Abstract
Cryosurgery dates back to the 19th century, with the description of the benefits of local application of cooling for conditions such as pain control. Once commercial liquefied gases became available, more progress was made in the use of cryotherapy for localized lesions. As understanding of disease response to freezing increased, safer techniques for performing freezing procedures helped prepare its clinical application in different clinical situations, such as prostate disease and bronchial cancers. Cryosurgical techniques are less invasive and have lower morbidity compared with surgical resection. However, the use of cryosurgery has been limited by a lack of good understanding of the underlying mechanisms of tissue destruction. To apply cryosurgery clinically, and to extend its use, it is important to understand the mechanisms of freeze injury on cells, and to control the thermal parameters.
Keywords: Cooling, Cryosurgery, Freezing, Mechanisms of freeze injury
HISTORICAL ASPECTS OF CRYOSURGERY
Cryosurgery dates back to the 19th century, when Arnott (1) first described the benefits of local application of cooling for various conditions, such as for pain control (Table 1). He used a salt solution containing crushed ice at −18°C to −24°C to treat advanced breast and uterine cancers. He considered this technique as a method for eradicating cancer cells to prolong life, if not as a cure in the early stages of disease.
TABLE 1.
Major events in the history of cryobiology
| Time | Events |
|---|---|
| 1845–1851 | Arnott (1) described the benefits of local application of cooling |
| 1877 | Cailletet and Pictet (2) developed systems for cooling gases |
| 1892 | Dewar (2) developed first vacuum flask for storage of liquefied gases |
| 1899 | First clinical application of liquid air on skin diseases by White (3) |
| 1907 | First clinical application of solid carbon dioxide by Pusey (4) |
| 1936–1940 | Application of cooling in deep-seated lesions, eg, brain lesions, using metal capsules by Fay (8) |
| 1950 | First clinical application of liquid nitrogen by Allington (6) |
| 1961 | First introduction of cryosurgical probe using liquid nitrogen by Cooper and Lee (9) |
| 1970s | Interest in cryosurgery diminished in favour of alternative therapies (eg, L-dopa) (2) |
| 1980s–1990s | Development of intraoperative ultrasound and modification of cryoprobe (2) |
| 2000s | Development of cryoplasty for treating atherosclerotic arterial diseases (2) |
Later, based on the principle that atmospheric gases heat up when compressed and cool down when expanded, Cailletet and Pictet (2) developed systems for cooling gases. In 1892, Dewar (2) developed the first vacuum flask to facilitate the storage of liquefied gases. Three years later, von Linde and Hampson (2) used the Joule-Thomson effect to produce continuously operating gas liquefiers. Once commercial liquefied gases became available, more progress was made in the use of cryotherapy (2).
The first clinical use of cryotherapy on skin diseases was reported by White (3) in the United States in 1899. He obtained liquid air from Professor Charles Tripler and applied it either as a swab, spray or liquid air-filled glass on the target lesion. Unfortunately, liquid air was not easily accessible and no further reports were available.
Solid carbon dioxide (−78.5°C) was first used by Pusey (4) in 1907. Liquid carbon dioxide freezes easily when released from a cylinder into air, and can be easily applied on various skin lesions (eg, warts, vascular nevi, epitheliomas).
In the 1930s, Lortat-Jocobs and Solente (5) applied liquid carbon dioxide through copper tips of various sizes, not only to treat skin lesions but also to treat gynecological lesions. They observed that the results were superior to electric cauterization in treating chronic endocervicitis and cervical erosions.
Difficulty removing the applicator tip while the tissue was frozen led to the development of other cryogenic agents. In 1950, Allington (6) first introduced liquid nitrogen (−196°C), which subsequently became popular in treating verrucae, keratoses and different noncancerous lesions (7). However, the use of liquid nitrogen and carbon dioxide were still limited by their delivery systems, which offered shallow tissue penetration and small tissue volume destruction. It was Fay (8) who devised a method of implanting metal capsules, which were connected to an external cold irrigation system, into the brain to treat tumours and abscesses. He also applied his refrigeration technique to treat breast cancers. As knowledge of disease response to freezing increased, safer techniques for introducing the cooling probe into brain tissue helped prepare its clinical application in the future.
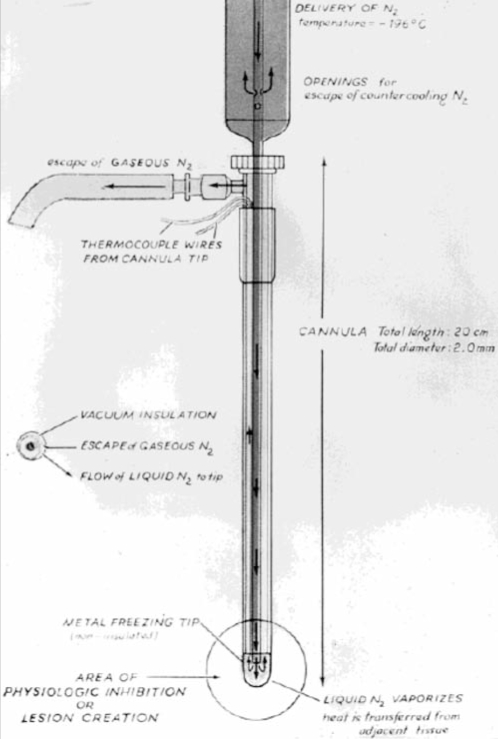
The modern era of cryosurgery arguably started in 1961, when Cooper and Lee (9) introduced their cryosurgical probe for treating Parkinsonism and other neural diseases (Figure 1). The probe consisted of three long concentric tubes, of which the inner tube served as the conduit for the liquid nitrogen to flow to the tip. The space between the inner and middle tube provided a pathway for gas to leave from the tip of the probe, while the space between the middle and outer tubes served as a vacuum-insulated layer to prevent heat loss to the tip of the probe. After the introduction of the cryoprobe, the use of cryosurgery was recognized and widely applied in different clinical situations, such as prostate disease and bronchial cancers (10). Animal and clinical studies soon demonstrated many possible clinical benefits from freezing, such as the development of antibodies (11).
Figure 1.
Diagram of the first cryoprobe, designed by Cooper and Lee (8) for treatment of Parkinsonism. The cryoprobe consists of outer and inner tubes. The inner tube allows liquid nitrogen to flow to the tip of probe. After the extraction of heat from the tissue, the liquid nitrogen becomes gaseous and escapes via the outer tube. Reprinted from reference 57 with permission
In the 1970s, cryosurgery began to fall from favour. One reason was the development of alternate techniques and new drugs. For example, the use of cryosurgery for Parkinson’s disease, the original stimulus for the cryoprobe, became passé after the introduction of L-dopa. The evolution of a simpler method of treating hemorrhoids by banding and injection also led to the diminished use of cryosurgery in this area. Other reasons for the diminishing role of cryosurgery in medicine were the pitfalls associated with its initial probes. Early prototypes featured probes that, when applied to a particular site, produced frozen domains which propagated from the site of application into surrounding tissue, making it difficult to control the extent of tissue destruction. Other drawbacks included prolonged drainage required after cryosurgery for prostate diseases, and the inability to see deep tissue when treating liver cancers. Because the effects of freezing varied with different cells, together with the inability to precisely define the area of tissue destruction, only treatment of cervical intraepithelial neoplasia, chronic cervicitis (12) and cardiac tachyarrhythmias (13,14) were considered standard in the 1980s.
Recent developments in intraoperative ultrasound and in the cryosurgical apparatus renewed attention in cryosurgery. Modern intraoperative ultrasound helped to accurately identify the site of lesions and the depth of probe placement, and can monitor the extent of the freezing process. Furthermore, the development of very small diameter vacuum-insulated cryoprobes, together with the use of liquid nitrogen, allowed supercooling down to approximately −200°C (15), resulting in a more efficient heat transfer. Recently, cryosurgical techniques have been extended to treat peripheral vascular disease. A novel device, the PolarCath (Boston Scientific, USA), was developed to combine the dilation force of a balloon with the delivery of cold thermal energy to the vessel wall. With the use of this technique, called cryoplasty, preliminary studies have shown that the primary patency rate in treating femoropopliteal arterial disease was up to 83.3% after 14 months, compared with 76% after one year of conventional percutaneous angioplasty (16,17). Although the underlying mechanisms are still not well understood, the role of vascular cell apoptosis in cryoplasty has been implicated (18).
To apply cryosurgery clinically and to extend its use, it is important to understand the mechanisms of freezing injury on cells and to control the thermal parameters. As a result, the development of cryoplasty is dependent on work from basic science and clinical research.
BASIC CONCEPTS IN CRYOBIOLOGY
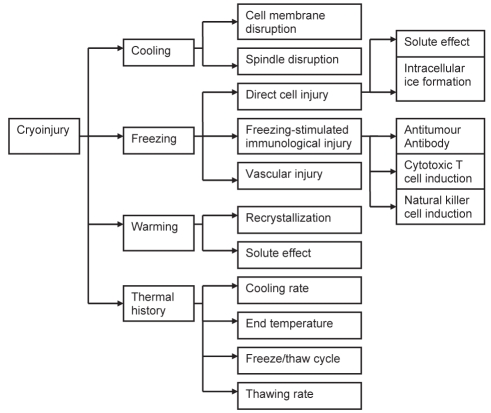
Cryosurgery involves tissue destruction under controlled freezing. The main advantages of this technique are that it is less invasive and has lower morbidity compared with surgical resection. However, the use of cryosurgery has been limited by a lack of good understanding of the underlying mechanisms of tissue destruction. The possible mechanisms of tissue injury are depicted in Figure 2 and discussed below.
Figure 2.
Flow chart showing the mechanisms of cryoinjury
Each cell in tissue is exposed to different thermal histories. The cells nearest to the cryosurgical probe experience the lowest temperature and the fastest cooling rates compared with cells farther away from the probe. The temperature decreases until the heat extracted from the tissue equals the heat in the cooling fluid. Low cryogenic temperature is observed at the tissue surface in contact with the probe; near-body temperature is observed on the outer edge of lesion. There is then a period of thawing during which the probe is removed. Because different cells at different locations may experience different cooling temperatures for various periods of time, thermal history is important in assessing tissue damage.
Effects of cooling
In general, most mammalian cells can withstand low, nonfreezing temperatures. However, these conditions can affect several aspects of cell function. The cell membrane is a lipid bilayer structure with proteins spanning across it. The cell membrane, in general, is impermeable except where membrane proteins allow mass transfer to occur. At low temperatures, the lipid transforms into a gel phase, or a structure with low free energy. During this process, the membrane proteins become separated and lose their ability to control mass transfer. The membrane becomes more permeable and allows ions to transfer in and out of cells more easily. As a result, the ionic composition of the cells changes and damage occurs.
The cytoskeleton is also affected by the cooling process. Meiotic spindles are known to be sensitive to hypothermia, resulting in tubulin depolymerization. In a study using human oocytes cooled to 0°C for 2 min to 3 min, the meiotic spindle shortened and disappeared after 10 min (19).
Effects of freezing
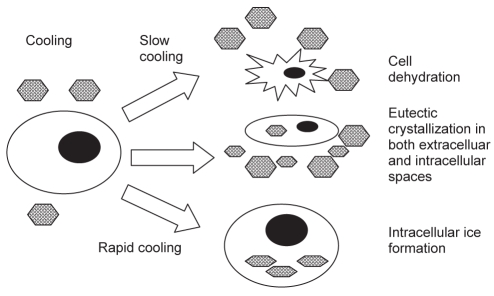
Extensive studies have been performed to understand the effect of freezing on biological tissue. One hypothesis is direct cellular injury from the extracellular space (Figure 3). The cell injury that occurs after freezing is thought to result from a high solute concentration causing cell dehydration (20). Intracellular ice formation causing intracellular organelle and cell membrane disruption has also been implicated (21).
Figure 3.
Diagram of the mechanisms of freezing injury. During slow cooling, ice forms in the extracellular space because of the elevated solute concentration in the unfrozen fraction. This leads to cell shrinkage. When the temperature is further decreased, allowing the initiation of eutectic crystallization in the extracellular space, the temperature and concentration of the intracellular space may allow eutectic crystallization to occur in the intracellular space. Alternatively, if the cooling rate is rapid, the cells are not able to lose water fast enough to maintain equilibrium, resulting in intracellular water becoming supercooled and eventually frozen. Hexagons represent ice crystals
Another theory is that freezing may stimulate immunological injury. It is believed that the immune system becomes sensitized to the destroyed frozen tissue, and any tissue left behind is attacked by the host’s own immune system after cryosurgery. However, the relevance of immunological injury is still controversial (22,23). Finally, it has been theorized that freezing involves vascular injury (24). The hypothesis is that freezing results in stasis of blood flow, particularly in the capillaries. The resulting ischemia leads to tissue necrosis.
It is important to bear in mind that, unlike cryopreservation, where cells are frozen in vitro under uniform conditions and then stored frozen for long periods of time, tissue subjected to cryosurgery is frozen in vivo and its cells experience a wide temperature gradient.
Effects of thawing
The effects of thawing depend on the previous cooling rate. It is known that slow thawing allows solute effects and maximum ice growth during recrystallization to take place. Because solute effects and ice growth are deleterious to cells, complete thawing before the start of another cycle is important in determining the success of cryosurgery for oncological conditions (25). However, rapid cooling followed by rapid thawing can also be beneficial. During rapid cooling, the ice crystals tend to be small, with high surface energies. The longer the time of thawing, the easier it is for the ice to recrystallize, especially for crystals with high surface energies. The larger ice crystals can be more destructive than the smaller ones, because of the size of the crystals or the forces generated during recrystallization. This is shown by the fact that red blood cells tend to have higher survival rates when cooled and warmed faster, or vice versa (26).
Effects of thermal history
Other parameters of thermal history, including cooling rate, end temperature and hold time, are also important in modulating the degree of cell injury. Traditional cryosurgical techniques include rapid cooling, slow thawing and repetition of the freeze/thaw cycle. In general, rapid cooling is more destructive than slow cooling, and the response and threshold of the cooling rate are cell-dependent. Under the same freezing rate, tumour cells tend to retain more cellular water and are less susceptible to dehydration than normal cells. Also, differences in sensitivity exist for different malignant cells. For example, a greater degree of dehydration is observed in normal liver cells, followed by metastatic colon carcinoma and primary hepatocellular carcinoma (27).
Apart from the freezing rate, the lethality of freezing increases with decreasing end temperature. In a study using Dunning AT-1 rat prostate tumour cells, exposed to the same cooling rate as normal cells, cell viability decreased as a function of the end temperature and became zero at −60°C (28). The sensitivity to end temperature is also cell-dependent. For instance, melanocytes and muscle cells are more sensitive to freezing, while other tumour cells, in general, are more resistant (29).
The hold time – the duration of time the tissue is in the frozen state – is also an important factor in determining the degree of cryoinjury. Cooling and holding human prostate cancer cells at −10°C for 20 min had a more destructive pattern than holding for 10 min (30). In a study of different thermal parameters on canine skin, keeping the tissue frozen had a progressive destructive effect on cells (31).
CELL INJURY DURING CRYOSURGERY
Direct cell injury
Direct cellular injury may occur through two different mechanisms. The first is the ‘minimum volume’ hypothesis. Under sufficiently slow cooling rates, freezing occurs in the extracellular space and the cells attempt to maintain equilibrium with the extracellular solution by osmosis (32). When cells are exposed to the high extracellular solute concentration during slow cooling, the cells shrink. As the solute concentration rises beyond a cell’s limit to shrink, the chemical potential gradient is decreased by extracellular salt transfer to the cytoplasm, resulting in a high intracellular solute concentration. During the thawing process, the cell contents are highly concentrated compared with the extracellular solution. Additionally, sudden exposure to the hypotonic solution leads to osmotic rupture of cells (33). There are a number of caveats with regard to this concept of ‘minimum volume’. One issue is that there is no actual minimum volume when precise measurements can be made. Second, it has been observed that the same degree of hemolysis can occur at different cell volumes using different concentrations of glycerol. Thus, while cell shrinkage and re-expansion are significant causes of cell damage, reduction of cell volume is probably not the predominant cause of cell injury (34).
The second putative mechanism of direct cell injury is membrane destabilization during freezing and thawing (35). Two different forms of injuries in cold nonacclimated protoplasts have been reported during freeze-induced dehydration. The first phenomenon occurs from 0°C to −5°C, when the protoplasts shrink to a minimum volume with almost 80% of cell water removed. During thawing, the are cells re-expanded but lysed before regaining their original volume. When the cells were initially cooled to lower temperatures, ie, at −10°C, approximately 90% of the cell water was removed osmotically. However, when the cells thawed, they were osmotically unresponsive and did not re-expand. The investigators proposed that the membrane was damaged during dehydration, leading to the failure of water and solute molecules to re-enter the membrane during osmotic re-expansion.
Intracellular ice formation
Supercooling is defined as the process by which liquid maintains its liquid state below its freezing point. Supercooled water in a cell has a higher chemical potential and higher driving force to leave the cell and freeze externally. Therefore, when the cooling rate is slow, the cell becomes dehydrated. In contrast, if the cooling rate is sufficiently high, intracellular ice forms. The mechanism by which this process occurs remains controversial. The protein-pore theory (36) proposes that extracellular ice propagates to the supercooled cytoplasm through the aqueous pore of the cell membrane. Ice growth during thawing is thought to be the cause of cell injury. This theory is supported by experiments in the salivary gland (37) and confluent cell monolayers (38) which demonstrated the propagation of ice via gap junctions with strong temperature dependence.
The surface-catalyzed theory hypothesizes that the interaction between extracellular ice and the plasma membrane, characterized by the contact angle between the cell membrane and ice, leads to the formation of intracellular ice. It is also posited that intracellular particles inside the cell can catalyze intracellular ice formation below −30°C (volume-catalyzed nucleation) (39).
The final theory is the membrane disruption theory. This proposes that intracellular ice formation occurs as a result of membrane disruption at the critical osmotic pressure gradient across the membrane during freezing (40).
While the exact mechanism of intracellular ice formation has still yet to be resolved, most cryobiologists agree that intracellular ice formation is lethal to cells. It is interesting, however, to note that on confluent monolayers of V-79W Chinese hamster fibroblasts and Madin-Darby canine kidney cells exposed to a slow cooling rate to temperatures as low as −40°C (41), intracellular ice formation may confer cryoprotective effects from dehydration. Thus, the role and significance of intracellular ice formation has yet to be defined.
MECHANISMS OF VASCULAR INJURY
A correlation between vascular injury and freezing was first proposed by Cohnheim (42) in 1877, when he hypothesized that necrosis in frostbitten tissue was caused by stasis of blood flow after thawing. Later, Lewis and Love (43) observed that vasculature in frozen human skin changed from stasis surrounded by an area of hyperemia at −5°C to the previous normal state with surrounding edema when rewarmed to room temperature. Subsequent investigators have also shown that changes to the vasculature after freezing and thawing, such as increased tissue edema, circulatory stasis and progressive thrombosis, lead to tissue necrosis after frostbite. Taken together, these results support the hypothesis that vascular injury plays an important role in tissue injury.
Endothelial damage in vascular injury
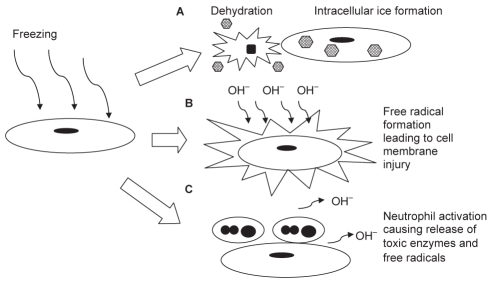
Extensive studies have been performed to investigate the role of the endothelium in mediating cryoinjury (Figure 4). Marzella et al (44) studied freezing injury to rabbit ears by microscopy. They showed that the microvasculature endothelium was destroyed within 1 h. Platelet aggregation was observed immediately on thawing. Interstitial swelling and neutrophil recruitment also occurred minutes after thawing, with extravasation of red blood cells by 6 h and endothelial separation by 24 h.
Figure 4.
Diagram of mechanisms of endothelial injury from freezing. (A) Direct cell injury from dehydration in slow cooling conditions or intracellular ice formation in rapid cooling conditions. (B) Free radical production from lipid peroxidation, reduction of the electron transport chain of the inner mitochondrial membrane, or metabolism of hypoxanthine via the xanthine oxidase pathway. (C) Neutrophil activation, together with the production of free radicals and toxic enzymes, leads to cell membrane injury
Several mechanisms have been proposed to explain endothelial injury after freezing. The first theory is direct cell injury as discussed above. The second theory deals with free radical production. In a study of the role of free radicals after freezing and thawing (45), the administration of superoxide dismutase and deferoxamine improved the viability of rabbit ears after frostbite. Electron microscopy demonstrated that endothelial injury, vascular stasis, neutrophil adhesion and erythrocyte aggregation were present. Several mechanisms were proposed to explain the free radical formation during ischemia and thawing. The first mechanism postulates that the electron transport chain of the inner mitochondrial membrane may be reduced during ischemia, leading to oxygen radical formation (46). The second possible source of free radicals is from lipid peroxidation. During ischemia, there is an increase in free fatty acid and arachidonic acid. On thawing, blood flow returns and the accumulated arachidonic acid is metabolized via the lipoxygenase and cyclooxygenase pathways, leading to increased formation of thromboxanes and superoxides (47). Another proposed mechanism is the production of oxygen radicals by the metabolism of hypoxanthine, via the xanthine oxidase pathway during thawing (48). Endothelial cells are the main source of xanthine oxidase in the blood vessels, implicating the importance of the interaction of endothelial cells with free radicals during freezing and thawing. The free radical theory is controversial because there are reports that fail to support this conclusion (42).
Another mechanism of post-thaw injury is by neutrophil activation. It is hypothesized that leukocytes may be trapped in the microvasculature, leading to obstruction. Leukocytes can also interact with the platelets and generate free radicals (29). The enzymes released by neutrophils damage the endothelial cells and increase the permeability of the endothelial cell layer via the production of active oxygen species (49). Gazzaniga et al (50) studied the inflammatory changes after cryosurgery using human melanoma xenografted into nude mice. They found that endothelial cell activation was the first noticeable event, followed by infiltration of polymorphonuclear cells, and then macrophages. In contrast, using an intravital muscle model to study the microcirculatory changes in frostbite injury of rat muscle (51), other investigators found no significant role of neutrophil adhesion in the early response. Likewise, other groups reported that a similar area of tissue destruction was achieved whether the vascular supply was clamped or unclamped in hepatic cryosurgery (52).
MECHANISMS OF IMMUNOLOGICAL INJURY
Several mechanisms of immunological injury have been proposed. The first theory is the production of antitumour antibodies (10). When the tumour cells die, the antigens inside the cells are released onto the membrane and phagocytosed by antigen-presenting cells. B cells with antibodies specific for the antigen are stimulated and transformed into plasma cells. Antibody formation induces complement fixation, leading to neutrophil and macrophage chemotaxis. These cells release free radicals and enzymes, which kill tumour cells left behind. This relationship of antibody production and cryosurgery is still controversial. Riera et al (53) found that the antibody level decreased in isoimmunized rabbits following cryosurgery.
The second mechanism of immunological involvement is through the induction of cytotoxic T cells. Normally, intracellular antigens are transferred to the cell membrane and recognized by cytotoxic T cells, which release enzymes and kill the cells. It was proposed that cryosurgery may sensitize the cytotoxic T cells or change the antigen presentation. In a study performed by Eskandari et al (54), T cell activation peaked at two weeks after cryosurgery in a R3327 tumour in the Copenhagen rat, and remained elevated compared with the control group.
The third possible mechanism is that cryosurgery may stimulate the activity of natural killer cells. However, the relationship of cryosurgery and the activity of natural killer cells is still undetermined. Nevertheless, the response of the immune system to cryosurgery seems to be cell type-dependent. For instance, a positive response was demonstrated in R3327 prostate adenocarcinoma, but no effect was observed in MRMT-1 mammary adenocarcinoma (55). It may also be that the amount of antigen is important in immune system stimulation. If the antigen amount is more than the immune system can bear, suppression of tumour immunity may occur. Roy et al (56) found that the survival time decreased if a greater amount of cryodestroyed tumour was injected into the animal.
CONCLUSIONS
Cryosurgery has developed over a long period of time and is still progressing slowly. The lack of complete knowledge regarding cryoinjury may be limiting its development. The mechanisms of cryoinjury are complex. The debate over whether the cell membrane or extracellular ice mediates intracellular ice formation is still not resolved, nor is the role of immunology in cryosurgery. Current literature focuses on cryopreservation, which is not directly relevant to cryosurgery. In cryosurgery, cells exhibit different thermal histories, making the effectiveness of cryosurgery unpredictable, variable and less controllable. With better understanding of cell injury mechanisms, we predict that cryosurgery will likely have a greater clinical impact and wider usage. The recent development of cryoplasty in treating peripheral vascular disease is a new area for exploration at the time of writing.
REFERENCES
- 1.Arnott J. Practical illustrations of the remedial efficacy of a very low or anaesthetic temperature. I. In cancer. Lancet. 1850;2:257–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper SM, Dawber RP. The history of cryosurgery. J R Soc Med. 2001;94:196–201. doi: 10.1177/014107680109400416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White AC. Liquid air: Its application in medicine and surgery. Med Rec. 1899;56:109–12. [Google Scholar]
- 4.Pusey WA. The use of carbon dioxide snow in the treatment of nevi and other lesions of the skin: A preliminary report. JAMA. 1907;49:1354–6. [Google Scholar]
- 5.Lortat-Jacobs L, Solente G. La cryotherapie. Paris: Maisson et Cie; 1930. [Google Scholar]
- 6.Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950;72:153–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Gage AA. History. In: Lucas L, editor. Cryosurgery: Mechanism and Applications. Paris: International Institute of Refrigeration; 1995. [Google Scholar]
- 8.Fay T. Early experiences with local and generalized refrigeration of the human brain. J Neurosurg. 1959;16:239–59. doi: 10.3171/jns.1959.16.3.0239. [DOI] [PubMed] [Google Scholar]
- 9.Cooper IS, Lee AS. Cryostatic congelation: A system for producing a limited, controlled region of cooling or freezing of biologic tissues. J Nerv Ment Dis. 1961;133:259–63. [PubMed] [Google Scholar]
- 10.Grana L, Kidd J, Swenson O. Cryogenic techniques within the tracheobronchial tree. J Cryosurg. 1969;2:62–7. [Google Scholar]
- 11.Yantorno C, Soanes WA, Gonder MJ, Shulman S. Studies in cryoimmunology. I. The production of antibodies to urogenital tissue in consequence of freezing treatment. Immunology. 1967;12:395–410. [PMC free article] [PubMed] [Google Scholar]
- 12.Benedet JL, Miller DM, Nickerson KG, Anderson GH. The results of cryosurgical treatment of cervical intraepithelial neoplasia at one, five, and ten years. Am J Obstet Gynecol. 1987;157:268–73. doi: 10.1016/s0002-9378(87)80149-7. [DOI] [PubMed] [Google Scholar]
- 13.Cox JL, Holman WL, Cain ME. Cryosurgical treatment of atrioventricular node reentrant tachycardia. Circulation. 1987;76:1329–36. doi: 10.1161/01.cir.76.6.1329. [DOI] [PubMed] [Google Scholar]
- 14.Ott DA, Garson A, Jr, Cooley DA, Smith RT, Moak J. Cryoablative techniques in the treatment of cardiac tachyarrhythmias. Ann Thorac Surg. 1987;43:138–43. doi: 10.1016/s0003-4975(10)60382-7. [DOI] [PubMed] [Google Scholar]
- 15.Baust J, Chang Z. Underlying mechanisms of damage and new concepts in cryosurgical instrumentation. In: Lucas L, editor. Cryosurgery: Mechanism and Applications. Paris: International Institute of Refrigeration; 1995. [Google Scholar]
- 16.Fava M, Loyola S, Polydorou A, et al. Cryoplasty for femoropopliteal arterial disease: Late angiographic results of initial human experience. J Vasc Interv Radiol. 2004;15:1239–43. doi: 10.1097/01.RVI.0000136291.07304.EA. [DOI] [PubMed] [Google Scholar]
- 17.Surowiec SM, Davies MG, Eberly SW, et al. Percutaneous angioplasty and stenting of the superficial femoral artery. J Vasc Surg. 2005;41:269–78. doi: 10.1016/j.jvs.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 18.Yiu W, Aruny JE, Cheng SWK, Sumpio BE. In-vitro model for evaluation of the effects of supercooling and re-warming on vascular cells. Int J Angiol. 2005;14:237–41. [Google Scholar]
- 19.Zenzes MT, Bielecki R, Casper RF, Leibo SP. Effects of chilling to 0 degrees C on the morphology of meiotic spindles in human metaphase II oocytes. Fertil Steril. 2001;75:769–77. doi: 10.1016/s0015-0282(00)01800-8. [DOI] [PubMed] [Google Scholar]
- 20.Lovelock JE. The haemolysis of human red blood-cells by freezing and thawing. Biochim Biophys Acta. 1953;10:414–26. doi: 10.1016/0006-3002(53)90273-x. [DOI] [PubMed] [Google Scholar]
- 21.Steponkus PL. Role of the plasma membrane in freezing injury and cold acclimation. Ann Rev Plant Physiol. 1984;35:543–84. [Google Scholar]
- 22.Ablin RJ. An appreciation and realization of the concept of cryoimmunology. In: Onik G, Rubinsky B, Watson G, et al., editors. Percutaneous Prostate Cryoablation. St Louis: Quality Medical Publishing; 1995. p. 136. [Google Scholar]
- 23.Hoffmann NE, Coad JE, Huot CS, Swanlund DJ, Bischof JC. Investigation of the mechanism and the effect of cryoimmunology in the Copenhagen rat. Cryobiology. 2001;42:59–68. doi: 10.1006/cryo.2001.2305. [DOI] [PubMed] [Google Scholar]
- 24.Fraser J, Gill W. Observations on ultra-frozen tissue. Br J Surg. 1967;54:770–6. doi: 10.1002/bjs.1800540907. [DOI] [PubMed] [Google Scholar]
- 25.Gage AA, Guest K, Montes M, Caruana JA, Whalen DA., Jr Effect of varying freezing and thawing rates in experimental cryosurgery. Cryobiology. 1985;22:175–82. doi: 10.1016/0011-2240(85)90172-5. [DOI] [PubMed] [Google Scholar]
- 26.Mazur P. Freezing of living cells: Mechanisms and implications. Am J Physiol. 1984;247:C125–42. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 27.Bischof J, Christov K, Rubinsky B. A morphological study of cooling rate response in normal and neoplastic human liver tissue: Cryosurgical implications. Cryobiology. 1993;30:482–92. doi: 10.1006/cryo.1993.1049. [DOI] [PubMed] [Google Scholar]
- 28.Bischof JC, Smith D, Pazhayannur PV, Manivel C, Hulbert J, Roberts KP. Cryosurgery of dunning AT-1 rat prostate tumor: Thermal, biophysical, and viability response at the cellular and tissue level. Cryobiology. 1997;34:42–69. doi: 10.1006/cryo.1996.1978. [DOI] [PubMed] [Google Scholar]
- 29.Weiss SJ. Oxygen, ischemia and inflammation. Acta Physiol Scand Suppl. 1986;548:9–37. [PubMed] [Google Scholar]
- 30.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171–86. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 31.Rubinsky B. The freezing process and mechanism of tissue damage. In: Onik G, Rubinsky B, Watson G, et al., editors. Percutaneous Prostate Cryoablation. St Louis: Quality Medical Publishing; 1995. [Google Scholar]
- 32.Mazur P. Kinetics of water loss from cells at subzero temperatures and the likelihood of intracellular freezing. J Gen Physiol. 1963;47:347–69. doi: 10.1085/jgp.47.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meryman HT. Modified model for the mechanism of freezing injury in erythrocytes. Nature. 1968;218:333–6. doi: 10.1038/218333a0. [DOI] [PubMed] [Google Scholar]
- 34.Pegg DE, Diaper MP. On the mechanism of injury to slowly frozen erythrocytes. Biophys J. 1988;54:471–88. doi: 10.1016/S0006-3495(88)82980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steponkus PL, Lynch DV. Freeze/thaw induced destabilization of the plasma membrane and the effects of cold acclimation. J Bioenerg Biomembr. 1989;21:21–41. doi: 10.1007/BF00762210. [DOI] [PubMed] [Google Scholar]
- 36.Mazur P. The role of cell membranes in the freezing of yeast and other single cells. Ann NY Acad Sci. 1965;125:658–76. doi: 10.1111/j.1749-6632.1965.tb45420.x. [DOI] [PubMed] [Google Scholar]
- 37.Berger WK, Uhrík B. Freeze-induced shrinkage of individual cells and cell-to-cell propagation of intracellular ice in cell chains from salivary gland. Experientia. 1996;52:843–50. doi: 10.1007/BF01938868. [DOI] [PubMed] [Google Scholar]
- 38.Acker JP, Elliott JA, McGann LE. Intercellular ice propagation: Experimental evidence for ice growth through membrane pores. Biophys J. 2001;81:1389–97. doi: 10.1016/S0006-3495(01)75794-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toner M, Cravalho EG, Karel M. Cellular response of mouse oocytes to freezing stress: Prediction of intracellular ice formation. J Biomech Eng. 1993;115:169–74. doi: 10.1115/1.2894117. [DOI] [PubMed] [Google Scholar]
- 40.Muldrew K, McGann LE. Mechanisms of intracellular ice formation. Biophys J. 1990;57:525–32. doi: 10.1016/S0006-3495(90)82568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acker JP, McGann LE. Protective effect of intracellular ice during freezing? Cryobiology. 2003;46:197–202. doi: 10.1016/s0011-2240(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 42.Cohnheim J. Lectures on general pathology: A handbook for practitioners and students. London: The New Sydenham Society; p. 1889. [Google Scholar]
- 43.Lewis T, Love WS. Vascular reactions of the skin to injury. Part III. Some effects of freezing, of cooling, and of warming. Heart. 1926;13:27–60. [Google Scholar]
- 44.Marzella L, Jesudass RR, Manson PN, Myers RA, Bulkley GB. Morphologic characterization of acute injury to vascular endothelium of skin after frostbite. Plast Reconstr Surg. 1989;83:67–76. doi: 10.1097/00006534-198901000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Manson PN, Jesudass R, Marzella L, Bulkley GB, Im MJ, Narayan KK. Evidence for an early free radical-mediated reperfusion injury in frostbite. Free Radic Biol Med. 1991;10:7–11. doi: 10.1016/0891-5849(91)90015-u. [DOI] [PubMed] [Google Scholar]
- 46.Cino M, Del Maestro RF. Generation of hydrogen peroxide by brain mitochondria: The effect of reoxygenation following postdecapitative ischemia. Arch Biochem Biophys. 1989;269:623–38. doi: 10.1016/0003-9861(89)90148-3. [DOI] [PubMed] [Google Scholar]
- 47.Hamberg M, Svensson J, Samuelsson B. Thromboxanes: A new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci USA. 1975;72:2994–8. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Granger DN, Rutili G, McCord JM. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–9. [PubMed] [Google Scholar]
- 49.Serhan CN, Broekman MJ, Korchak HM, Marcus AJ, Weissmann G. Endogenous phospholipid metabolism in stimulated neutrophils differential activation by FMLP and PMA. Biochem Biophys Res Commun. 1982;107:951–8. doi: 10.1016/0006-291x(82)90615-5. [DOI] [PubMed] [Google Scholar]
- 50.Gazzaniga S, Bravo A, Goldszmid SR, et al. Inflammatory changes after cryosurgery-induced necrosis in human melanoma xenografted in nude mice. J Invest Dermatol. 2001;116:664–71. doi: 10.1046/j.0022-202x.2001.01313.x. [DOI] [PubMed] [Google Scholar]
- 51.Zook N, Hussmann J, Brown R, et al. Microcirculatory studies of frostbite injury. Ann Plast Surg. 1998;40:246–53. doi: 10.1097/00000637-199803000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Dilley AV, Dy DY, Warlters A, et al. Laboratory and animal model evaluation of the Cryotech LCS 2000 in hepatic cryotherapy. Cryobiology. 1993;30:74–85. doi: 10.1006/cryo.1993.1007. [DOI] [PubMed] [Google Scholar]
- 53.Riera CM, Brandt EJ, Shulman S. Studies in cryo-immunology. IV. Antibody development in rabbits after iso-immunization followed by freezing. Immunology. 1968;15:779–87. [PMC free article] [PubMed] [Google Scholar]
- 54.Eskandari H, Ablin RJ, Bhatti RA. Immunologic responsiveness & tumour growth of the Dunning R3327 rat prostatic adenocarcinoma following cryosurgery & orchiectomy. Indian J Exp Biol. 1982;20:872–4. [PubMed] [Google Scholar]
- 55.Matsumura K, Sakata K, Saji S, Misao A, Kunieda T. Antitumor immunologic reactivity in the relatively early period after cryosurgery: Experimental studies in the rat. Cryobiology. 1982;19:263–72. doi: 10.1016/0011-2240(82)90152-3. [DOI] [PubMed] [Google Scholar]
- 56.Roy A, Lahiri S, Lahiri P, Pal S, Ghosh S, Roy B. Immunologic and survival studies in mice immunised with cryodestroyed ascites fibrosarcoma (AFS) cells. Indian J Exp Biol. 1990;28:1026–30. [PubMed] [Google Scholar]
- 57.Cooper IS. Cryogenic surgery of the basal ganglia. JAMA. 1962;181:600–4. doi: 10.1001/jama.1962.03050330030006. [DOI] [PubMed] [Google Scholar]