR fragments of 268 bp for the β-globin gene and 156 bp for the 208F→L mutation were separated using 2% agarose gel electrophoresis (Figure 1).
Figure 1.
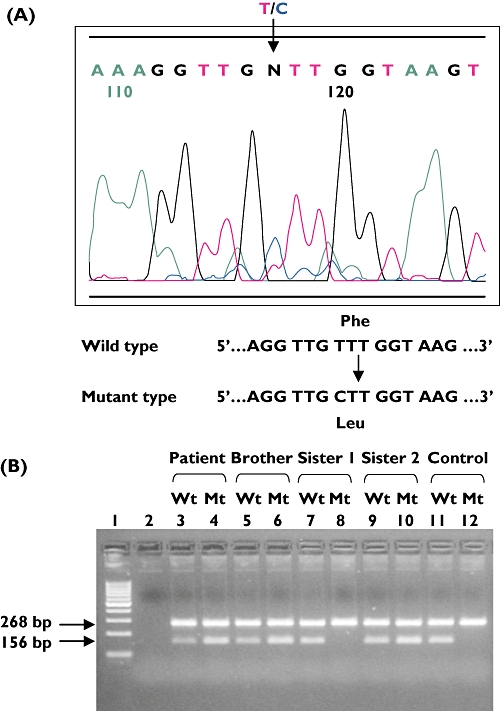
A novel mutation detected at T622C nt position, exon 9 of the TPMT gene by (a) sequencing, (b) Amplified Refractory Mutation System-polymerase chain reaction (ARMS-PCR). Wt, wild type; Mt, mutant
Results
A 61-year-old man was diagnosed with severe eczema and was treated with an initial dose of 50 mg of azathiopurine. After 4 weeks' treatment with a satisfactory clinical response, full blood count showed signs of azathiopurine-related toxicity with a drop in absolute neutrophil count from 6.58 to 2.93 × 109 l−1 and white blood cells from 9.05 to 3.66 × 109 l−1 (Table 1). The patient was then taken off therapy and blood counts returned to normal, suggesting patient's sensitivity to azathiopurine. Radiochemical assay showed the patient had an intermediate TPMT activity level of 9.1 nmol h−1 ml−1 peripheral red blood cells (pRBC). However, genotyping for TPMT *3A, *3C and *6 was not detected. The discordance in the TPMT phenotype–genotype correlation led us to investigate novel mutations in the open reading frame of the TPMT gene.
Table 1.
The full blood counts and dosings when patient is treated with azathioprine
| Dose | 25 mg | 50 mg | 50 mg | Off therapy |
|---|---|---|---|---|
| Duration, weeks | 1 | 1 | 3 | 2 |
| WBC (×109 l−1) | 9.05 | 9.79 | 3.66 | 7.37 |
| ANC (×109 l−1) | 6.58 | 8 | 2.93 | 5.63 |
| Haemoglobin (g dl−1) | 11.2 | 11.5 | 9.5 | 10.9 |
| Platelets (×109 l−1) | 189 | 263 | 151 | 278 |
| Eczema status | Improved | Cleared | Cleared, stopped treatment | Flared |
WBC, white blood cells; ANC, absolute neutrophil count.
A novel mutation was detected in exon 9 that resulted in an amino acid change at amino acid position 208 (phenylalanine→leucine) (Figure 1). We confirmed the reduced TPMT activity conferred by this novel mutation in the patient and his siblings by measuring TPMT activity in pRBC and DNA analysis. Based on the cut-off values of our assay (deficient <5 nmol h−1 ml−1 pRBC, intermediate = 5–10 nmol h−1 ml−1 pRBC, normal/high >10 nmol h−1 ml−1 pRBC) [12], two of the three siblings who harboured 208F→L mutation had intermediate TPMT activity (brother 8.9 nmol h−1 ml−1 pRBC, sister 8.8 nmol h−1 ml−1 pRBC), suggesting that the novel mutation is associated with the decreased TPMT activity. The other sister showed normal TPMT activity (11.9 nmol h−1 ml−1 pRBC) and did not harbour the mutation. This further confirms the effect of the novel mutation 208F→L on enzyme activity. Screening was carried out in 100 healthy adult Chinese controls did not reveal any other carriers of this variant.
Discussion
In the Asian population, TPMT activity shows a bimodal distribution compared with the White population, which displays a trimodal distribution. About 3–5% of Asians have intermediate TPMT enzyme activity and, most commonly, they are heterozygous for TPMT*3C. In contrast, 11% of White populations have intermediate TPMT activity, and 0.03% have extremely low or absent activity, and its effects can be fatal due to severe myelosuppression. However, the latter is occasionally seen in Asians.
The three TPMT genetic variants reported in Asians are mostly *3C, *6 and *3A at very low frequencies. However, there remain a small percentage of individuals in whom we cannot account for the phenotype, and this may be revealed only by resequencing the TPMT gene. Here, we have reported a novel mutation of 208F→L in exon 9 of the TPMT gene detected in a 61-year-old Chinese man who is sensitive to azathioprine. The phenotype–genotype relationship was validated by measuring his TPMT activity and family studies on his three siblings. We have demonstrated the use of phenotype–genotype correlation strategy to potentially identify novel mutations associated with decreased or absent TPMT enzyme activity. Adding to the pool of TPMT variants, at least 28 variant alleles of TPMT have been detected (TPMT*2-*25) that are associated with decreased or absent TPMT activity [13, 14].
Acknowledgments
We would like to thank our funding agencies NMRC/0582/2001 and SCS-EN14 for their support in this project.
Competing interests
None declared.
REFERENCES
- 1.Mason M, Currey HL, Barnes CG, Dunne JF, Hazleman BL, Strickland ID. Azathioprine in rheumatoid arthritis. BMJ. 1969;1:420–2. doi: 10.1136/bmj.1.5641.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Shakra M, Shoenfeld Y. Azathioprine therapy for patients with systemic lupus erythematosus. Lupus. 2001;10:152–3. doi: 10.1191/096120301676669495. [DOI] [PubMed] [Google Scholar]
- 3.Lennard L, Murphy MF, Maddocks JL. Severe megaloblastic anaemia associated with abnormal azathioprine metabolism. Br J Clin Pharmacol. 1984;17:171–2. doi: 10.1111/j.1365-2125.1984.tb02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maddocks JL, Lennard L, Amess J, Amos R, Thomas RM. Azathioprine and severe bone marrow depression. Lancet. 1986;I:156. doi: 10.1016/s0140-6736(86)92291-9. [DOI] [PubMed] [Google Scholar]
- 5.Lennard L, Van Loon JA, Weinshilboum RM. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther. 1989;46:149–54. doi: 10.1038/clpt.1989.119. [DOI] [PubMed] [Google Scholar]
- 6.Swann PF, Waters TR, Moulton DC, Xu Y, Zheng Q, Edwards M, Mace R. Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science. 1996;273:1109–11. doi: 10.1126/science.273.5278.1109. [DOI] [PubMed] [Google Scholar]
- 7.Somerville L, Krynetski EY, Krynetskaia NF, Beger RD, Zhang W, Marhefka CA, Evans WE, Kriwacki RW. Structure and dynamics of thioguanine-modified duplex DNA. J Biol Chem. 2003;278:1005–11. doi: 10.1074/jbc.M204243200. [DOI] [PubMed] [Google Scholar]
- 8.Weinshilboum RM, Raymond FA, Pazmino PA. Human erythrocyte thiopurine methyltransferase: radiochemical microassay and biochemical properties. Clin Chem Acta. 1978;85:323–33. doi: 10.1016/0009-8981(78)90311-x. [DOI] [PubMed] [Google Scholar]
- 9.Yates CR, Krynetski EY, Loennechen T, Fessing MY, Tai HL, Pui CH, Relling MV, Evans WE. Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med. 1997;126:608–14. doi: 10.7326/0003-4819-126-8-199704150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Tai HL, Krynetski EY, Yates CR, Loennechen T, Fessing MY, Krynetskaia NF, Evans WE. Thiopurine S-methyltransferase deficiency: two nucleotide transitions define the most prevalent mutant allele associated with loss of catalytic activity in Caucasians. Am J Hum Genet. 1996;58:694–702. [PMC free article] [PubMed] [Google Scholar]
- 11.Otterness D, Szumlanski C, Lennard L, Klemetsdal B, Aarbakke J, Park-Hah JO, Iven H, Schmiegelow K, Branum E, O'Brien J, Weinshilboum R. Human thiopurine methyltransferase pharmacogenetics: gene sequence polymorphisms. Clin Pharmacol Ther. 1997;62:60–73. doi: 10.1016/S0009-9236(97)90152-1. [DOI] [PubMed] [Google Scholar]
- 12.Kham SK, Soh CK, Liu TC, Chan YH, Ariffin H, Tan PL, Yeoh AE. Thiopurine S-methyltransferase activity in three major Asian populations: a population-based study in Singapore. Eur J Clin Pharmacol. 2008;64:373–9. doi: 10.1007/s00228-007-0426-x. [DOI] [PubMed] [Google Scholar]
- 13.Ujiie S, Sasaki T, Mizugaki M, Ishikawa M, Hiratsuka M. Functional characterization of 23 allelic variants of thiopurine S-methyltransferase gene (TPMT*2–*24) Pharmacogenet Genomics. 2008;10:887–93. doi: 10.1097/FPC.0b013e3283097328. [DOI] [PubMed] [Google Scholar]
- 14.Garat A, Cauffiez C, Renault N, Lo-Guidice JM, Allorge D, Chevalier D, Houdret N, Chavatte P, Loriot MA, Gala JL, Broly F. Characterisation of novel defective thiopurine S-methyltransferase allelic variants. Biochem Pharmacol. 2008;76:404–15. doi: 10.1016/j.bcp.2008.05.009. [DOI] [PubMed] [Google Scholar]


