Abstract
Statins are recognized as the principal and most effective class of drugs for reducing serum cholesterol levels and, therefore, significantly reducing cardiovascular events and mortality. Statins may have a wide range of beneficial biological effects in addition to lipid lowering, a phenomenon commonly termed a ‘pleiotropic effect’. However, the dose-dependency of these effects remains unclear. The present study evaluated whether atorvastatin, a potent statin, ameliorates the serum markers of pleiotropic effects, with a focus on dose-dependency. The pleiotropic effects of treatment with atorvastatin 5 mg/day and 10 mg/day for six months each in 15 patients with primary hypercholesterolemia were assessed in a prospective, randomized, open-label, crossover, single-centre study. Atorvastatin treatment dose-dependently decreased a serum marker of oxidative stress as well as the serum low-density lipoprotein cholesterol level. However, serum markers of inflammation and fibrinolysis decreased independently of dose. In conclusion, the dose-dependency of atorvastatin’s pleiotropic effects differs among individual biological effects.
Keywords: Fibrinolysis, Inflammation, Oxidative stress, Statins
Currently, statins (3-hydroxymethyl-glutaryl coenzyme A [HMG-CoA] reductase inhibitors) are widely used in the treatment of patients with hypercholesterolemia. Indeed, the results of large-scale clinical trials indicate that patients may benefit from statin treatment regardless of their initial cholesterol levels (1,2). Statins also have a wide range of beneficial biological effects in addition to lipid lowering, a phenomenon commonly termed a ‘pleiotropic effect’ (3–6). These effects include anti-inflammatory, fibrinolytic and antioxidant functions. The beneficial effects of statins may be, at least in part, attributable to their pleiotropic actions (7,8). However, the dose-dependency of a statin’s pleiotropic effects remains unclear. In the present study, we assessed whether atorvastatin, a potent statin, ameliorates the serum markers of pleiotropic effects, with a focus on its dose-dependency.
METHODS
Study subjects and design
Fifteen patients (eight men and seven women, mean [± SD] age 69±9 years) were recruited from the outpatient clinic of Kyoto University hospital (Kyoto, Japan). All patients had primary hypercholesterolemia (total cholesterol 5.69 mmol/L or greater) insufficiently responsive to dietary therapy. The exclusion criteria were clinical evidence of secondary hypercholesterolemia (eg, hypothyroidism, nephrotic syndrome), a medical history of statin incompatibility or any acute illness. All study subjects were randomly assigned to either 5 mg or 10 mg of atorvastatin daily after dinner for six months, at which time they switched doses for a further six months. Blood samples were drawn at baseline and after each treatment period. Ten age- and sex-matched subjects (six men and four women, mean age 64±12 years) with normal serum total cholesterol levels were selected as the control group. The study was approved by the review committee of the Kyoto University Hospital, and all patients gave their informed consent.
Measurements of blood samples
After an overnight fast, blood was collected in a serum tube. The tubes were centrifuged, and the serum was separated and stored at −70°C. All samples were measured in batches. Baseline, six-month and 12-month evaluations included the following measurements: serum total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), high-sensitivity C-reactive protein (hs-CRP), plasminogen activator inhibitor type-1 (PAI-1) and 8-hydroxy-2′-deoxyguanosine (8-OHdG). Total cholesterol, HDL-C and triglycerides were measured enzymatically using assay kits from Kyowa Medix (Japan) and Diatoron (Japan). The LDL-C concentration was calculated according to the Friedewald equation (9). Serum hs-CRP levels were measured by automated immunoassays using the Behring Nephelometer 100 system (Dade, Behring, Japan) (10), which is sensitive enough to detect levels as low as 0.2 ng/mL. Serum concentrations of PAI-1 were measured by ELISA with rabbit antihuman PAI-1 immunoglobulin (Biopool AB, Sweden) with a detection limit of 0.5 ng/mL (11). The serum level of 8-OHdG was determined by a competitive ELISA (8-OHdG Check; Japan Institute for the Control of Aging, Fukuroi, Japan) (12). The determination range was 0.64 pg/mL to 2000 pg/mL.
Statistical analysis
Numerical data are expressed as mean ± SD. An unpaired Student’s t test was used to compare the results between different groups. The pre- and post-treatment values were compared by one-way ANOVA followed by multiple comparisons using the Bonferroni/Dunn’s test. Spearman’s correlation coefficients for relations between the extent of reduction in the LDL-C levels and either the hs-CRP, PAI-1 or 8-OHdG levels were determined. All statistical analyses were performed using Statview software (SAS Inc, USA). P<0.05 was considered to be statistically significant.
RESULTS
All 15 patients fulfilled the study protocol with no adverse effects including blood chemistries.
Baseline characteristics of study patients
Table 1 summarizes the patient characteristics, including the lipid profiles, parameters of pleiotropic effects of atorvastatin, and other characteristics for the control groups and patients at baseline. Total cholesterol, triglycerides, LDL-C, PAI-1 and 8-OHdG concentrations were significantly higher at baseline in the hypercholesterolemic patients than in the control group. The prevalence of complications and drug use was well balanced between the two groups.
TABLE 1.
Patient characteristics
| Controls (n=10) | Hypercholesterolemic patients (n=15) | |
|---|---|---|
| Age, years | 64±12 | 69±9 |
| Men/women | 6/4 | 8/7 |
| Total cholesterol, mmol/L | 4.42±0.70 | 6.85±0.85** |
| HDL cholesterol, mmol/L | 1.40±0.28 | 1.47±0.49 |
| Triglyceride, mmol/L | 0.93±0.37 | 1.27±0.42* |
| LDL cholesterol, mmol/L | 2.61±0.59 | 4.78±0.59** |
| hs-CRP, ng/mL | 591±480 | 1090±844 |
| PAI-1, ng/mL | 19±8 | 29±13* |
| 8-OHdG, pg/mL | 479±524 | 1115±340** |
| Complications | ||
| Hypertension | 6 (60) | 8 (53) |
| Angina | 1 (10) | 1 (7) |
| Arrhythmia | 0 (0) | 2 (13) |
| Peripheral artery disease | 0 (0) | 1 (7) |
| Diabetes mellitus | 0 (0) | 0 (0) |
| Drug use for complications | ||
| Angiotensin receptor blocker | 3 (30) | 5 (33) |
| Calcium channel blocker | 4 (40) | 3 (20) |
| Disopyramide | 0 (0) | 1 (7) |
| Nitrate | 1 (10) | 1 (7) |
P<0.05,
P<0.01 versus controls. Data are presented as n (%) or mean ± SD. 8-OHdG 8-hydroxy-2′-deoxyguanosine; HDL High-density lipoprotein; hs-CRP High-sensitivity C-reactive protein; LDL Low-density lipoprotein; PAI-1 Plasminogen activator inhibitor type-1
Changes in lipid profile
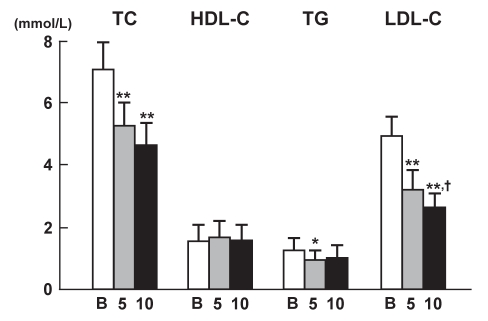
Figure 1 depicts the changes in total cholesterol, HDL-C, triglyceride and LDL-C at baseline and after 5 mg/day or 10 mg/day atorvastatin treatment. Atorvastatin significantly decreased the serum LDL-C in a dose-dependent manner; concentrations at 5 mg/day and 10 mg/day doses of atorvastatin were significantly different. The small dose (5 mg) of atorvastatin significantly decreased triglyceride concentrations, but the higher (10 mg) dose of the statin did not further significantly decrease triglycerides. HDL-C levels remained unchanged, despite the statin treatment.
Figure 1.
Changes in serum lipid levels after atorvastatin therapy (baseline values [B], and after 5 mg/day and 10 mg/day treatment). Data are shown as mean ± SD. *P<0.05, **P<0.01 versus baseline values; †P<0.05 versus values at 5 mg/day atorvastatin treatment. LDL-C Low-density lipoprotein cholesterol; HDL-C High-density lipoprotein cholesterol; TC Total cholesterol; TG Triglyceride
Changes in parameters of pleiotropic effects
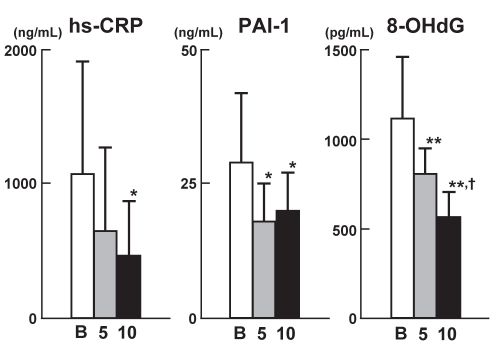
Figure 2 shows the changes in the parameters (hs-CRP, PAI-1 and 8-OHdG levels) of the pleiotropic effects of atorvastatin. Atorvastatin significantly decreased serum hs-CRP levels only at the higher dose (10 mg/day). Both 5 mg and 10 mg doses of atorvastatin significantly decreased the serum PAI-1 levels compared with baseline values, although the decreases were comparable between the two treatment regimens. Of the three parameters, only 8-OHdG concentrations were significantly decreased dose-dependently by atorvastatin, with values at 5 mg and 10 mg doses of atorvastatin significantly different. No significant correlations were observed between the extent of reduction in the LDL-C levels and either the hs-CRP or PAI-1 levels. There was a significantly positive correlation between the extent of reduction in the LDL-C concentrations and that in the 8-OHdG concentrations (n=30, r=0.37, P<0.05).
Figure 2.
Changes in serum levels of markers of pleiotropic effects after atorvastatin therapy (baseline values [B], and after 5 mg/day and 10 mg/day treatment). Data are shown as mean ± SD. *P<0.05, **P<0.01 versus baseline values; †P<0.05 versus values at 5 mg/day atorvastatin treatment. 8-OHdG 8-hydroxy-2′-deoxyguanosine; hs-CRP High-sensitivity C-reactive protein; PAI-1 Plasminogen activator inhibitor-1
DISCUSSION
Our study is the first to compare the pleiotropic effects of atorvastatin at two doses in the same patient. Whereas atorvastatin’s effect on the hs-CRP and PAI-1 levels was independent of the change in LDL-C, the 8-OHdG reduction observed with the statin was directly related to the change in LDL-C. It is tempting to speculate about the mechanistic relationships between the lipid-lowering effect and the pleiotropic effects of atorvastatin. The effect of atorvastatin on hs-CRP levels was independent of its effect on LDL-C. Although the precise mechanism of the statin-induced hs-CRP reduction is unclear, our findings of atorvastatin’s effect on hs-CRP levels are in agreement with previous studies (13,14). Because statins suppress PAI-1 production by a mechanism that relies on HMG-CoA reductase inhibition (15), the reduction in PAI-1 levels may correlate with the LDL-C reduction, which is also dose-dependent and relies on HMG-CoA reductase inhibition. However, our findings conflict with the aforementioned hypothesis. Our data suggest that the atorvastatin dose needed for reductions in LDL-C and PAI-1 levels may be different, ie, the lower dose may be sufficient for PAI-1 reduction. In the current study, the reduction in the serum 8-OHdG level was significantly correlated with reductions in LDL-C levels. Although some of the antioxidative effects of statins appear to be cholesterol-independent (16), our findings may support the conceptual framework that statins suppress the production of reactive oxygen species by inhibiting HMG-CoA reductase activity (11).
REFERENCES
- 1.Collins R, Armitage J, Parish S, Sleight P, Peto R Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363:757–67. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 2.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 3.Albert MA, Danielson E, Rifai N, Ridker PM PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 4.Liao JK. Isoprenoids as mediators of the biological effects of statins. J Clin Invest. 2002;110:285–8. doi: 10.1172/JCI16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–9. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 6.Topol EJ. Intensive statin therapy – a sea change in cardiovascular prevention. N Engl J Med. 2004;350:1562–4. doi: 10.1056/NEJMe048061. [DOI] [PubMed] [Google Scholar]
- 7.Brown BG, Zhao XQ, Sacco DE, Albers JJ. Lipid lowering and plaque regression: New insights into prevention of plaque disruption and clinical events in coronary disease. Circulation. 1993;87:1781–91. doi: 10.1161/01.cir.87.6.1781. [DOI] [PubMed] [Google Scholar]
- 8.Packard CJ. Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS) Circulation. 1998;97:1440–5. doi: 10.1161/01.cir.97.15.1440. [DOI] [PubMed] [Google Scholar]
- 9.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 10.McWhorter VC, Ford LC, Butch AW. Analytical performance of the Synchron LX20 Pro, BN trade mark II and IMMAGE high sensitivity C-creative protein assays and concordance in cardiovascular risk stratification. Clin Chim Acta. 2004;347:71–9. doi: 10.1016/j.cccn.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 11.Wassmann S, Laufs U, Baumer AT, et al. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–7. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- 12.Yin B, Whyatt RM, Perera FP, Randall MC, Cooper TB, Santella RM. Determination of 8-hydroxydeoxyguanosine by an immunoaffinity chromatography-monoclonal antibody-based ELISA. Free Radic Biol Med. 1992;18:1023–32. doi: 10.1016/0891-5849(95)00003-g. [DOI] [PubMed] [Google Scholar]
- 13.Rosenson RS, Koenig W. Utility of inflammatory markers in the management of coronary artery disease. Am J Cardiol. 2003;92:10i–18i. doi: 10.1016/s0002-9149(03)00504-6. [DOI] [PubMed] [Google Scholar]
- 14.Tekin A, Tekin G, Güzelsoy D, et al. Effects of atorvastatin (10 mg) on hemostatic and inflammatory parameters in hyperlipidemic patients with angiographically proven coronary artery disease. Am J Cardiol. 2004;94:206–9. doi: 10.1016/j.amjcard.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 15.Demir M, Acarturk E, Saamaz I, Cayli M, Kikinc Y. Effects of atorvastatin on lipid profile and coagulation parameters. Curr Ther Res Clin Exp. 2001;62:691–8. [Google Scholar]
- 16.Rikitake Y, Kawashima S, Takeshita S, et al. Anti-oxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 2001;154:87–96. doi: 10.1016/s0021-9150(00)00468-8. [DOI] [PubMed] [Google Scholar]