Abstract
The authors used data from a nationally representative survey of 933 adults aged 54 years or older (mean age = 66.2 years; standard deviation, 8.0) in Taiwan to explore whether mortality prediction at older ages is improved by the use of 3 clusters of biomarkers: 1) standard cardiovascular and metabolic risk factors; 2) markers of disease progression; and 3) nonclinical (neuroendocrine and immune) markers. They also evaluated the extent to which these biomarkers account for the female advantage in survival. Estimates from logistic regression models of the probability of dying between 2000 and 2006 (162 deaths; mean length of follow-up = 5.8 years) showed that inclusion of each of the 3 sets of markers significantly (P = 0.024, P = 0.002, and P = 0.003, respectively) improved discriminatory power in comparison with a base model that adjusted for demographic characteristics, smoking, and baseline health status. The set of disease progression markers and the set of nonclinical markers each provided more discriminatory power than standard risk factors. Most of the excess male mortality resulted from the men being more likely than women to smoke, but each of 3 markers related to disease progression or inflammation (albumin, neutrophils, and interleukin-6) explained more than 10% of excess male mortality.
Keywords: biological markers, mortality, risk factors, sex factors, Taiwan
John Graunt, often considered the father of both demography and epidemiology, is renowned for his systematic analysis of deaths listed in London's “bills of mortality” (1). More than 3 centuries later, scholars in both fields are still engaged in mortality prediction, with demographers focusing on the influence of social, demographic, and behavioral factors and epidemiologists on risk factors for chronic disease. The recent proliferation of “biosocial surveys” that obtain sociodemographic information through interviews along with biologic markers based on physical assessments and laboratory analyses (2) provides new opportunities for enhancing mortality prediction.
Most population-based studies of all-cause mortality (3–7) focus on a set of risk factors for cardiovascular disease and metabolic syndrome that are typically measured in preventive health examinations. More recent population-based studies have included “new” measures, but most focus on cardiovascular mortality, and many include only a small number of biologic measures (8–14). Although cardiovascular disease remains a leading cause of death in many countries, including Taiwan, an examination of a single set of causes is unlikely to provide a sufficiently general explanation of overall mortality.
In this study, we used data from a national sample of older adults in Taiwan to integrate biologic and self-reported measures into models of all-cause mortality over a 6-year period. Our first goal was to evaluate the links between mortality and 3 sets of biologic measures: 1) standard risk factors related to cardiovascular and metabolic function; 2) markers of disease progression; and 3) markers of neuroendocrine and immune function. We focused on the extent to which the latter 2 clusters enhanced mortality prediction. Our second objective was to provide insight into the sex difference in mortality at older ages. A vast body of literature documents the female survival advantage in industrialized nations and a widening of this advantage through most of the 20th century (15–18). Numerous investigators have also used cause-of-death data and self-reports of morbidity to examine the sources of the sex difference (15, 19–21). By contrast, few investigators have used biologic measures to identify the physiologic pathways that underlie excess male mortality.
MATERIALS AND METHODS
Data
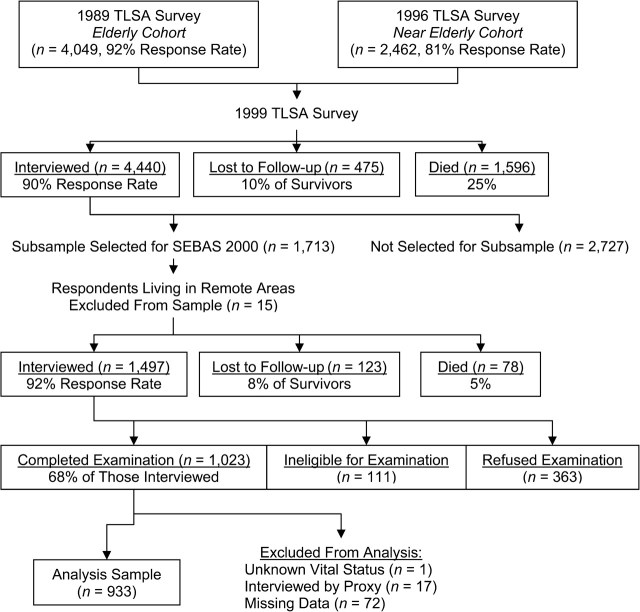
The 2000 Social Environment and Biomarkers of Aging Study (SEBAS) comprised a nationally representative sample of persons aged 54 years or older in Taiwan; elderly persons (ages ≥71 years) and urban residents were oversampled (22). As Figure 1 shows, SEBAS was based on a random subsample of respondents interviewed during the 1999 wave of the Taiwan Longitudinal Survey of Aging (sometimes referred to as the Survey of Health and Living Status of the Near-Elderly and Elderly in Taiwan). Written informed consent was obtained for participation in both the in-home interview and the hospital visit; all protocols were approved by human subjects committees in Taiwan and at Georgetown University (Washington, DC) and Princeton University (Princeton, New Jersey).
Figure 1.
Attrition across waves of the Taiwan Longitudinal Survey of Aging (TLSA) and the Social Environment and Biomarkers of Aging Study (SEBAS), Taiwan, 1989–2000. The 15 respondents living in remote areas were excluded from the SEBAS subsample because they lived too far from the hospitals contracted to participate in the physical examination portion of the study.
On a scheduled day several weeks after a household interview, participants collected a 12-hour overnight urine sample (7 PM to 7 AM). They fasted overnight and visited a nearby hospital the following morning, where medical personnel drew a blood specimen and took blood pressure and anthropometric measurements. Compliance was extremely high: 96% of participants fasted overnight and provided a urine specimen deemed suitable for analysis.
Among the 1,713 respondents selected for SEBAS, 1,497 provided interviews (92% of survivors) and 1,023 underwent the physical examination (68% of those interviewed). Of the 474 who were not examined, 111 were not asked to participate based on exclusion criteria (e.g., serious illness). Disproportionately high nonparticipation rates were found among the healthiest and least healthy respondents, with persons who underwent the medical examination reporting the same average health status as those who did not. Results presented elsewhere (23) demonstrate that, in the presence of controls for age, estimates from the medical examination portion of SEBAS are unlikely to be seriously biased.
Blood and urine specimens were analyzed at Union Clinical Laboratories in Taipei, Taiwan. In addition to the routine standardization and calibration tests performed by the laboratory, 9 persons (outside the target sample) contributed triplicate sets of specimens. The results indicated intralaboratory reliability of 0.86 or higher for duplicates sent to Union Clinical Laboratories and interlaboratory correlations of 0.65 or higher (≥0.92 in most cases) between results from Union Clinical Laboratories and results from the US laboratory Quest Diagnostics (San Juan Capistrano, California). The original assays of interleukin-6 (Endogen enzyme immunoassay; Pierce Biotechnology, Rockford, Illinois) performed in 2000 had a high proportion (32%) of values below the limit of detection. Here we used measures based on new assays of the stored frozen specimens conducted in 2007 (enzyme-linked immunosorbent assay (R&D Systems, Inc., Minneapolis, Minnesota); lower detection limit = 0.7 pg/mL, interassay coefficient of variation = 12.6%). Serum creatinine was assayed using the alkaline picrate method (Beckman CX7 (Beckman Coulter, Inc., Fullerton, California); detection limit = 0.1 mg/dL, coefficient of variation = 3.3%). Serum albumin was assayed using the bromcresol green method (detection limit = 1.0 g/dL, coefficient of variation = 1.5%). Leukocyte (white blood cell) count was determined by direct current using a Sysmex Cell Counter SE-9000 (Sysmex Corporation, Kobe, Japan; detection limit = 0.02 × 103 cells/μL, coefficient of variation = 1.5%). Percentage of neutrophils was calculated from white blood cells, eosinophils, basophils, lymphocytes, and monocytes (coefficient of variation = 3.3%). Details regarding the remaining assays are provided elsewhere (22).
Survival status was ascertained through linkage with the death certificate file maintained by the Taiwan Department of Health and the household registration file maintained by the Ministry of the Interior. After exclusion of 1 person with unknown vital status, those with proxy interviews (n = 17), and respondents with missing data on covariates (n = 72), the analysis sample comprised 933 respondents (162 of whom died by December 31, 2006).
Biomarker selection
The first cluster of biomarkers comprised 6 standard risk factors related to cardiovascular and metabolic function: hypertension, total cholesterol, high density lipoprotein cholesterol, body mass index (weight (kg)/height (m)2), waist circumference, and glycosylated hemoglobin. Many population-based studies have established a link between these risk factors and all-cause mortality (4, 24–27).
The 4 markers in the second group are used to evaluate and monitor disease. Creatinine clearance is a key indicator of kidney function. Albumin, the major protein found in plasma, represents a nonspecific but highly sensitive measure of disease progression (28). Leukocyte (white blood cell) count is an indicator of cellular response to inflammation (29). Neutrophils, the most abundant type of white blood cell in humans, are generally associated with acute inflammation. All 4 of these measures have been found to be associated with mortality in population-based samples (30–33).
The third group of biomarkers included 4 neuroendocrine measures—epinephrine, norepinephrine, cortisol, and dehydroepiandrosterone sulfate—and 2 immune markers—interleukin-6 and insulin-like growth factor 1. We refer to this group as nonclinical because these measures are not widely used in clinical practice and do not have well-established cutoffs. Nevertheless, recent population-based studies have shown that these markers are associated with mortality (34–38), and a 3-year follow-up in Taiwan suggested that they may be stronger predictors than the standard risk factors (39).
Biomarker and control variables
Variables based on the 3 groups of biomarkers are shown in Table 1. Classifications for the standard risk factors were based on established cutoffs where appropriate (40, 41). Diastolic and systolic blood pressure were calculated as the average of 2 seated readings (1 minute apart) taken by a registered nurse (using a mercury sphygmomanometer on the right arm) at least 20 minutes after the respondent arrived at the hospital. The classification for body mass index conformed to the categories used by the Taiwan Department of Health (42). The cutoffs for waist circumference were those recommended for Asian populations (43, 44). Glycosylated hemoglobin was parameterized as a continuous variable because reference ranges vary across laboratories (45). Two dichotomous measures captured data on the use of antihypertensive and hypoglycemic medications.
Table 1.
Biomarkers, Control Variables, and Outcome Variable Included in Mortality Models (n = 933), Social Environment and Biomarkers of Aging Study, Taiwan, 2000a
| Mean (SD) | % | |
| Death by December 31, 2006 | 17.4 | |
| Sociodemographic variables | ||
| Age in 2000, years (observed range, 54–91) | 66.2 (8.0) | |
| Male sex | 57.9 | |
| Mainlander | 13.2 | |
| Education, years (observed range, 0–17) | 5.2 (4.6) | |
| Urban resident | 43.5 | |
| Baseline health status | ||
| No. of current health conditions (potential range, 0–12)b | 1.3 (1.3) | |
| No. of mobility limitations (potential range, 0–9)c | 1.8 (2.3) | |
| Cognitive function (potential range, 0–24)d | 16.7 (3.5) | |
| CES-D score (potential range, 0–30)e | 5.4 (5.2) | |
| Self-assessed health status (potential range, 1–5; 5 = excellent)f | 3.1 (1.0) | |
| Any smoking in past 6 months | 24.3 | |
| Standard risk factors | ||
| Hypertension | ||
| Normal blood pressure (SBP <120 mm Hg and DBP <80 mm Hg) | 15.5 | |
| Prehypertension (SBP 120–139 or DBP 80–89) | 34.9 | |
| Stage 1 hypertension (SBP 140–159 or DBP 90–99) | 32.2 | |
| Stage 2 hypertension (SBP ≥160 or DBP ≥100) | 17.3 | |
| Use of antihypertensive medication | 22.7 | |
| Total cholesterol, mg/dL | ||
| Desirable (<200) | 50.9 | |
| Borderline-high (200–239) | 33.9 | |
| High (≥240) | 15.2 | |
| High density lipoprotein cholesterol, mg/dL | ||
| Low (<40) | 26.9 | |
| Normal (40–59) | 51.8 | |
| High (≥60) | 21.3 | |
| Body mass indexg | ||
| Underweight (<18.5) | 3.3 | |
| Normal (18.5–23.9) | 45.1 | |
| Overweight (24–26.9) | 30.1 | |
| Obese (≥27) | 21.5 | |
| High waist circumference (females: >80 cm; males: >90 cm) | 49.0 | |
| Glycosylated hemoglobin, %h | 5.8 (1.4) | |
| Use of hypoglycemic agents | 11.7 | |
| Markers of disease progression | ||
| Creatinine clearance, mL/minutei | 62.4 (17.9) | |
| Serum albumin, g/dL | 4.5 (0.3) | |
| White blood cell count, 103 cells/μLh | 6.1 (1.6) | |
| Neutrophils, % | 56.3 (9.4) | |
| Nonclinical markers | ||
| Urinary epinephrine, μg/g creatininehj | 2.5 (2.5) | |
| Urinary norepinephrine, μg/g creatinineh | 21.7 (9.6) | |
| Urinary cortisol, μg/g creatinineh | 26.7 (29.7) | |
| Serum dehydroepiandrosterone sulfate, μg/dLhj | 80.8 (58.1) | |
| Interleukin-6, pg/mLhj | 3.3 (3.5) | |
| Insulin-like growth factor 1, ng/mLh | 107.0 (48.3) |
Abbreviations: CES-D, Center for Epidemiologic Studies Depression Scale; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
Descriptive statistics are based on weighted data.
Current illness was measured by counting the following 12 self-reported conditions: high blood pressure, diabetes mellitus, heart disease, cancer or malignant tumor, lower respiratory tract disease, arthritis or rheumatism, gastric ulcer or stomach ailment, liver or gallbladder disease, cataracts, kidney disease, gout, and spinal or vertebral spurs.
The measure of mobility limitations counted how many of the following physical tasks the respondent reported difficulty in performing without aid: standing continuously for 15 minutes and for 2 hours, squatting, raising both hands over one's head, grasping or turning objects with one's fingers, lifting or carrying an object weighing 11–12 kg, running a short distance (20–30 m), walking 200–300 m, and climbing 2 or 3 flights of stairs.
The measure of cognitive function counted the number of cognitive tasks completed incorrectly, including basic orientation questions, a series of 4 subtractions, and immediate memory recall.
Depressive symptoms were measured by means of a 10-item short form of the full Center for Epidemiologic Studies Depression Scale (47), coded according to standard practice based on both the number and severity of symptoms.
Global self-assessed health status was based on the following question: “Regarding your current state of health, do you feel it is excellent, good, average, not so good, or poor?”
Weight (kg)/height (m)2.
Outliers (i.e., values greater than 5 standard deviations above the mean) were recoded to that cutpoint (i.e., trimmed). Values were trimmed for glycosylated hemoglobin (n = 4), white blood cell count (n = 1), epinephrine (n = 1), norepinephrine (n = 1), cortisol (n = 3), dehydroepiandrosterone sulfate (n = 4), interleukin-6 (n = 2), and insulin-like growth factor 1 (n = 1).
Creatinine clearance was estimated on the basis of the Cockcroft-Gault formula (46); outliers (n = 3) for serum creatinine were trimmed before calculation of this measure.
Approximately 11% of values on interleukin-6, 20% of values on epinephrine, and 1% of values on dehydroepiandrosterone sulfate were below assay sensitivity; these cases were assigned a value of 0.
Four markers of disease progression were obtained from the fasting blood sample. Creatinine clearance was estimated from the formula of Cockcroft and Gault (46), which is based on the level of serum creatinine, taking into account age, sex, and body weight. Serum albumin, white blood cell count, and the percentage of white blood cells comprised of neutrophils were measured as continuous variables.
Because there are no established cutoffs, the nonclinical measures were also treated as continuous variables. Epinephrine, norepinephrine, and cortisol measurements were obtained from the overnight urine specimen, which provided integrated values of basal operating levels during a period when most participants were resting; values are reported in micrograms per gram of urinary creatinine to adjust for body size. Levels of dehydroepiandrosterone sulfate, interleukin-6, and insulin-like growth factor 1 were based on the fasting blood sample.
Sociodemographic control factors included in each model comprised age, sex, ethnicity (mainlander vs. Taiwanese), education, and urban residence. We tested a quadratic term for age to capture possible nonlinear effects; because it was not significant, we excluded it from the final models. We also included 6 measures of baseline health status: 1) number of current health conditions; 2) number of mobility limitations; 3) cognitive function score; 4) Center for Epidemiologic Studies Depression Scale (47) score; 5) global self-assessed health; and 6) recent smoking status.
Analytical strategy
In the initial stage of the analysis, we fitted both logistic regression models of the probability of dying between 2000 and 2006 and Cox proportional hazards models based on age-specific mortality rates over the 6-year period. Diagnostic checks indicated that the proportional hazards assumption was violated for sex and several other covariates. Thus, although a hazard model has the advantage that age can be used as the time scale, there would be no well-identified procedure for obtaining estimates of biomarker-specific contributions to excess male mortality in a model that accounted for nonproportional hazards. Moreover, because we did not have additional information on biomarkers within the 6-year period to consider time-varying covariates and because all respondents were followed for the same time period, the logistic model did not present serious drawbacks. Thus, we focused the analysis on estimates derived from logistic regression, but below we describe the similarity of results from the 2 approaches.
In order to adjust for the clustered sampling design of the Taiwan Longitudinal Survey of Aging and SEBAS, we incorporated into the logistic regression models a random effect for the primary sampling units. Model 1 included sociodemographic variables and measures of baseline health status that may affect both biomarkers and mortality. Each of the subsequent 3 models added 1 cluster of biomarkers: cardiovascular/metabolic markers (model 2), markers of disease progression (model 3), and nonclinical markers (model 4). Model 5 included all 3 clusters of biomarkers. Because previous research has demonstrated that risk is often associated with both low and high levels of biomarkers or, more generally, that the associations between some biomarkers and mortality are nonlinear, we tested quadratic terms for the continuous biomarkers. We retained the 4 terms that were statistically significant (P < 0.05, 2-sided): creatinine clearance, white blood cell count, epinephrine, and interleukin-6. To test the robustness of our findings, we recoded our continuous biomarkers into categories based on quintiles of the observed distributions and found that the substantive results were unchanged (results not shown).
We calculated the receiver operating characteristic curve to evaluate the accuracy of the models in discriminating between decedents and survivors. The area under the receiver operating characteristic curve (AUC) summarizes the performance of a model, with higher values indicating better accuracy. Chi-square tests based on the AUC values were used to determine whether inclusion of a given set of markers yielded a significant improvement over the base model.
Finally, we examined the contribution of each biomarker in accounting for the sex difference in mortality. Before determining the model to use for this exercise, we added interaction terms between each biomarker variable and sex to models 2–4 to assess whether the effects of the biomarkers differed by sex. Because the interaction terms were significant (P < 0.05, 2-sided) for only 2 biomarkers (white blood cells and interleukin-6)—little more than what we would expect by chance—we did not include these interactions in the model.
In light of a substantial body of literature that attributes a large fraction of excess male mortality in industrialized countries to smoking, we calculated the contribution attributable to smoking as a benchmark. Thus, our base model for determining the mortality odds ratio (OR) for males relative to females (ORbase) included all of the sociodemographic and health-status variables shown in model 1 except smoking. Because males have higher mortality than females in Taiwan, ORbase exceeds 1 and provides a measure of the degree of excess male mortality after adjustment for the control variables. Subsequently, we added a biomarker, which may have comprised more than 1 variable for biomarkers that were categorical, had a quadratic term, or included an associated variable denoting medication use, to the base model. The resulting odds ratio for males (ORw/marker) was used to calculate the percentage change in excess male mortality attributable to that biomarker:
A negative percent change implied that inclusion of the biomarker accounted for some of the excess male mortality, whereas a positive percent change indicated that inclusion of the biomarker exaggerated the sex difference. Corresponding calculations were performed for the smoking variable and for the 3 clusters of biomarkers.
To examine the robustness of our findings, we repeated this analysis including information in the base model on whether the respondent smoked. Because the effect of smoking on excess mortality could not be estimated in this exercise, the estimates of percent change were larger for almost all of the biomarkers. However, the relative importance of the biomarkers remained essentially unaltered—for example, the 3 biomarkers associated with the largest reduction in the sex difference were the same regardless of whether smoking was included in the model. Stata 10.1 was used for all analyses (Stata Corporation, College Station, Texas).
RESULTS
Table 2 presents estimated odds ratios for the 5 logistic models. Among the standard risk factors, only low body mass index (underweight) was significantly associated with mortality (P = 0.017, 2-sided). All 4 markers of disease progression and 3 nonclinical measures (epinephrine, interleukin-6, and, in model 5, cortisol) were significantly associated with 6-year mortality.
Table 2.
Odds Ratios From Logistic Regression Modelsa for the Probability of Dying Between 2000 and 2006 (n = 933), Social Environment and Biomarkers of Aging Study, Taiwan
| Baseline (Model 1) |
Standard Risk Factors (Model 2) |
Markers of Disease Progression (Model 3) |
Nonclinical Markers (Model 4) |
All Biomarkers (Model 5) |
||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Sociodemographic factors | ||||||||||
| Age | 1.117** | 1.084, 1.151 | 1.118** | 1.083, 1.155 | 1.074** | 1.036, 1.113 | 1.105** | 1.071, 1.140 | 1.062** | 1.020, 1.106 |
| Male sex | 1.579 | 0.964, 2.589 | 1.759* | 1.016, 3.046 | 1.438 | 0.857, 2.414 | 1.894* | 1.102, 3.254 | 2.410** | 1.277, 4.546 |
| Any smoking in past 6 months | 2.600** | 1.617, 4.181 | 2.401** | 1.467, 3.929 | 2.634** | 1.594, 4.351 | 2.502** | 1.523, 4.110 | 2.612** | 1.510, 4.516 |
| Standard risk factors | ||||||||||
| Hypertension | ||||||||||
| Normal (referent) | 1 | 1 | ||||||||
| Prehypertension | 0.903 | 0.471, 1.731 | 0.948 | 0.471, 1.911 | ||||||
| Stage 1 hypertension | 0.746 | 0.383, 1.452 | 0.728 | 0.354, 1.496 | ||||||
| Stage 2 hypertension | 1.404 | 0.691, 2.854 | 1.544 | 0.716, 3.329 | ||||||
| Use of antihypertensive medication | 0.834 | 0.499, 1.395 | 0.821 | 0.467, 1.445 | ||||||
| Total cholesterol | ||||||||||
| Desirable (referent) | 1 | 1 | ||||||||
| Borderline high | 0.772 | 0.486, 1.224 | 0.866 | 0.525, 1.429 | ||||||
| High | 1.359 | 0.755, 2.444 | 1.630 | 0.843, 3.153 | ||||||
| High density lipoprotein cholesterol | ||||||||||
| Low (referent) | 1 | 1 | ||||||||
| Normal | 0.707 | 0.449, 1.114 | 0.755 | 0.460, 1.240 | ||||||
| High | 0.745 | 0.403, 1.376 | 0.614 | 0.314, 1.201 | ||||||
| Body mass indexb | ||||||||||
| Underweight | 2.808* | 1.205, 6.542 | 1.840 | 0.728, 4.652 | ||||||
| Normal (referent) | 1 | 1 | ||||||||
| Overweight | 0.891 | 0.525, 1.514 | 1.188 | 0.655, 2.156 | ||||||
| Obese | 0.743 | 0.381, 1.449 | 1.332 | 0.616, 2.879 | ||||||
| High waist circumference | 1.262 | 0.739, 2.154 | 1.606 | 0.887, 2.908 | ||||||
| Glycosylated hemoglobin | 1.192 | 0.988, 1.440 | 1.156 | 0.939, 1.423 | ||||||
| Use of hypoglycemic agents | 0.989 | 0.466, 2.096 | 1.156 | 0.515, 2.595 | ||||||
| Markers of disease progression | ||||||||||
| Creatinine clearance | 0.922** | 0.874, 0.971 | 0.914** | 0.864, 0.968 | ||||||
| Creatinine clearance squared | 1.000* | 1.000, 1.001 | 1.000* | 1.000, 1.001 | ||||||
| Albumin | 0.355** | 0.177, 0.715 | 0.428* | 0.197, 0.930 | ||||||
| White blood cell count | 0.581 | 0.325, 1.040 | 0.557 | 0.297, 1.044 | ||||||
| White blood cell count squared | 1.047* | 1.006, 1.089 | 1.042 | 0.998, 1.087 | ||||||
| Neutrophils | 1.027* | 1.004, 1.050 | 1.021 | 0.996, 1.047 | ||||||
| Nonclinical markers | ||||||||||
| Epinephrine | 0.884 | 0.741, 1.054 | 0.906 | 0.749, 1.094 | ||||||
| Epinephrine squared | 1.020** | 1.005, 1.035 | 1.018* | 1.002, 1.034 | ||||||
| Norepinephrine | 1.004 | 0.984, 1.025 | 1.020 | 0.997, 1.043 | ||||||
| Cortisol | 1.005 | 0.999, 1.011 | 1.006* | 1.000, 1.013 | ||||||
| Dehydroepiandrosterone sulfate | 1.000 | 0.995, 1.004 | 1.000 | 0.996, 1.005 | ||||||
| Interleukin-6 | 1.350** | 1.183, 1.541 | 1.235** | 1.064, 1.434 | ||||||
| Interleukin-6 squared | 0.991** | 0.984, 0.997 | 0.993 | 0.987, 1.000 | ||||||
| Insulin-like growth factor 1 | 0.999 | 0.995, 1.004 | 0.999 | 0.994, 1.004 | ||||||
| Log-likelihood | −350.7 | −339.6 | −327.2 | −326.2 | −299.8 | |||||
| AUC | 0.79 | 0.80 | 0.82 | 0.82 | 0.85 | |||||
| P value from AUC test (vs. model 1) | 0.0240 | 0.0017 | 0.0027 | 0.0000 | ||||||
Abbreviations: AUC, area under the receiver operating characteristic curve; CI, confidence interval; OR, odds ratio.
* P < 0.05 (2-sided); **P < 0.01 (2-sided).
Models included a random-effect term for the primary sampling units and adjustment for mainlander status, education, urban residence, current chronic conditions, mobility limitations, cognitive function, Center for Epidemiologic Studies Depression Scale (47) score, and self-assessed health status. For definitions of variables, see Table 1.
Weight (kg)/height (m)2.
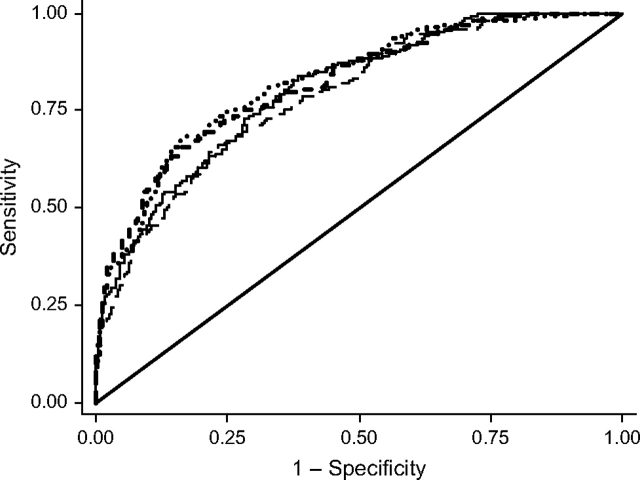
The receiver operating characteristic curves for models 1–4 are presented in Figure 2. Chi-square tests based on the AUC values (bottom of Table 2) indicated that inclusion of each of the 3 sets of markers significantly improved discriminatory power in comparison with the base model (model 2: P = 0.024; model 3: P = 0.002; model 4: P = 0.003). The disease progression and nonclinical models (models 3 and 4) had larger AUC values than the standard risk factor model (model 2). Additional comparisons (not shown) between the AUC value for model 2 and the AUC values for models that added the disease progression or nonclinical markers to model 2 revealed that both sets of markers significantly increased the predictive power of model 2 (P = 0.003 and P = 0.002, respectively). Comparisons across the models revealed that only the disease progression variables accounted for a substantial fraction of the increased risk of dying with age (the odds ratio for age decreased from 1.12 in model 1 to 1.07 in model 3) or the level of excess male mortality (the odds ratio for male sex decreased from 1.58 in model 1 to 1.44 in model 3).
Figure 2.
Receiver operating characteristic curves for models of the probability of dying between 2000 and 2006, Social Environment and Biomarkers of Aging Study, Taiwan. – – –, baseline (model 1); —, standard risk factors (model 2); • • •, markers of disease progression (model 3); - - -, nonclinical markers (model 4); —, referent.
We fitted models similar to those in Table 2 using Cox proportional hazards (results not shown). The Cox models were stratified to permit different baseline hazards for men and women, and standard errors were adjusted for clustering within primary sampling units; in addition, quadratic terms were included for only those biomarkers with significant quadratic terms in the Cox models. The 2 sets of estimates were very similar. For example, the AUC values for each of the 5 models differed by less than 0.01 between the 2 approaches. Similarly, for both the logistic models and the Cox models, the disease progression and nonclinical models yielded larger improvements over the baseline model than the standard risk factor model.
Table 3 reveals that an indicator of whether the respondent had smoked during the past 6 months accounted for more than half of excess male mortality: The base model implied that the odds of dying were 2.3 times greater for men than for women, but ORmale was reduced to 1.6 (and no longer significant) after the model controlled for smoking status. That is, more than half of excess male mortality resulted from a higher prevalence of smoking among men than among women in this sample (41% vs. 2%). Two markers of disease progression (albumin and neutrophils) and 1 nonclinical marker (interleukin-6) each explained more than 10% of the sex difference in mortality, but these reductions were much more modest than those from smoking.
Table 3.
Contributions of Various Biomarkers to the Sex Difference in Mortalitya (n = 933), Social Environment and Biomarkers of Aging Study, Taiwan, 2000–2006
| Odds Ratio for Male Sex | 95% Confidence Interval | Change From Base Model, % | |
| Base modelb | 2.28* | 1.46, 3.57 | |
| Any smoking in past 6 months | 1.58 | 0.96, 2.59 | −54.8 |
| Standard risk factors | |||
| Hypertension and use of antihypertensive agents | 2.31* | 1.47, 3.62 | 2.1 |
| Total cholesterol | 2.26* | 1.43, 3.56 | −1.7 |
| High density lipoprotein cholesterol | 2.21* | 1.40, 3.47 | −5.8 |
| Body mass indexc | 2.20* | 1.40, 3.46 | −6.5 |
| High waist circumference | 2.32* | 1.45, 3.69 | 2.6 |
| Glycoslyated hemoglobin and use of hypoglycemic agents | 2.51* | 1.59, 3.97 | 17.8 |
| All biomarkers in this cluster | 2.51* | 1.52, 4.14 | 17.6 |
| Markers of disease progression | |||
| Creatinine clearance and its quadratic term | 2.33* | 1.47, 3.67 | 3.3 |
| Albumin | 2.12* | 1.35, 3.34 | −12.4 |
| White blood cell count and its quadratic term | 2.33* | 1.48, 3.66 | 3.6 |
| Neutrophils | 2.14* | 1.36, 3.36 | −11.4 |
| All biomarkers in this cluster | 2.09* | 1.31, 3.34 | −15.0 |
| Nonclinical markers | |||
| Epinephrine and its quadratic term | 2.80* | 1.75, 4.48 | 40.5 |
| Norepinephrine | 2.40* | 1.53, 3.79 | 9.4 |
| Cortisol | 2.34* | 1.49, 3.68 | 4.8 |
| Dehydroepiandrosterone sulfate | 2.34* | 1.48, 3.69 | 4.5 |
| Interleukin-6 and its quadratic term | 2.12* | 1.34, 3.36 | −12.7 |
| Insulin-like growth factor 1 | 2.30* | 1.47, 3.60 | 1.5 |
| All biomarkers in this cluster | 2.69* | 1.64, 4.43 | 32.0 |
* P < 0.01 (2-sided).
Each row represents the effect of adding the specified biomarker or cluster of biomarkers to the base model. A negative percent change implies that inclusion of the selected biomarker accounts for some of the excess male mortality, whereas a positive percent change indicates that inclusion of the biomarker exaggerates the sex difference.
The base model excluded smoking from model 1 (Table 2).
Weight (kg)/height (m)2.
In contrast, ORmale increased after adjustment for many of the standard risk factors and for all of the nonclinical markers except interleukin-6. Because women are generally more likely than men to have high risk levels of these markers, the model predicts that excess male mortality would be even greater if men and women had the same average levels of these biomarkers.
DISCUSSION
Our analyses addressed 2 important questions. First, do various clusters of biomarkers improve our prediction of survival over a base model that adjusts for demographic characteristics, smoking, and baseline health status? Second, do measurements of these biomarkers shed any light on differences in mortality between men and women at older ages?
We found that each of the 3 clusters of biomarkers improved discriminatory power in comparison with the base model. In addition, after adjustment for the standard risk factors, the markers of disease progression and the nonclinical markers each significantly improved mortality prediction.
We also determined that smoking accounted for 55% of excess male mortality. This estimate is consistent with several studies in the United States and Western Europe that found that, averaged over a broad range of adult ages, the sex difference in all-cause mortality attributable to smoking ranged between 40% and 60% (48–50). Although no biomarker in our study had such a dramatic effect, 3 markers—interleukin-6, albumin, and neutrophils—each accounted for more than 10% of the excess male mortality in our sample. Thus, measures related to inflammation and the presence or progression of disease may provide some insight into why males experience higher mortality at these ages. For example, markers of kidney (creatinine and albumin) and liver (albumin) function may reflect life-threatening conditions that are more common among men than among women.
This study had several advantages over earlier efforts to enhance mortality prediction in general populations. Foremost, SEBAS was based on a nationally representative sample of older Taiwanese. The survey collected data on an extensive set of biomarkers, was linked with verified mortality information for a 6-year follow-up period, and obtained detailed information about potential confounders.
At the same time, there were several important limitations of this analysis. Biomarkers were measured on a single occasion—either overnight prior to the medical examination or during the examination—and may have been affected by medication use or diet. The reference periods associated with these markers vary enormously (e.g., glycosylated hemoglobin reflects the previous several months, whereas interleukin-6 can vary widely within a single day). Moreover, the restriction of SEBAS to persons aged 54 years or older may limit the generalizability of these results to younger age groups. For example, the estimates in Table 2 demonstrated that low body mass index predicted mortality whereas high body mass index did not; this finding probably arose because of the association between disease and weight loss at older ages and is consistent with other studies of older adults (51–53). The relations between mortality and other cardiovascular risk factors—particularly cholesterol and blood pressure—have been shown to weaken or reverse direction with increasing age (53–55). However, estimates based on separate analyses of respondents aged 54–69 and ≥70 years (not shown) indicated that the standard risk factors, considered as a group, were equally strong predictors for both age cohorts in Taiwan.
Another potential shortcoming is that our estimates were based on the assumption that the effects of the biomarkers on survival are the same for men and women. This assumption is consistent with findings from 2 large-scale population-based studies (13, 53) and leads to a parsimonious model, but it may understate the contributions associated with indicators of disease and inflammation. This speculation is based on exploratory analyses which suggested that both white blood cell count and interleukin-6 have larger effects on mortality among men than among women and the fact that the Cockcroft-Gault formula used to estimate creatinine clearance incorporates an implicit sex interaction. Had we used unadjusted values, serum creatinine would have accounted for a nontrivial proportion of excess male mortality. In future studies with sufficient statistical power, investigators need to reexamine sex-specific associations between the biomarkers and survival despite the potential complexity (i.e., the estimated contribution to excess male mortality would vary by the levels of the biomarkers).
Our findings are consistent with previous research in 2 respects. First, our results suggest that measures beyond standard risk factors are predictive of survival in generally healthy populations—a conclusion supported by a growing body of literature on the association between interleukin-6 and mortality in Western population-based samples (12, 35, 38, 56) and links between overall mortality and 1 or more of the following markers: epinephrine, creatinine, albumin, and white blood cell count (10, 13, 37, 53, 57). Second, our results demonstrate that these biomarkers cannot account for the observed sex difference in mortality. Although surprising at first glance, this outcome is in line with a comparative study that demonstrated that the female advantage regarding standard risk factors was smaller in Taiwan than in the United States and that sex differences in hormonal markers embodied a substantial female disadvantage in both countries (58). Fried et al. (53) reached a similar conclusion regarding the failure of biomarkers to explain excess US male mortality, despite adjustment for cardiovascular risk factors, measures of clinical and subclinical disease, and functional and cognitive impairments.
These results suggest that we and other researchers may be missing critical physiologic systems or pathways that underlie excess male mortality. Alternatively, the sex difference in survival may operate through huge numbers of potentially unobservable or unmeasurable physiologic effects. Our results underscore the importance not only of including an expansive set of biologic measures in survival models but also of examining the “social pathways” through which biologic effects may operate differently for men and women, such as risk-taking behaviors (other than smoking), utilization of health services, and compliance with medical protocols.
Whether the additional explanatory power of any set of biomarkers is substantively important lies not in any statistical analysis or test but in the eye of the beholder. For example, if the standard risk factor model is viewed as an improvement over a demographic model of mortality, then we need to recognize that the nonclinical and disease progression biomarkers provide enhancements in prediction that are at least as notable. Some researchers have argued that selected “new biomarkers” yield only a minor improvement in all-cause mortality prediction (13) and that the estimated cost of measurement does not justify their use as a general screening tool (59), whereas others have reported that such biomarkers substantially improve mortality prediction (14). In the present study, we found that the set of disease progression markers and the cluster of markers reflecting neuroendocrine and immune function each 1) significantly improved mortality prediction over a model that included standard risk factors and 2) provided more discriminatory power than the standard risk factors. From a theoretical perspective, the inflammation and disease progression markers provide a foothold for understanding the sources of sex differentials in mortality in industrialized countries, as well as increases in mortality risk with age. On the other hand, from a practical perspective, we recognize that some of these measures may not be causally linked to survival, that we lack information on preventive interventions based on these markers, and that potential interventions may not be cost-effective.
Acknowledgments
Author affiliations: Office of Population Research, Princeton University, Princeton, New Jersey (Noreen Goldman); Department of Demography, University of California, Berkeley, Berkeley, California (Dana A. Glei); Bureau of Health Promotion, Population and Health Research Center, Department of Health, Taiwan, Republic of China (Yu-Hsuan Lin); and Center for Population and Health, Georgetown University, Washington, DC (Maxine Weinstein).
This work was supported by the Demography and Epidemiology Unit of the Behavioral and Social Research Program of the US National Institute on Aging (grants R01AG16790 and R01AG16661).
The authors are grateful to Dr. Min-Long Lai and Susana Ong at Union Clinical Laboratory in Taipei, Taiwan, for their assistance with the laboratory assays. The authors thank Dr. Tristan Gorrindo for his comments and suggestions.
This paper was presented at the 2008 Annual Meeting of the Population Association of America in New Orleans, Louisiana.
Data and documentation for the 2000 Social Environment and Biomarkers of Aging Study are available from the Inter-University Consortium for Political and Social Research at http://www.icpsr.umich.edu/cocoon/ICPSR/STUDY/03792.xml.
Conflict of interest: none declared.
Glossary
Abbreviations
- AUC
area under the receiver operating characteristic curve
- OR
odds ratio
- SEBAS
Social Environment and Biomarkers of Aging Study
References
- 1.Graunt J. Natural and Political Observations Mentioned in a Following Index, and Made Upon the Bills of Mortality. London, United Kingdom: Martyn and Allestry; 1662. (Republished, with an introduction by B. Benjamin, in the Journal of the Institute of Actuaries (J Inst Actuar. 1964;90:1–61)) [Google Scholar]
- 2.Weinstein M, Vaupel JW, Wachter KW, editors. Biosocial Surveys: Committee on Advances in Collecting and Utilizing Biological Indicators and Genetic Information in Social Science Surveys. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- 3.Menotti A, Blackburn H, Kromhout D, et al. Cardiovascular risk factors as determinants of 25-year all-cause mortality in the Seven Countries Study. Eur J Epidemiol. 2001;17(4):337–346. doi: 10.1023/a:1012757616119. [DOI] [PubMed] [Google Scholar]
- 4.Norrish A, North D, Yee RL, et al. Do cardiovascular disease risk factors predict all-cause mortality? Int J Epidemiol. 1995;24(5):908–914. doi: 10.1093/ije/24.5.908. [DOI] [PubMed] [Google Scholar]
- 5.Kronmal RA, Cain KC, Ye Z, et al. Total serum cholesterol levels and mortality risk as a function of age. A report based on the Framingham data. Arch Intern Med. 1993;153(9):1065–1073. [PubMed] [Google Scholar]
- 6.Wilson PW, Abbott RD, Castelli WP. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis. 1988;8(6):737–741. doi: 10.1161/01.atv.8.6.737. [DOI] [PubMed] [Google Scholar]
- 7.Harris T, Cook EF, Garrison R, et al. Body mass index and mortality among nonsmoking older persons. The Framingham Heart Study. JAMA. 1988;259(10):1520–1524. [PubMed] [Google Scholar]
- 8.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145(1):21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 9.Jenny NS, Yanez ND, Psaty BM, et al. Inflammation biomarkers and near-term death in older men. Am J Epidemiol. 2007;165(6):684–695. doi: 10.1093/aje/kwk057. [DOI] [PubMed] [Google Scholar]
- 10.Kistorp C, Raymond I, Pedersen F, et al. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293(13):1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 11.Lee KW, Hill JS, Walley KR, et al. Relative value of multiple plasma biomarkers as risk factors for coronary artery disease and death in an angiography cohort. CMAJ. 2006;174(4):461–466. doi: 10.1503/cmaj.050880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stork S, Feelders RA, van den Beld AW, et al. Prediction of mortality risk in the elderly. Am J Med. 2006;119(6):519–525. doi: 10.1016/j.amjmed.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355(25):2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 14.Zethelius B, Berglund L, Sundstrom J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358(20):2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 15.Lopez AD. The sex mortality differentials in developed countries. In: Lopez AD, Ruzicka LT, editors. Sex Differentials in Mortality: Trends, Determinants and Consequences. Canberra, Australia: Australian National University; 1983. pp. 53–120. [Google Scholar]
- 16.Pampel FC. Cigarette use and the narrowing sex differential in mortality. Popul Dev Rev. 2002;28(1):77–104. [Google Scholar]
- 17.Department of International Economic and Social Affairs, United Nations. Sex differentials in life expectancy and mortality in developed countries: an analysis by age groups and causes of death from recent and historical data. Popul Bull UN. 1988;25:65–107. [PubMed] [Google Scholar]
- 18.Glei DA, Horiuchi S. The narrowing sex differential in life expectancy in high-income populations: effects of differences in the age pattern of mortality. Popul Stud (Camb) 2007;61(2):141–159. doi: 10.1080/00324720701331433. [DOI] [PubMed] [Google Scholar]
- 19.Trovato F, Lalu NM. Contribution of cause-specific mortality to changing sex differences in life expectancy: seven nations case study. Soc Biol. 1998;45(1-2):1–20. doi: 10.1080/19485565.1998.9988961. [DOI] [PubMed] [Google Scholar]
- 20.Wong MD, Chung AK, Boscardin WJ, et al. The contribution of specific causes of death to sex differences in mortality. Public Health Rep. 2006;121(6):746–754. doi: 10.1177/003335490612100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Case A, Paxson C. Sex differences in morbidity and mortality. Demography. 2005;42(2):189–214. doi: 10.1353/dem.2005.0011. [DOI] [PubMed] [Google Scholar]
- 22.Goldman N, Weinstein M, Chang MC, et al. 2000 Social Environment and Biomarkers of Aging Study in Taiwan (SEBAS): Main Documentation for SEBAS Public Use Data. Ann Arbor, MI: Inter-University Consortium for Political and Social Research; 2003. ( http://www.icpsr.umich.edu). (Accessed December 5, 2006) [Google Scholar]
- 23.Goldman N, Lin IF, Weinstein M, et al. Evaluating the quality of self-reports of hypertension and diabetes. J Clin Epidemiol. 2003;56(2):148–154. doi: 10.1016/s0895-4356(02)00580-2. [DOI] [PubMed] [Google Scholar]
- 24.Poulter N. Global risk of cardiovascular disease. Heart. 2003;89(suppl 2):ii2–ii5. doi: 10.1136/heart.89.suppl_2.ii2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon CG, Manson JE. Obesity and mortality: a review of the epidemiologic data. Am J Clin Nutr. 1997;66(4 suppl):1044S–1050S. doi: 10.1093/ajcn/66.4.1044S. [DOI] [PubMed] [Google Scholar]
- 26.Turra CM, Goldman N, Seplaki CL, et al. Determinants of mortality at older ages: the role of biological markers of chronic disease. Popul Dev Rev. 2005;31(4):677–701. [Google Scholar]
- 27.Keil U, Liese AD, Hense HW, et al. Classical risk factors and their impact on incident nonfatal and fatal myocardial infarction and all-cause mortality in southern Germany. Results from the MONICA Augsburg cohort study 1984–1992. Eur Heart J. 1998;19(8):1197–1207. doi: 10.1053/euhj.1998.1089. [DOI] [PubMed] [Google Scholar]
- 28.Volpato S, Leveille SG, Corti MC, et al. The value of serum albumin and high density lipoprotein cholesterol in defining mortality risk in older persons with low serum cholesterol. J Am Geriatr Soc. 2001;49(9):1142–1147. doi: 10.1046/j.1532-5415.2001.49229.x. [DOI] [PubMed] [Google Scholar]
- 29.Margolis KL, Manson JE, Greenland P, et al. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: The Women's Health Initiative Observational Study. Arch Intern Med. 2005;165(5):500–508. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 31.Djoussé L, Rothman KJ, Cupples LA, et al. Serum albumin and risk of myocardial infarction and all-cause mortality in the Framingham Offspring Study. Circulation. 2002;106(23):2919–2924. doi: 10.1161/01.cir.0000042673.07632.76. [DOI] [PubMed] [Google Scholar]
- 32.Lee CD, Folsom AR, Nieto FJ, et al. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and white men and women: Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2001;154(8):758–764. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- 33.Gillum RF, Mussolino ME, Madans JH. Counts of neutrophils, lymphocytes, and monocytes, cause-specific mortality and coronary heart disease: The NHANES-I Epidemiologic Follow-up Study. Ann Epidemiol. 2005;15(4):266–271. doi: 10.1016/j.annepidem.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Bruunsgaard H, Ladelund S, Pedersen AN, et al. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003;132(1):24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cappola AR, Xue QL, Ferrucci L, et al. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88(5):2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 36.Mazat L, Lafont S, Berr C, et al. Prospective measurements of dehydroepiandrosterone sulfate in a cohort of elderly subjects: relationship to gender, subjective health, smoking habits, and 10-year mortality. Proc Natl Acad Sci U S A. 2001;98(14):8145–8150. doi: 10.1073/pnas.121177998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reuben DB, Talvi SL, Rowe JW, et al. High urinary catecholamine excretion predicts mortality and functional decline in high-functioning, community-dwelling older persons: MacArthur Studies of Successful Aging. J Gerontol A Biol Sci Med Sci. 2000;55(10):M618–M624. doi: 10.1093/gerona/55.10.m618. [DOI] [PubMed] [Google Scholar]
- 38.Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: The Framingham Heart Study. Am J Med. 2003;115(6):429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Goldman N, Turra CM, Glei DA, et al. Predicting mortality from clinical and nonclinical biomarkers. J Gerontol A Biol Sci Med Sci. 2006;61(10):1070–1074. doi: 10.1093/gerona/61.10.1070. [DOI] [PubMed] [Google Scholar]
- 40.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 41.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive Summary. Bethesda, MD: National Heart, Lung, and Blood Institute; 2001. National Cholesterol Education Program, National Heart, Lung, and Blood Institute. (NIH publication no. 01-3670). ( http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3xsum.pdf). (Accessed January 8, 2007) [Google Scholar]
- 42.Department of Health, Executive Yuan, Taiwan. Taipei, Taiwan: Taiwan Department of Health; 2002. Definition of adult obesity [in Chinese] ( http://food.doh.gov.tw/foodnew/Files/Health/AdultDefinition.jpg). (Accessed December 17, 2008) [Google Scholar]
- 43.Inuoe S, Zimmet P, Caterson I, et al. The Asia-Pacific Perspective: Redefining Obesity and its Treatment. Sydney, Australia: Health Communications Australia Pty Ltd; 2000. [Google Scholar]
- 44.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Geneva, Switzerland: World Health Organization; 1998. [PubMed] [Google Scholar]
- 45.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 46.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 47.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):149–166. [Google Scholar]
- 48.Retherford RD. Tobacco smoking and the sex mortality differential. Demography. 1972;9(2):203–216. [PubMed] [Google Scholar]
- 49.Waldron I. The contribution of smoking to sex differences in mortality. Public Health Rep. 1986;101(2):163–173. [PMC free article] [PubMed] [Google Scholar]
- 50.Valkonen T, Poppel FV. The contribution of smoking to sex differences in life expectancy: four Nordic countries and the Netherlands 1970–1989. Eur J Public Health. 1997;7(3):302–310. [Google Scholar]
- 51.Visscher TL, Seidell JC, Menotti A, et al. Underweight and overweight in relation to mortality among men aged 40–59 and 50–69 years: The Seven Countries Study. Am J Epidemiol. 2000;151(7):660–666. doi: 10.1093/oxfordjournals.aje.a010260. [DOI] [PubMed] [Google Scholar]
- 52.Grabowski DC, Ellis JE. High body mass index does not predict mortality in older people: analysis of the Longitudinal Study of Aging. J Am Geriatr Soc. 2001;49(7):968–979. doi: 10.1046/j.1532-5415.2001.49189.x. [DOI] [PubMed] [Google Scholar]
- 53.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: The Cardiovascular Health Study. JAMA. 1998;279(8):585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 54.Schatz IJ, Masaki K, Yano K, et al. Cholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: a cohort study. Lancet. 2001;358(9279):351–355. doi: 10.1016/S0140-6736(01)05553-2. [DOI] [PubMed] [Google Scholar]
- 55.Satish S, Freeman DH, Jr, Ray L, et al. The relationship between blood pressure and mortality in the oldest old. J Am Geriatr Soc. 2001;49(4):367–374. doi: 10.1046/j.1532-5415.2001.49078.x. [DOI] [PubMed] [Google Scholar]
- 56.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 57.Gruenewald TL, Seeman TE, Ryff CD, et al. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci U S A. 2006;103(38):14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldman N, Weinstein M, Cornman J, et al. Sex differentials in biological risk factors for chronic disease: estimates from population-based surveys. J Womens Health (Larchmt) 2004;13(4):393–403. doi: 10.1089/154099904323087088. [DOI] [PubMed] [Google Scholar]
- 59.Lippi G, Salvagno GL, Targher G, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death: considerable costs and limited benefits [electronic article] MedGenMed. 2007;9(1):34. [PMC free article] [PubMed] [Google Scholar]