Abstract
Adverse health effects of exposures to acute air pollution have been well studied. Fewer studies have examined effects of chronic exposure. Previous studies used exposure estimates for narrow time periods and were limited by the geographic distribution of pollution monitors. This study examined the association of chronic particulate exposures with all-cause mortality, incident nonfatal myocardial infarction, and fatal coronary heart disease (CHD) in a prospective cohort of 66,250 women from the Nurses’ Health Study in northeastern US metropolitan areas. Nonfatal outcomes were assessed through self-report and medical record review and fatalities through death certificates and medical record review. During follow-up (1992–2002), 3,785 deaths and 1,348 incident fatal CHD and nonfatal myocardial infarctions occurred. In age- and calendar-time-adjusted models, 10-μg/m3 increases in 12-month average exposures to particulate matter <10 μm in diameter were associated with increased all-cause mortality (16%, 95% confidence interval: 5, 28) and fatal CHD (43%, 95% confidence interval: 10, 86). Adjustment for body mass index and physical activity weakened these associations. Body mass index and smoking modified the association between exposure to particulate matter <10 μm in diameter and fatal CHD. In this population, increases in such exposures were associated with increases in all-cause and CHD mortality. Never smokers with higher body mass indexes were at greatest risk of fatal CHD.
Keywords: air pollution, cohort studies, coronary disease, environmental exposure, incidence, particulate matter, risk factors
Adverse effects associated with acute air pollution exposures have been well studied; however, evidence of chronic exposure effects has been more limited. The Harvard Six Cities Study (1) and the American Cancer Society Study (2), prominent early studies of long-term exposure to air pollution, both found an increased risk of mortality associated with long-term exposure to particulates. More recent key studies have shown an association between adverse health outcomes and chronic particulate exposure, even as particulate levels have decreased over time (3–6). Despite these findings, questions remain regarding the persistence of effects because particulate exposures accumulate over longer time periods. Additionally, gaps in current knowledge regarding susceptible subgroups of the population add to the importance of investigating the impact of risk factors that may change over time. Answers to these questions would inform targeted public health prevention strategies, as well as environmental policy.
The current study, using data from the Nurses’ Health Study, an ongoing prospective cohort, examined the relation of chronic particulate exposures with all-cause mortality and incident and fatal coronary heart disease (CHD), addressing these questions by combining improved exposure, covariate, and outcome assessment. We estimated particulate matter less than 10 μm in diameter (PM10) at every residential address for each nurse (updated biennially) during each month of the study period by using a statistical model combining spatial smoothing and land use regression. The model used PM10 monitoring data and variables that affect pollution generation and dispersion, such as population density, distance to roadways, elevation, urban land use, point-source PM10 emissions, precipitation, and wind speed. Repeated information on lifestyle and behavioral risk factors enabled us to examine potential confounding and effect modification by a variety of time-varying, individual-level risk factors.
MATERIALS AND METHODS
The Nurses’ Health Study was initiated in 1976 when 121,700 US registered female nurses, aged 30–55 years, completed a mailed questionnaire. At study inception, they resided in 11 states scattered over the United States (California, Connecticut, Florida, Massachusetts, Maryland, Michigan, New Jersey, New York, Ohio, Pennsylvania, Texas). Since that time, participants have moved into all 50 states. The study population for this analysis was limited to nurses living in Metropolitan Statistical Areas in 13 states in the northeastern region of the United States (Maine, New Hampshire, Vermont, Massachusetts, Connecticut, Rhode Island, New York, New Jersey, Pennsylvania, Delaware, Ohio, Maryland, Michigan). This contiguous region contains 63% of the Nurses’ Health Study population. The density of PM10 monitoring locations was inadequate for detailed exposure assessment prior to 1988. Therefore, we began follow-up in 1992 to allow for as long as 4 years of exposure estimation prior to the outcomes of interest. Since the baseline and subsequent follow-up Nurses’ Health Study questionnaires are mailed to participants in June of even years, cohort follow-up began on June 30, 1992.
From 1976 until the present, study participants have completed biennial questionnaires on behavioral and lifestyle exposures and health outcomes. Only 6% of nurses available for follow-up no longer respond to questionnaires. Questions have addressed such risk factors as physical activity, body mass index (weight (kg)/height (m)2), smoking status, and medical history. Our outcomes of interest were fatal CHD, nonfatal myocardial infarction, incident CHD (defined as first incident nonfatal myocardial infarction or fatal CHD), and all-cause mortality. These outcomes were selected on the basis of prior Nurses’ Health Study research and were verified (7–10). Women who self-reported nonfatal outcomes were asked for permission to release their medical records, which were reviewed by a physician blinded to exposure status.
Confirmed cases of nonfatal myocardial infarction met World Health Organization criteria: symptoms and diagnostic electrocardiographic changes or elevations in cardiac enzymes (11). Cases of nonfatal myocardial infarctions were designated as probable if an interview or letter confirming hospitalization for the infarction was obtained and medical records were unavailable. We included probable and confirmed cases in the analysis. Information on deaths was obtained through next of kin, death certificates, postal authority reports, or the National Death Index. Fatal CHD was confirmed by hospital records, autopsy, or death certificate if CHD was the underlying and most plausible cause. We also included sudden deaths if CHD was the plausible cause listed on the death certificate. For all-cause mortality analyses, we excluded 7,884 women with a history of cancer (other than nonmelanoma skin cancer) before 1992, the beginning of the follow-up period. For analyses of incident nonfatal myocardial infarction, we excluded women with a history of nonfatal myocardial infarction. Analyses of all-cause mortality excluded accidental deaths. For our analysis of fatal CHD, women with nonfatal myocardial infarctions prior to baseline were excluded, and women experiencing nonfatal myocardial infarctions during follow-up were censored at the time of the event.
Exposure assessment
PM10 exposures were estimated for each participant by using a geographic information system–based spatial smoothing model that predicted residence-specific monthly outdoor PM10 concentrations in the northeastern region of the United States (12). This model, a generalized additive mixed model, used PM10 data from monitoring sites in the US Environmental Protection Agency's Air Quality System, the IMPROVE network (Interagency Monitoring of PROtected Visual Environments, a network of monitors located in national parks and wilderness areas), and Harvard University research studies to estimate monthly smooth spatial terms and smooth regression terms of geographic information system–derived and meteorologic covariates.
The model was fit in 2 stages. In the first-stage equation, , yi,t is the natural-log-transformed PM10 monthly site average, t is the number of months, ui is an estimated site-specific intercept that represents the long-term adjusted mean, gt(si) is the time-period-specific residual spatial variability, si is the projected spatial coordinate pair for the ith location, and Zi,t,1 through Zi,t,P are time-varying covariates derived from the geographic information system. This model stage estimates site-specific terms (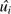 ), adjusting for time-varying covariates and time-varying residual spatial variability. The second-stage equation,
), adjusting for time-varying covariates and time-varying residual spatial variability. The second-stage equation, 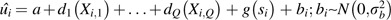 , where bi is a site-specific random effect, models the site-specific
, where bi is a site-specific random effect, models the site-specific 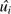 terms by using smooth functions of site-specific, time-invariant geographic information system–derived covariates (Xi,1 through Xi,Q) and residual time-invariant spatial variability (g(si)). This model structure allows for highly spatially and temporally resolved predictions of chronic PM10 levels, even for individuals living in areas with no nearby monitors (albeit with greater uncertainty for locations with distant monitors).
terms by using smooth functions of site-specific, time-invariant geographic information system–derived covariates (Xi,1 through Xi,Q) and residual time-invariant spatial variability (g(si)). This model structure allows for highly spatially and temporally resolved predictions of chronic PM10 levels, even for individuals living in areas with no nearby monitors (albeit with greater uncertainty for locations with distant monitors).
Geographic information system–derived model covariates included block group and county-level population density, distance to the nearest road by Census Feature Class Code road class for A1–A3 roads, elevation from the US Geological Survey National Elevation Dataset, urban land use from the US Geological Survey National Land Cover Dataset, point- and area-source primary PM10 emissions information from the US Environmental Protection Agency National Emissions Inventory, and meteorologic information on wind speed and precipitation from the National Climatic Data Center (12). Cross-validation results showed that this geographic information system–based spatial smoothing model (cross-validation R2 = 0.62) performed substantially better than other approaches using inverse distance weighting (cross-validation R2 = 0.29) or nearest-neighbor spatial interpolators (cross-validation R2 = 0.22).
Statistical analysis
Person-time was calculated from baseline (June 30, 1992) until the end of follow-up (June 30, 2002), death, or disease outcome, whichever occurred first. Those who died, reported cancer, or reported an outcome of interest (e.g., nonfatal myocardial infarction) prior to June 30, 1992, were excluded from the analysis. Nurses who moved outside a Metropolitan Statistical Area or the northeastern region of the United States were excluded from the period of follow-up during which these conditions were true but were allowed to reenter the risk set if the situation reversed. Incidence rates were determined as the number of new cases divided by person-months of follow-up. Time-varying Cox proportional hazards models on a monthly time scale were used to calculate hazard ratios and 95% confidence intervals evaluating the association between each outcome of interest and average PM10 exposure. In separate models, PM10 exposure was modeled as the month prior to the outcome; a 3-month moving average (calculated as the 3 months of exposure previous to the month in which an outcome occurred); and 12-, 24-, 36-, and 48-month moving averages. We stratified by age in months, as determined at the beginning of each month, and adjusted for state of residence (indicator variables), year (linear term), and season (indicator variables). State-level indicator variables were included to control for large-scale spatial patterns in mortality that might be caused by factors other than pollution, thereby estimating the effect of pollution based on within-state variation (6, 13). All statistical analyses used SAS version 9.1 software (SAS Institute, Inc., Cary, North Carolina).
Evaluation of confounders and effect modifiers
Although data for outcomes and pollutant exposures were available on a monthly basis, information on potential confounders and effect modifiers were available on a biennial basis only. Therefore, each woman was assigned the same covariate values for all of the months following a questionnaire return until updated values were available from the next questionnaire. Hypertension (yes, no), physician-diagnosed diabetes (yes, no), high cholesterol level (yes, no), physical activity (<3, 3–<9, 9–<18, 18–<27, or ≥27 metabolic-equivalent hours/week), body mass index (continuous), and smoking status (never, former, or current) and pack-years of smoking were updated every 2 years. Family history of myocardial infarction (yes, no) was assessed at enrollment in 1976 and again in 1984. Previous studies (14, 15) have found an influence of socioeconomic status on the relation between air pollution exposure and health outcomes. Given that adequate individual-level socioeconomic status information was unavailable from the Nurses’ Health Study questionnaires, we developed 2 area-level socioeconomic status variables that were evaluated as potential confounders. Median household value and median family income were determined from 2000 US Census data for the census tract of residence. Effect modification by body mass index, physical activity, diabetes, smoking, hypercholesterolemia, and hypertension was evaluated through stratification.
RESULTS
In 1992, the Nurses’ Health Study consisted of 104,645 living nurses who had no reported history of cancer (excluding nonmelanoma skin cancer). Of these nurses, 75,809 were residing in our 13 selected states (referred to as the northeast region), with 66,250 living in Metropolitan Statistical Areas. Most women lived in Pennsylvania, New York, Ohio, and Massachusetts (4 of the original states at inception of the cohort), while fewer than 1% resided in Vermont, Rhode Island, New Hampshire, Delaware, or Maine. Table 1 shows little difference in the characteristics of nurses in the main cohort, in the northeastern region, and in northeastern region Metropolitan Statistical Areas. Mean age during follow-up was approximately 62 years. Forty-four percent of women living in the northeastern region reported never smoking, and 58% met the World Health Organization's defined category of being overweight (body mass index >25) (16–18). During follow-up, the mean 12-month moving average of predicted PM10 exposure in the northeastern region was 21.6 μg/m3, with a standard deviation of 4.3 and an interquartile range of 6.0. Mean values of 12-month-moving-average PM10 were compared across categories of characteristics, and differences among categories were slight (data not shown). For example, the mean values ranged from 21.4 μg/m3 to 21.6 μg/m3 across categories of physical activity and 21.0 μg/m3 to 22.8 μg/m3 across median census tract family income.
Table 1.
Characteristics of the US Study Population in Selected Categories From the Nurses’ Health Study During the Follow-up Period 1992–2002a
| Characteristic | Nation | Northeast | Northeast MSAs |
| No. of women | 104,645 | 75,809 | 66,250 |
| Mean (SD) age, years | 62.2 (7.7) | 62.5 (7.6) | 62.4 (7.6) |
| Body mass index in kg/m2, % | |||
| <25.0 | 43.7 | 41.9 | 42.3 |
| 25.0–<30.0 | 33.9 | 34.4 | 34.2 |
| ≥30.0 | 22.4 | 23.7 | 23.5 |
| Smoking status, % | |||
| Never | 45.0 | 44.1 | 43.6 |
| Current | 12.7 | 13.4 | 13.5 |
| Former | 42.3 | 42.5 | 42.9 |
| Mean (SD) pack-years of smoking | 25.0 (21.3) | 24.8 (21.0) | 24.8 (21.0) |
| High cholesterol level, % | 49.7 | 49.9 | 49.5 |
| Diabetes, % | 7.4 | 7.5 | 7.4 |
| Hypertension, % | 40.5 | 40.6 | 40.4 |
| Family history of myocardial infarction, % | 33.5 | 34.2 | 34.2 |
| Physical activity in MET-hours/week, % | |||
| <3 | 21.9 | 22.1 | 22.1 |
| 3–<9 | 23.2 | 23.7 | 23.8 |
| 9–<18 | 20.8 | 21.0 | 21.0 |
| 18–<27 | 12.8 | 13.0 | 12.8 |
| ≥27 | 21.2 | 20.5 | 20.3 |
| Mean (SD) predicted PM10 exposure, μg/m3 | 21.2 (4.5) | 21.6 (4.3) | |
| Mean (SD) median family income in US $, thousandsb | 64.2 (23.8) | 67.0 (24.1) | |
| Mean (SD) median house value in US $, 10 thousandsb | 15.7 (10.1) | 16.6 (10.4) |
Abbreviations: MET, metabolic equivalent; MSA, Metropolitan Statistical Area; PM10, particulate matter <10 μm in diameter; SD, standard deviation.
Percentages are based on complete information for participants.
Estimated for census tract of residence using 2000 US Census data.
During the follow-up period from 1992 to 2002, 3,785 nonaccidental deaths occurred. When we stratified by age and adjusted for state of residence, year, and season, each increase of 10 μg/m3 in PM10 in the 12 months prior to death was associated with a hazard ratio of 1.16 (95% confidence interval (CI): 1.05, 1.28) (Table 2). Risk was not significantly elevated for exposure in the month prior to death; however, risk appeared to increase as the window of exposure increased to 24 months prior to the event (data not shown).
Table 2.
Hazard Ratios and 95% CIs for All-Cause and Cause-Specific Mortality Associated With a 10-μg/m3 Change in Predicted PM10 Exposurea for the US Nurses’ Health Study During the Follow-up Period 1992–2002
| No. of Cases | No. of Person-months | The Month Before |
3-Month Moving Average |
12-Month Moving Average |
48-Month Moving Average |
|||||
| Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | |||
| All-cause mortality | 3,785 | 600,752 | 1.04 | 0.98, 1.11 | 1.14 | 1.05, 1.23 | 1.16 | 1.05, 1.28 | 1.15 | 1.04, 1.28 |
| Myocardial infarction | ||||||||||
| First CHD event | 1,348 | 597,456 | 1.08 | 0.98, 1.19 | 0.96 | 0.84, 1.09 | 1.10 | 0.94, 1.29 | 1.09 | 0.92, 1.29 |
| Fatal CHDb | 494 | 597,456 | 1.16 | 0.98, 1.36 | 1.21 | 0.98, 1.48 | 1.43 | 1.10, 1.86 | 1.43 | 1.09, 1.88 |
| Nonfatal myocardial infarction | 854 | 597,458 | 1.03 | 0.91, 1.18 | 0.83 | 0.71, 0.98 | 0.94 | 0.77, 1.15 | 0.93 | 0.75, 1.15 |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; PM10, particulate matter <10 μm in diameter.
Modeled stratifying by age in months, adjusting for state of residence, year, and season.
Including sudden deaths, excluding prior nonfatal myocardial infarction.
From 1992 until 2002, 854 nonfatal myocardial infarctions and 494 CHD deaths occurred, excluding prior nonfatal myocardial infarctions (Table 2). The hazard ratios for first incident nonfatal myocardial infarction or fatal CHD events associated with an increase of 10 μg/m3 in PM10 were elevated, but not statistically significant, for the majority of the exposure time periods considered. Each 10-μg/m3 increase in PM10 in the 12 months prior to CHD death was associated with a hazard ratio of 1.43 (95% CI: 1.10, 1.86). Risk of CHD death was also elevated significantly for PM10 exposure periods longer than 12 months. The risk associated with PM10 in the month prior to a nonfatal myocardial infarction was elevated slightly; however, none of the other time periods of exposure showed an association.
To assess the impact of potential confounders or modifying risk factors, univariate and multivariate models were adjusted for each potential confounder (Table 3). Physical activity and smoking each attenuated the risk estimates for all-cause mortality, whereas median census tract house value increased the risk estimate. In the full model, after adjustment for smoking status and pack-years, body mass index, diabetes, family history of myocardial infarction, hypercholesterolemia, hypertension, physical activity, and median family income and median house value in the census tract of residence, the hazard ratio was attenuated to 1.07 (95% CI: 0.97, 1.18). If physical activity was not included in the full model, then the hazard ratio was 1.11 (95% CI: 1.01, 1.23). The hazard ratio for CHD death remained statistically significant regardless of which potential confounder was included in the model (Table 3). However, physical activity and smoking attenuated and census tract median house value increased the strength of the association. In the fully adjusted multivariate model, the hazard ratio was 1.30 (95% CI: 1.00, 1.71); in the fully adjusted model, the hazard ratio, excluding physical activity, was 1.35 (95% CI: 1.03, 1.77).
Table 3.
Hazard Ratios and 95% CIs for Adjusted Associations of All-Cause Mortality and Fatal CHDa With a 10-μg/m3 Change in Average Predicted PM10 in the 12 Months Prior to Death for the US Nurses’ Health Study During the Follow-up Period 1992–2002
| Model | All-Cause Mortality |
Fatal CHD |
||
| Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | |
| Basicb | 1.16 | 1.05, 1.28 | 1.43 | 1.10, 1.86 |
| Smoking | 1.12 | 1.02, 1.23 | 1.39 | 1.07, 1.81 |
| Body mass index | 1.15 | 1.05, 1.27 | 1.40 | 1.08, 1.82 |
| Diabetes | 1.15 | 1.04, 1.27 | 1.41 | 1.08, 1.83 |
| Family history of myocardial infarction | 1.16 | 1.05, 1.28 | 1.44 | 1.11, 1.87 |
| Hypercholesterolemia | 1.16 | 1.05, 1.28 | 1.43 | 1.10, 1.86 |
| Hypertension | 1.15 | 1.05, 1.27 | 1.42 | 1.09, 1.84 |
| Physical activity | 1.08 | 0.98, 1.19 | 1.34 | 1.03, 1.74 |
| Median family income | 1.16 | 1.05, 1.28 | 1.42 | 1.09, 1.84 |
| Median house value | 1.19 | 1.08, 1.31 | 1.47 | 1.13, 1.93 |
| Fullc | 1.07 | 0.97, 1.18 | 1.30 | 1.00, 1.71 |
| Full excluding physical activity | 1.11 | 1.01, 1.23 | 1.35 | 1.03, 1.77 |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; PM10, particulate matter <10 μm in diameter.
Including sudden deaths.
Modeled stratifying by age in months, adjusting for state of residence, year, and season.
Modeled stratifying by age in months, adjusting for state of residence, year, season, smoking status, pack-years of smoking, family history of myocardial infarction, high cholesterol level, diabetes, hypertension, median family income in the census tract of residence, physical activity, and median house value in the census tract of residence.
The effect of PM10 exposure on all-cause mortality was not modified by body mass index, physical activity, hypercholesterolemia, hypertension, or diabetes. However, the association between CHD death and PM10 exposure was modified by body mass index. For CHD death, stratified results revealed a stronger risk of fatal CHD for women with a body mass index of 30 or higher (Table 4). Stratified results also indicated that never smokers were at higher risk of fatal CHD associated with PM10 exposure than former or current smokers (Table 5). Furthermore, among women with a body mass index of less than 30, the hazard ratio for those who had never smoked was 1.41 (95% CI: 0.82, 2.42) for a 10-μg/m3 change in PM10, whereas hazard ratios for former (hazard ratio = 0.98, 95% CI: 0.58, 1.64) and current (hazard ratio = 0.85, 95% CI: 0.47, 1.53) smokers were not elevated. Women with body mass indexes of 30 or higher showed a similar, but stronger pattern of risk, with a hazard ratio of 2.82 (95% CI: 1.40, 5.71) for never smokers.
Table 4.
Hazard Ratios and 95% CIs for the Association Between Fatal CHDa and a 10-μg/m3 Change in Average Predicted PM10 in the 12 Months Prior to Death Stratified by Body Mass Indexb for the US Nurses’ Health Study During the Follow-up Period 1992–2002
| Body Mass Index <30 | Body Mass Index ≥30 | |||
| No. of casesc | 310 | 149 | ||
| No. of person-months | 432,557 |
132,250 |
||
| Hazard Ratio |
95% CI |
Hazard Ratio |
95% CI |
|
| Base modeld | 1.20 | 0.86, 1.68 | 2.16 | 1.35, 3.45 |
| Adjusted modele | 1.08 | 0.76, 1.52 | 1.99 | 1.23, 3.22 |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; PM10, particulate matter <10 μm in diameter.
Including sudden deaths.
Weight (kg)/height (m)2.
Risks are based on complete information for participants.
Modeled stratifying by age in months, adjusting for state of residence, year, and season.
Modeled stratifying by age in months, adjusting for state of residence, year, season, smoking status, family history of myocardial infarction, high cholesterol level, diabetes, hypertension, median family income in the census tract of residence, physical activity, and median house value in the census tract of residence.
Table 5.
Hazard Ratios and 95% CIs for the Association Between Fatal CHDa and a 10-μg/m3 Change in Average Predicted PM10 in the 12 Months Prior to Death Stratified by Body Mass Indexb and Smoking Statusc for the US Nurses’ Health Study During the Follow-up Period 1992–2002
| Never Smoker | Former Smoker | Current Smoker | ||||
| All | ||||||
| No. of casesa | 160 | 190 | 125 | |||
| No. of person-months | 251,153 |
244,466 |
77,153 |
|||
| Hazard Ratio |
95% CI |
Hazard Ratio |
95% CI |
Hazard Ratio |
95% CI |
|
| Basic modeld | 1.87 | 1.24, 2.81 | 1.32 | 0.89, 1.96 | 0.90 | 0.54, 1.51 |
| Adjusted modele | 1.83 | 1.20, 2.79 | 1.22 | 0.82, 1.83 | 0.88 | 0.52, 1.48 |
| Body mass index <30 | ||||||
| No. of casesa | 97 | 120 | 100 | |||
| No. of person-months | 181,451 |
180,120 |
64,912 |
|||
| Hazard Ratio |
95% CI |
Hazard Ratio |
95% CI |
Hazard Ratio |
95% CI |
|
| Basic modeld | 1.42 | 0.83, 2.41 | 1.01 | 0.60, 1.68 | 0.88 | 0.50, 1.58 |
| Adjusted modele | 1.41 | 0.82, 2.42 | 0.98 | 0.58, 1.64 | 0.85 | 0.47, 1.53 |
| Body mass index ≥30 | ||||||
| No. of casesa | 59 | 67 | 23 | |||
| No. of person-months | 59,425 |
59,897 |
11,614 |
|||
| Hazard Ratio |
95% CI |
Hazard Ratio |
95% CI |
Hazard Ratio |
95% CI |
|
| Basic modeld | 2.85 | 1.44, 5.65 | 1.89 | 0.99, 3.58 | 0.98 | 0.31, 3.14 |
| Adjusted modele | 2.82 | 1.40, 5.71 | 1.64 | 0.86, 3.13 | 1.03 | 0.31, 3.42 |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; PM10, particulate matter <10 μm in diameter.
Including sudden deaths.
Weight (kg)/height (m)2.
Risks are based on complete information for participants.
Modeled stratifying by age in months, adjusting for state of residence, year, and season.
Modeled stratifying by age in months, adjusting for state of residence, year, season, smoking status, family history of MI, high cholesterol level, diabetes, hypertension, median family income in census tract of residence, physical activity, and median house value in census tract of residence.
DISCUSSION
In a population of women residing in Metropolitan Statistical Areas in the northeastern region of the United States, all-cause mortality was statistically significantly associated with average PM10 exposures in the time period 3–48 months prior to death. The association was strongest with average PM10 exposure in the 24 months prior to death (hazard ratio = 1.16, 95% CI: 1.05, 1.28) and weakest with exposure in the month prior to death (hazard ratio = 1.04, 95% CI: 0.98, 1.11). Risk of fatal CHD was significantly associated with chronic exposure to PM10. The association of greatest magnitude occurred with mean exposure in the 24 months prior to death (hazard ratio = 1.42, 95% CI: 1.11, 1.81). We did not find an association between nonfatal myocardial infarctions and PM10 exposure in this population.
We examined the effects of potential confounders on these health outcomes, with physical activity and smoking attenuating the strength of the relation. Risk of all-cause mortality and fatal CHD associated with PM10 in the 12 months before death was significantly elevated after adjustment for smoking status and pack-years, body mass index, diabetes, family history of myocardial infarction, hypercholesterolemia, hypertension, and median census tract family income and house value. Controlling for physical activity in addition to these factors resulted in elevated, but nonsignificant risks of all-cause mortality and fatal CHD. Few previous studies have controlled for physical activity, and mechanisms underlying this attenuation are unclear.
Examination of possible effect modification showed that the association between fatal CHD and long-term PM10 exposure was influenced by body mass index and smoking. Women with a higher body mass index were at increased risk of fatal CHD associated with PM10. Women who had never smoked showed the strongest risk, regardless of body mass index. These results suggest that the health effects of smoking may mask the impact of air pollution. Reductions in PM10 levels may produce greater benefits in healthier populations.
Our observations are consistent with a growing body of literature on chronic particulate matter exposure, cardiovascular events, and mortality. The size fraction in these studies varies (PM10 or particulate matter <2.5 μm in diameter (PM2.5)), precluding direct comparisons in some cases. In the extended Harvard Six Cities Study (4) and the American Cancer Society Study (6), the adjusted hazard ratios for a 10-μg/m3 change in PM2.5 exposure were 1.14 (95% CI: 1.06, 1.22) and 1.06 (95% CI: 1.02, 1.11), respectively. Eftim et al. (3) evaluated the association between all-cause mortality and fine particulates by using Medicare data in the same counties. They observed a 10.9% (95% CI: 9.0, 12.8) increase in all-cause mortality for a 10-μg/m3 increase in PM2.5 in the American Cancer Society Study counties and a 20.8% (95% CI: 14.8, 27.1) increase in the Harvard Six Cities Study counties.
In a case-control study of Swedish men and women using 30-year averaged, traffic-related PM10, Rosenlund et al. (19) did not find an association with nonfatal myocardial infarctions (odds ratio = 0.92, 95% CI: 0.71, 1.19 for an approximately 5-μg/m3 change in PM10). For fatal myocardial infarction, there was a nonsignificant elevated risk (odds ratio = 1.39, 95% CI: 0.94, 2.07) (19). Results from our study were stronger than those in this study, which included both genders. The Adventist Health Study on Health Effects of Smog showed associations of fatal CHD with PM10, PM2.5, and PM10–2.5 for women but not for men (20).
The relation between chronic PM2.5 exposure and cardiovascular events was examined in the Women's Health Initiative observational study (5). Although results are not directly comparable, a significant increase in deaths from CHD and a nonsignificant increased risk of first myocardial infarction were also found. Similar to our study, the authors of this study observed effect modification by body mass index. The overall associations were higher than those reported here, likely because of differences in the particle size studied.
The time pattern of the association we found is interesting. Distributed lag-time series analyses of PM10 exposure out to a month prior to death (21, 22) reported larger associations than those seen a few days preceding death, very similar to our findings regarding monthly exposure variables. Recent reanalyses of the Harvard Six Cities Study (23, 24) examined whether previous findings are due to decades of exposure or shorter-term changes. The reported association was with exposure in the previous 2 years. These results indicate a developing coherence of the air pollution mortality literature, and the mortality risk benefits from reducing air pollution would be expected within a few years of intervention.
Most previous cohort studies have not examined the effect of physical activity on the association of particulate exposure with all-cause mortality and fatal CHD (1, 2). It was assessed in the Women's Health Initiative study, which did not find evidence of confounding (5). Whether physical activity is a confounder, is a risk modifier, or is in the causal pathway warrants further exploration.
PM10 can deposit in the thoracic area of the respiratory system. The biologic mechanisms through which PM10 contributes to mortality and CHD are not clearly understood. Hypothesized pathways include oxidative stress (25) and inflammation leading to accelerated atherosclerosis (26, 27) or endothelial dysfunction (28), and ischemic responses in the myocardium (28).
Limitations of our study include potential bias from obtaining initial information through self-report. Use of medical records, interviews, autopsy reports, and death certificate information reduces the possibility, and it is unlikely that underreporting would be associated with exposure. Findings may differ for other geographic areas. Selecting the northeastern region of the United States enabled us to focus on contiguous states with a denser and more evenly distributed study population than in other regions of the country. The similarity of sources and model complexity increased accuracy but necessitated initial assessment and validation in a smaller area. We did not adjust for additional ambient pollutants. Particulate exposures were assigned by geocoded residential locations; however, work locations were unavailable. In addition, we were unable to determine time spent outdoors, housing characteristics, or whether nurses were living at reported addresses year-round. Some addresses were unable to be geocoded because of abbreviations and the like. High correlations between time windows of exposure (ρ > 0.95 for correlations of all measures between 12 and 48 months) and potential residual confounding by incomplete adjustment for season limited our ability to determine the most relevant time period. Although estimates of pollution exposure were available on a monthly basis, covariates were assessed only every 2 years.
Through a combination of updated covariates, improved outcome assessment, and more accurate long-term exposure assessment, the current study provides valuable information on the associations of chronic PM10 exposure with all-cause mortality and CHD. We identified women nonsmokers with a higher body mass index as a potentially susceptible population. Most importantly, with residential addresses that were updated every 2 years and monthly predictions from our geographic information system–based exposure model, we had the unique opportunity to assess exposures on a finer spatial and temporal scale than in previous long-term studies. Finally, our findings add to a growing coherence of the literature across multiple time scales indicating that the public health benefits of reducing particle concentrations will be realized within years, not decades, of the reduction. This study also suggests that measures taken to limit particulate air pollution should benefit population health over extended periods of time.
Acknowledgments
Author affiliations: Cancer Prevention and Control Program, Department of Environmental Health Sciences, Department of Epidemiology and Biostatistics, Arnold School of Public Health, University of South Carolina, Columbia, South Carolina (Robin C. Puett); Exposure, Epidemiology, and Risk Program, Department of Environmental Health, Harvard School of Public Health, Boston, Massachusetts (Joel Schwartz, Jaime E. Hart, Jeff D. Yanosky, Frank E. Speizer, Helen Suh, Francine Laden); Channing Laboratory, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Jaime E. Hart, Frank E Speizer, Francine Laden); Department of Biostatistics, Harvard School of Public Health, Boston, Massachusetts (Christopher J. Paciorek); and National Health and Environmental Effects Research Laboratory, US Environmental Protection Agency, Chapel Hill, North Carolina (Lucas M. Neas).
This research was supported by a grant (83054501-0) from the US Environmental Protection Agency's Science to Achieve Results (STAR) program; a PPG grant (CA87969) from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services; and a grant (1F32 HL083648) from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services.
The authors acknowledge members of the Channing Laboratory, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, particularly Ellen Hertzmark, Dr. Diane Feskanich, and Dr. Lisa Li, for their programming assistance. They also thank Marcia Goetsch for information technology support.
This manuscript was subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Conflict of interest: none declared.
Glossary
Abbreviations
- CHD
coronary heart disease
- CI
confidence interval
- PM2.5
particulate matter <2.5 μm in diameter
- PM10
particulate matter <10 μm in diameter
References
- 1.Dockery DW, Pope CA, III, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24):1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 2.Pope CA, III, Thun MJ, Namboodiri MM, et al. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151(3 pt 1):669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- 3.Eftim SE, Samet JM, Janes H, et al. Fine particulate matter and mortality: a comparison of the six cities and American Cancer Society cohorts with a Medicare cohort. Epidemiology. 2008;19(2):209–216. doi: 10.1097/EDE.0b013e3181632c09. [DOI] [PubMed] [Google Scholar]
- 4.Laden F, Schwartz J, Speizer FE, et al. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;173(6):667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356(5):447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 6.Pope CA, III, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pischon T, Hu FB, Rexrode KM, et al. Inflammation, the metabolic syndrome, and risk of coronary heart disease in women and men. Atherosclerosis. 2008;197(1):392–399. doi: 10.1016/j.atherosclerosis.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li TY, Rana JS, Manson JE, et al. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;113(4):499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280(21):1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 10.Oh K, Hu FB, Manson JE, et al. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the Nurses’ Health Study. Am J Epidemiol. 2005;161(7):672–679. doi: 10.1093/aje/kwi085. [DOI] [PubMed] [Google Scholar]
- 11.Rose G, Blackburn H. Cardiovascular Survey Methods. Geneva, Switzerland: World Health Organization; 1982. [PubMed] [Google Scholar]
- 12.Yanosky JD, Paciorek C, Schwartz J, et al. Spatio-temporal modeling of chronic PM10 exposure for the Nurses’ Health Study. Atmos Environ. 2008;42(18):4047–4062. doi: 10.1016/j.atmosenv.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerrett M, Burnett RT, Brook J, et al. Do socioeconomic characteristics modify the short term association between air pollution and mortality? Evidence from a zonal time series in Hamilton, Canada. J Epidemiol Community Health. 2004;58(1):31–40. doi: 10.1136/jech.58.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krewski D, Burnett RT, Goldberg MS, et al. Reanalysis of the Harvard Six Cities Study and the American Cancer Society Study of Particulate Air Pollution and Mortality: A Special Report of the Institute's Particle Epidemiology Reanalysis Project. Cambridge, MA: Health Effects Institute; 2000. [Google Scholar]
- 16.World Health Organization. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. Geneva, Switzerland: World Health Organization; 1995. (WHO technical report series 854) [PubMed] [Google Scholar]
- 17.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. Geneva, Switzerland: World Health Organization; 2000. (WHO technical report series 894) [PubMed] [Google Scholar]
- 18.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 19.Rosenlund M, Berglind N, Pershagen G, et al. Long-term exposure to urban air pollution and myocardial infarction. Epidemiology. 2006;17(4):383–390. doi: 10.1097/01.ede.0000219722.25569.0f. [DOI] [PubMed] [Google Scholar]
- 20.Chen LH, Knutsen SF, Shavlik D, et al. The association between fatal coronary heart disease and ambient particulate air pollution: are females at greater risk? Environ Health Perspect. 2005;113(12):1723–1729. doi: 10.1289/ehp.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanobetti A, Schwartz J, Samoli E, et al. The temporal pattern of mortality responses to air pollution: a multicity assessment of mortality displacement. Epidemiology. 2002;13(1):87–93. doi: 10.1097/00001648-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology. 2000;11(3):320–326. doi: 10.1097/00001648-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Laden F, Schwartz J, Speizer FE, et al. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;173(6):667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz J, Coull B, Laden F, et al. The effect of dose and timing of dose on the association between airborne particles and survival. Environ Health Perspect. 2008;116(1):64–69. doi: 10.1289/ehp.9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donaldson K, Stone V, Seaton A, et al. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect. 2001;109(suppl 4):523–527. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pope CA, III, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109(1):71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 27.Pope CA, III, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56(6):709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 28.Utell MJ, Frampton MW, Zareba W, et al. Cardiovascular effects associated with air pollution: potential mechanisms and methods of testing. Inhal Toxicol. 2002;14(12):1231–1247. doi: 10.1080/08958370290084881. [DOI] [PubMed] [Google Scholar]


