Abstract
Microfilarial periodicity of Dirofilaria immitis (the dog heartworm) was determined at two hr intervals for 72 consecutive hrs in 10 naturally infected war dogs, 3-9 years old, in Korea to facilitate harvest of the microfilariae for possible use in laboratory works and to elucidate further the periodicity of the microfilaria depending on geographic location. Although the periodicity had been observed as being low-grade nocturnal, maximal microfilarial counts were found at 21:00 hr and minimal at 11:00 hr, giving rise to an evident peak in fluctuation of the larval counts. This is the first record of the periodicity of the microfilariae identified as D. immitis in Korea.
Keywords: Dirofilaria immitis, microfilarial periodicity, dog, Korea
INTRODUCTION
Dirofilaria immitis, the dog heartworm parasitic in the right ventricle and pulmonary artery of the dog, fox, wolf and various other wild carnivores, is common in warm countries particularly in the tropics (Rhee, 1987). In Korea the prevalence rates have been shown to be 23%, 17% or 28% in dogs by means of microfilarial detection, autopsy or antigen test, respectively (Rhee, 1966; Rhee and Rim, 1970; Lee et al., 1996).
Periodicity is a well-known phenomenon which occurs with many filarioid worms, and various hypotheses put forward to explain periodicity have been comprehensively reviewed by Oishi (1959), Hawking (1967), Katamine (1972) and Masuya (1976). Females of D. immitis are ovoviviparous and the naked microfilariae may be found in the blood at all times, but there appears to be a periodicity superimposed on this which varies with geographic location (Rhee, 1987). The microfilarial periodicity of D. immitis in the peripheral blood stream has already been reported in different countries by various investigators mentioned in discussion. However, the periodicity as well as precise identification of D. immitis microfilariae in Korea, so far, has not been recorded. In order to facilitate harvest of the microfilariae for possible use in immunological, laboratory diagnosis and chemotherapeutic studies, the present investigation was designed to examine this aspect. It can be further elucidated the wave pattern of the microfilaria depending on different localities.
MATERIALS AND METHODS
Ten war dogs, 3-9 years old, with varying levels of the microfilariae of D. immitis were used in the experiment from June to July 1998. The dogs were screened by a modified Knott's test from 35 individuals in an air base of the Republic of Korea Air Force, born and reared only in Korea. In counting the degree of the microfilariae by using an automatic pipette, 0.1 ml of blood was withdrawn from the ear vein at 2 hr intervals over a period of 72 consecutive hrs and thickly smeared on three clean glass slides, respectively. After complete drying the thick blood films, they were dehemoglobinized in water, fixed in methanol, and stained with 0.02% brilliant cresyl blue for one hr. A count was made of arithmetic mean of the numbers of worms in the three thick blood films from each animal and stage. Meanwhile, the differentiation of microfilariae was based on the morphologic characteristics and on the distance of certain fixed points from the anterior extremity as percentages of the total length as a criteria for identification (Rhee, 1987).
All examinations for 72 hrs were repeated, at weekly interval, at least twice with similar results. Data obtained as percentages of mean microfilarial counts at 2 hr intervals were analyzed by Non-Linear Regression procedure in SAS package.
RESULTS
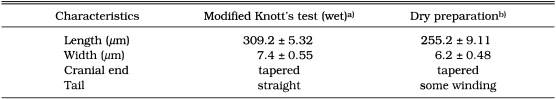
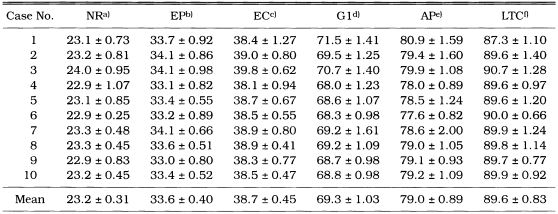
As shown in Table 1, dimension of the naked microfilariae differed between wet and dry preparations. They were 309.2 ± 5.32 × 7.4 ± 0.55 µm in a modified Knott's method fixed in 2% formalin and 255.2 ± 9.11 × 6.2 ± 0.48 µm in dry preparation fixed in methanol. The microfilariae fixed in 2% formalin had a long straight tail and a tapering cranial end. The percentage distance from the anterior end to the fixed points is depicted in Table 2. Thus the mean percentages of the distance of these reference points were: nerve ring, 23.2; excretory pore, 33.6; excretory cell, 38.7; first genital cell, 69.3; anal pore, 79.0 and last tail cell, 89.6.
Table 1.
Comparison of morphological characteristics of Dirofilaria immitis microfilariae by modified Knott's test and dry preparation
a)fixed in 2% formalin; b)fixed in methanol.
Each value represents the mean of 10 determinations per individual with the standard deviations.
Table 2.
Percentage distance from the anterior end to the fixed points of Dirofilaria immitis microfilariae in dry preparations
a)nerve ring; b)excretory pore; c)excretory cell; d)first genital cell; e)anal pore; f)last tail cell.
Each value represents the mean of 10 determinations with the standard deviations.
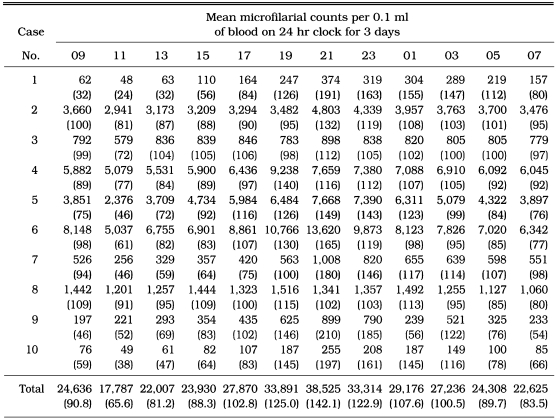
The chronological changes in the number and percentage of the mean microfilarial counts at 2 hr intervals in peripheral blood of each animal are depicted in Table 3. A significant difference of microfilarial counts was not found among the thick blood films prepared from each animal and stage. Of ten infected dogs, high microfilaraemia - 103 unit microfilaria/0.1 ml blood - was observed from five dogs, while low microfilaraemia - 102 unit microfilaria/0.1 ml blood - from another five dogs. Maximal microfilarial counts were found at 21:00 hr from eight dogs and at 19:00 hr from another two dogs, while minimal at 11:00 hr except two dogs at 07:00 and 09:00 hr, respectively. The percentage of peak count at 21:00 hr was 142.1 and minimal count at 11:00 hr 65.6. The ratio of the minimum to the maximum of the percentage of microfilarial counts (Max./Min.) was 2.17. The periodicity was observed as lowgrade nocturnal, and in agreement with the equation, Y = 98.99 + 33.97 × Sin (15.46 × X), showing that the hour (X) was a parameter (Asymptotic property, 95%; SE, B0-1.342, B1-1.917, B2-0.001).
Table 3.
Mean microfilarial counts of Dirofilaria immitis at 2 hr intervals in dogs from Korea
Percentages of mean microfilarial counts per 0.1 ml of blood in each stage on 24 hr clock for 3 days were shown in the parentheses (Asymptotic property, 95%).
DISCUSSION
There is no definite theory to explain the mechanism of microfilarial periodicity of many filarioid worms as yet. Much work had been carried out on this phenomenon, and two dominating hypotheses were proposed to explain the mechanism before. One is periodic parturition of larva and the other is of migration of larva itself in the body (Katamine, 1972). Thereafter, a diffuse autofluorescence and numerous fluorescent granules comprising Vitamin A were detected in the highly nocturnal microfilariae of Wuchereria bancrofti because the larvae were probably injured by sun light. However, in lowgrade nocturnal larvae of D. immitis, were observed less granules (Masuya, 1976). Meanwhile, the physiologic basis for periodicity is unknown; however, several investigators suggest that one or a variety of host factors influence the periodic trend of some microfilaraemias (Hawking, 1967).
The dog heartworm produces microfilariae that circulate in the peripheral blood stream as well as the blood of all other parts of canine body. There is a tendency towards microfilarial periodicity in a day (circadian rhythm) as well as seasonal periodicity showing a summit in summer throughout a year (Oishi, 1959). This appears to vary in different countries. Thus Tarplee and Bradley (1982) found maximal numbers at midnight in the USA; Euzeby and Laine (1951) found the lowest number at 08:00 hr and the greatest at 20:00 hr in France; Webber and Hawking (1955) found minimum parasitemia at 06:00 hr and maximum at 18:00 hr in a Chinese strain of D. immitis in England. Moreover, several investigators in Japan have referred to the periodicity of D. immitis as being lowgrade nocturnal (Masuya, 1976), and a distinct nocturnal: maximal numbers were found at 24:00 hr and minimal numbers at 10:00 hr and the number of maximum was 6.5 times of minimal count from 28 dogs naturally infected with D. immtis (Oishi, 1959).
On the contrary, Angus (1981) reported that there were both a distinct diurnal (16:00 hr) and lowgrade nocturnal (from 24:00 to 01:00 hr) peaks in the periodicity of D. immitis microfilariae in cephalic venous blood of dogs in South East Queensland. There was diurnal periodicity - maximal microfilarial counts of D. immitis were found at 11:00 hr and minimal at 22:00 hr in a dog from Tanzania (Matola, 1991). Moreover, the microfilaraemia in a dog experimentally infected with D. immitis was diurnally subperiodic with maximum microfilaria numbers between 12:00 and 16:00 hr (Grieve and Lauria, 1983) and Schnelle and Young (1944) observed minimum microfilaraemia at 11:00 hr and maximum at 16:30 hr in the USA.
In Korea, the present observation made on 10 cases has shown nocturnal subperiodicity, giving rise to one evident peak in fluctuation of the larval counts. This observation, along with those of other investigators who described nocturnal periodicity, differed markedly with that of Matola (1991), who found maximal numbers at approximately midday in Tanzania and those of Grieve and Lauria (1983) and Schnelle and Young (1944), who reported diurnal subperiodicity in the USA. However, the present observation is nearly in line with the observations of Tarplee and Bradley (1982) in the USA, Euzeby and Laine (1951) in France, Webber and Hawking (1955) in England, Oishi (1959) and Masuya (1976) in Japan, who found nocturnal periodicity in different countries. Overall, it was concluded that the periodicity of D. immitis microfilaria in dogs of Korea exhibited lowgrade nocturnal (Asymptotic property, 95%) and closely resembles that in the strain of Japan. It is likely that this geographical variation appears to be influenced by the environmental conditions such as sun light, atmospheric temperature and humidity upon migration of the larva itself rather than host factors, as indicated by Oishi (1959).
Our present findings and those recorded previously by others indicate that the periodicities of D. immitis microfilariae are diurnal, nocturnal or both which may be influenced by the geographical location, although the factors for such variation in the periodicity are still poorly understood.
Interestingly, it has been reported that the prevalence of Dipetalonema recon-ditum was associated with that of D. immitis in the peripheral blood of dogs in some countries. However, we could found only D. immitis, referring to the morphological characteristics in Table 1 and the corresponding description in Table 2 of that given by Newton and Wright (1956), in this study.
References
- 1.Angus BM. Periodicity exhibited by microfilariae of Dirofilaria immitis in South East Queensland. Austral Vet J. 1981;57:101–102. doi: 10.1111/j.1751-0813.1981.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 2.Euzeby J, Laine B. Sur la periodicite des microfilaires de Dirofilaria immitis. Ses variations sous l'influence de divers facteurs. Revue Vet Med Ioulouse. 1951;102:231–238. [Google Scholar]
- 3.Grieve RB, Lauria S. Periodicity of Dirofilaria immitis microfilariae in canine and murine hosts. Acta Tropica. 1983;40:121–127. [PubMed] [Google Scholar]
- 4.Hawking F. The 24-hour periodicity of microfilariae: biological mechanisms responsible for its production and control. Proc Roy Soc London. 1967;B169:59–76. [Google Scholar]
- 5.Katamine D. Mechanism of microfilarial periodicity (Turnus) Prog Med Parasit in Japan. 1972;IV:395–420. [Google Scholar]
- 6.Lee JC, Lee CY, Shin SS, Lee CG. A survey of canine heartworm infections among German shepherds in South Korea. Korean J Parasitol. 1996;34(4):225–231. doi: 10.3347/kjp.1996.34.4.225. [DOI] [PubMed] [Google Scholar]
- 7.Masuya T. Studies on the mechanism of the filarial periodicity - The autofluorescence in the microfilariae and their periodicity. Jap J Parasitol. 1976;25(4):283–312. [Google Scholar]
- 8.Matola YG. Periodicity of Dirofilaria immitis microfilariae in a dog from Muheza district, Tanzania. J Helminthol. 1991;65:76–78. [PubMed] [Google Scholar]
- 9.Newton WL, Wright WH. The occurrence of a dog filariid other than Dirofilaria immitis in the United States. J Parasitol. 1956;42:246–258. [PubMed] [Google Scholar]
- 10.Oishi I. Fundamental studies on the periodicity of the microfilariae. Nippon Eiseigaku Zasshi. 1959;14(4):549–565. [Google Scholar]
- 11.Rhee JK. Studies on the infection rate of Dirofilaria immitis of Korean dogs in Chonju and its vicinity by means of Kume's acetone-concentrating method. Korean J Vet Res. 1966;6(1):42–44. [Google Scholar]
- 12.Rhee JK. Advanced Veterinary Parasitology. 1st ed. Seoul, Korea: Daehan Printing & Publishing Co.; 1987. pp. 234–239. [Google Scholar]
- 13.Rhee JK, Rim BM. Observation on the infection rate of helminths in Korean autochthonal dogs with special reference to the viewpoint of public health. Theses Coll Chonbuk Nat Univ. 1970;12:27–38. [Google Scholar]
- 14.Schnelle GB, Young RM. Clinical studies on microfilarial periodicity in war dogs. Bull US Army Med Dept. 1944;80:52–59. [Google Scholar]
- 15.Tarplee FA, Bradley RE. The periodicity of D. immitis microfilariae and the effect of intravenous administration of acepromazine maleate on this microfilariaemia. Florida Vet J. 1982;11:35–39. [Google Scholar]
- 16.Webber WAF, Hawking F. Experimental maintenance of Dirofilaria repens and D. immitis in dogs. Exp Parasitol. 1955;4:143–164. doi: 10.1016/0014-4894(55)90007-2. [DOI] [PubMed] [Google Scholar]