Abstract
The myocardial performance index (MPI) was assessed in 30 patients with limb girdle muscle dystrophy (LGMD) with a normal left ventricular ejection fraction (greater than 50%), as well as in 30 age- and sex-matched healthy adults with a left ventricular ejection fraction greater than 50%. MPIs derived by pulsed-wave Doppler and tissue Doppler were also compared. The MPI was 0.37±0.09 in the LGMD patients and 0.29±0.09 in the control group (P=0.003). These data show that patients with LGMD have occult cardiac dysfunction as evidenced by a higher MPI than the controls. There was good agreement between the MPIs measured by pulsed-wave Doppler and tissue Doppler methods in these patients.
Keywords: Left ventricular dysfunction, Limb girdle muscle dystrophy, Myocardial performance index, Tei index
Muscular dystrophy is a heterogeneous group of muscle disorders that are heritable, genetically determined, and characterized by progressive and debilitating weakness. Although the characteristic involvement is that of skeletal muscle, cardiac involvement is seen with varying frequency in most of these disorders. In fact, in Duchenne’s, Becker’s and myotonic dystrophies, cardiac involvement contributes to significant morbidity and mortality (1,2). Limb girdle muscle dystrophy (LGMD) is a genetically and phenotypically heterogeneous group characterized by predominant involvement of muscles of the pelvic and shoulder girdles, and sparing of the facial and distal muscles.
Contrary to previous reports in which cardiac involvement was reported to be exceedingly rare, many recent studies have shown that a significant number of LGMD patients have cardiac abnormalities (3–10). Clinically relevant cardiac problems are seen only in 10% of these patients. However, subclinical involvement evidenced by electrocardiographic and echocardiographic abnormalities may be as high as 80% (5,6). The echocardiographic abnormalities frequently noted are regional wall motion abnormality and abnormal left ventricular ejection fraction (LVEF). While some of the autosomal dominant types of LGMD are associated with rhythm and conduction abnormalities, dilated cardiomyopathy is common with the more frequent autosomal recessive types, such as the sarcoglycanopathies (6,7).
The myocardial performance index (MPI) (Tei index) has been increasingly used over the past decade as a measure of global cardiac function. Numerous studies (11–17) have demonstrated its diagnostic and prognostic utility in a variety of clinical settings. It has been shown to be independent of heart rate and blood pressure, and is a highly reproducible measure (11,13). We hypothesized that because the MPI is an index of global cardiac function, it may be a suitable and sensitive marker of cardiac involvement in patients with LGMD. To our knowledge, there have been no studies of the MPI in patients with this disorder. The original method described for calculating the MPI used mitral and aortic flow patterns. Tissue Doppler (TD) imaging of the mitral annulus has, however, gained acceptance and is widely used (18).
In the present study, we evaluated cardiac function in patients with LGMD who had no overt cardiac symptoms and normal LVEF using the MPI, and compared it with healthy age- and sex-matched controls who also had a normal LVEF. We also studied the degree of agreement between the MPIs calculated by the conventional pulsed-wave Doppler (PWD) method and the TD method in this group of patients.
METHODS
Thirty patients diagnosed with LGMD formed the study group. Patients with an LVEF less than 50% were excluded. The control group consisted of 30 age- and sex-matched healthy people recruited from the employees of the hospital.
The diagnosis of LGMD was based on a detailed history, physical examination, and complete neuromuscular workup that included a biochemical screen, nerve conduction study, electromyography and muscle biopsy. All LGMD patients presented with weakness, predominantly in the limb girdle distribution (pelvic, shoulder or both). Detailed family charting and pedigree analysis were performed in all patients. A muscle biopsy was performed in all patients using standard open biopsy technique in one of the proximal and moderately affected muscles. All biopsies were subjected to routine histochemical stains, enzyme histochemistry and immunostaining. Cryostat sections from frozen muscle blocks were initially stained for dystrophin and then with various sarcoglycan antibodies.
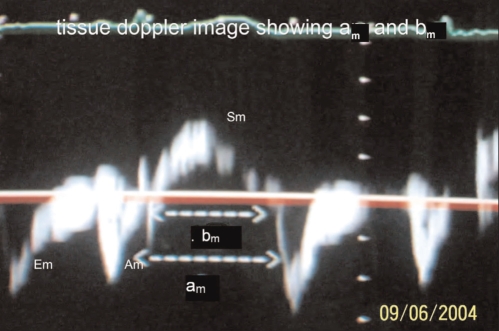
All 30 patients and 30 controls underwent echocardiographic evaluation after a detailed history and clinical examination. Echocardiography was completed using a Hewlett-Packard SONOS 5500 machine with TD facility with the subjects in the left lateral position. Left ventricular dimensions were M-mode derived from the parasternal long axis two-dimensional view, and LVEFs were obtained by planimetry. Note was made of any regional wall motion abnormality. Mitral inflow velocity and aortic outflow velocity patterns were recorded using PWD with the sample volume between the tips of the mitral leaflets and just below the aortic valve, respectively, in the apical five-chamber view. All echocardiographic and Doppler measurements were performed in accordance with the respective guidelines of the American Society of Echocardiography (19,20). Time from end of A to beginning of E (a) and aortic ejection time (b) were noted. In the patients with LGMD, mitral annulus TD tracings were also obtained in the apical four-chamber view, placing the sample volume at the level of the mitral annulus on the interventricular septum. The duration from the end of Am to beginning of Em (am) and duration of the systolic wave (bm) were noted (Figure 1). All measurements were performed in triplicate, and the mean of the three values was used for all calculations and analyses. From these measured variables, the left ventricular MPI-PWD was calculated as (a−b)/b in both the study and control groups. Additionally, in patients with LGMD, the left ventricular MPI-TD was calculated as (am−bm)/bm.
Figure 1.
Image of tissue Doppler recording showing time between end of atrial component of mitral annular diastolic waveform (Am) to start of early component of mitral annular diastolic waveform (Em) representing the sum of isovolumetric contraction time, isovolumetric relaxation time and ejection time (am) and duration of systolic wave form of medial mitral annulus (Sm) representing aortic ejection time (bm)
All measurements were done on-line by one of the authors (RG), who was experienced in echocardiograpy. Intraobserver variability was assessed by repeating echocardiography in eight patients within two to seven days of the initial examination under the same basal conditions. Variability was calculated as the mean percentage error, derived as the difference between the two sets of measurements divided by the mean of the two.
All data were expressed as the mean ± SD. Differences between the control and study groups in the various echocardiographic variables were analyzed using the unpaired Student’s t test. Within the patient group, the paired t test was used for difference in paired samples of MPI and time intervals measured using conventional and TD methods. The agreement between the MPIs derived from the two methods was studied using a Bland-Altman plot, and the correlation between the time intervals and MPIs was derived by the two methods using Pearson’s correlation. Differences were considered significant at P<0.05. Statistical analyses and graphing were performed using Graph Pad statistical software (Graph Pad Software Inc, USA).
RESULTS
The study group consisted of 30 LGMD patients from 24 families. There were 17 men and 13 women, with a mean age of 28±9 years. Fifteen of 30 patients (50%) had sarcoglycanopathy (alpha-sarcoglycanopathy, n=8; beta-sarcoglycanopathy, n=5; delta-sarcoglycanopathy, n=2), and the other 15 patients (50%) were grouped as having nonsarcoglycanopathic LGMD because they were positive for all sarcoglycan components. Biopsies of all 30 patients were strongly positive for dystrophin, thus excluding patients with Becker’s muscular dystrophy. One patient had diabetes, and none had hypertension or any other significant medical illness. A history of parental consanguinity was present in 17 patients (57%). The pattern of inheritance was autosomal recessive in 17 patients (57%) from 13 families, autosomal dominant in three patients (10%) of a single family, and sporadic in the remaining 10 patients (33%). All 30 patients had predominant weakness of the pelvic girdle, with 22 of them (73%) having associated shoulder girdle involvement. Trunk muscle weakness was seen in 18 patients (60%) and lordosis was noted in five patients (17%). Only five patients (17%) had a power of three of five or less according to the Medical Research Council grading system in one or more areas. The control group consisted of 30 age- and sex-matched healthy adults.
Biochemical analysis included a complete blood count, basic metabolic panel, and creatine kinase (CPK) and thyroid stimulating hormone. The results of all of the above tests except for CPK were normal in all patients. Serum CPK was elevated in all patients. The mean level was 2325 U/L, and the range was 117 U/L to 13,000 U/L.
Nerve conduction studies were normal in all patients except the lone patient with diabetes, in whom there was evidence of mild sensorimotor axonal polyneuropathy. The electromyograms of all patients showed myopathic features. Muscle biopsies showed a dystrophic pattern in 23 patients, a mixed myopathic and dystrophic pattern in six patients, and end-stage fibrosis in one patient. None had neuropathic changes.
The echocardiographic data of the patients and controls are shown in Table 1. The MPI was significantly higher in the LGMD patients and was primarily driven by a shorter ejection time (b). None of the patients had an LVEF of less than 50%, a regional wall motion abnormality or echocardiographic evidence of left ventricular hypertrophy.
TABLE 1.
Doppler echocardiographic variables in limb girdle muscle dystrophy (LGMD) patients and controls
| Variable | LGMD patients (n=30) | Controls (n=30) | P |
|---|---|---|---|
| LVEDD (cm) | 4.67±0.5 | 4.54±0.4 | NS |
| LVESD (cm) | 3.17±0.5 | 2.84±0.5 | 0.01 |
| LVEF (%) | 60±7 | 68 ±6. | 0.001 |
| E/A ratio | 1.5±0.4 | 1.6±0.3 | NS |
| Early deceleration time (ms) | 138±43 | 144 ±26 | NS |
| a (ms) | 366±29 | 366±43 | NS |
| Aortic ejection time (ms) | 268±24 | 287±25 | 0.002 |
| Myocardial performance index | 0.37±0.09 | 0.29±0.09 | 0.003 |
Data presented as the mean ± SD. a Time interval between end of A to start of E in mitral inflow recording; LVEDD Left ventricular end-diastolic dimension; LVESD Left ventricular end-systolic dimension; LVEF Left ventricular ejection fraction; NS Not significant
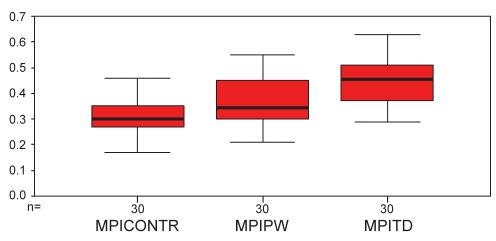
The comparison between the MPI-PW and that calculated by the MPI-TD in the LGMD patients is shown in Table 2. The MPI-TD results were significantly higher than the MPI-PW results. This difference was primarily driven by a higher am compared with a. There was no significant difference between the ejection times calculated by the two methods (b, bm). A box-and-whisker plot depicting the mean MPIs with 95% confidence limits in the cases and controls is shown in Figure 2. There was moderate correlation between a and am (r=0.6, P<0.0001), between b and bm (r=0.6, P<0.0001) and between MPI-PW and MPI-TD (r=0.6, P<0.001). The Bland-Altman plot (Figure 3) between MPI-PW and MPI-TD revealed good agreement between the two. The mean difference was 0.07 (95% CI –0.09 to 0.24). The intraobserver variability for MPI-PW and MPI-TD was 3.7%±2.2% and 3.3%±3.2%, respectively.
TABLE 2.
Comparison between conventional and tissue Doppler indexes in limb girdle muscle dystrophy patients
| Parameter | Conventional | Tissue Doppler | P |
|---|---|---|---|
| IVCT+ET+IVRT (ms) | 366±29 | 401±30 | 0.0001 |
| ET (ms) | 268±23 | 278±22 | NS |
| MPI | 0.37±0.09 | 0.44±0.09 | 0.0001 |
Data presented as the mean ± SD. ET Ejection time; IVCT Isovolumetric contraction time; IVRT Isovolumic relaxation time; MPI Myocardial performance index; NS Not significant
Figure 2.
Box-and-whisker plot of myocardial performance indexes (MPIs) depicting means and 95% CIs. MPICONTR MPI of controls using pulsed-wave Doppler; MPIPW MPI of limb girdle muscle dystrophy (LGMD) patients using pulsed-wave Doppler; MPITD MPI of LGMD patients using tissue Doppler
Figure 3.
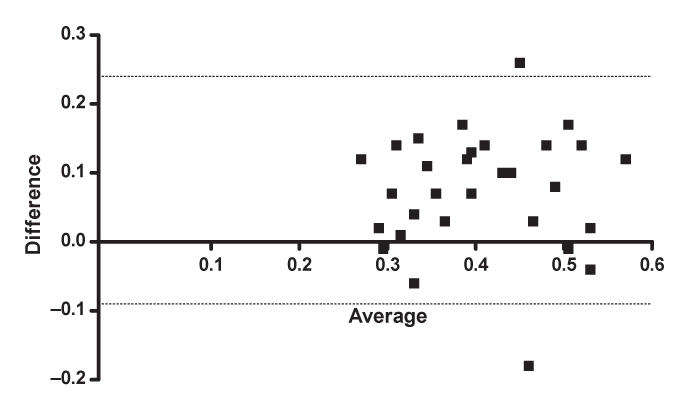
Bland-Altman plot of pulsed-wave myocardial performance index and tissue Doppler myocardial performance index in patients with limb girdle muscle dystrophy
The mean MPI-PW was 0.36±0.09 in the 15 patients with sarcoglycanopathy and 0.38±0.09 in the 15 nonsarcoglycanopathy patients (P not significant). The mean MPI-TD was 0.42±0.08 in the 15 patients with sarcoglycanopathy and 0.47±0.09 in the 15 nonsarcoglycanopathy patients (P not significant).
DISCUSSION
There have been important advances in our understanding of LGMD after its initial description and classification in the mid-20th century (21). The current classification of LGMD describes up to 15 different subtypes (22). The autosomal dominant varieties are classified as type 1 and the autosomal recessive varieties as type 2. Cardiac involvement in muscular dystrophies is well known, and the manifestations range from nonspecific electrocardiographic abnormalities to life-threatening arrhythmias, and from asymptomatic left ventricular dysfunction to frank congestive heart failure. The frequency, severity and nature of involvement varies among the different types of dystrophies. Studies on well-characterized patients of LGMD have revealed clinically relevant cardiac problems in up to 10% of these patients and subclinical involvement in approximately three-quarters of them (5,7).
In our study, we found that there exists a significant difference in the MPI of LGMD patients compared with age- and sex-matched controls, even when they had no symptoms and had an LVEF of greater than 50%. Studies have shown that a cut-off of 50% for the LVEF has good sensitivity and specificity for discriminating between normal and impaired myocardial performance (23). The mean MPI was 0.37±0.09 in LGMD patients compared with 0.29±0.09 in the controls. It is important to note that the MPI, despite being significantly higher in the study group, was still below the cut-off for normal in all but five of the cases. It remained significantly higher than the controls even after exclusion of these five cases. It is known that cardiomyopathy is more common in patients with sarcoglycanopathy than in those with other types of LGMD. However, in our study, there was no significant difference between the MPIs of patients in the sarcoglycanopathy and nonsarcoglycanopathy groups. The MPIs of patients in each of these subgroups analyzed separately remained significantly different from those of the controls. This makes it unlikely that our results were predominantly influenced by patients in the sarcoglycanopathy group.
In our study, we also used TD imaging of the medial mitral annulus to measure the MPI in the study group, apart from the conventional method, which uses mitral inflow and aortic outflow patterns. All the data required for calculating the MPI cannot be derived from a single cardiac cycle using the conventional method. Thus, variability due to heart rate and posture is a pitfall when using the conventional method to measure MPI. TD imaging of the mitral annulus potentially overcomes this disadvantage, because all of the necessary variables are obtained in a single cardiac cycle. It is also relatively independent of preload and heart rate. The other advantages of the MPI-TD are its better curve/signal definition and its ability to study regional differences in cardiac function (23–25). In our study, the variables calculated by this method had good correlation with those of their counterparts derived by the conventional method. The mean difference between the MPIs was 0.07 (95% CI −0.09 to 0.24). Despite the significant difference between the MPIs calculated by these two methods, there was good agreement between the two in any given patient, as demonstrated by the Bland-Altman plot. This is in agreement with other studies in which agreement between the conventional and TD method was studied (18,23–26). The findings of our study also agree with the suggestion that the cut-off for defining normality should be raised by approximately 0.1 whenever the MPI-TD is used for discriminating between normal and impaired left ventricular function.
The MPI is a measure of global cardiac function, and is a more apt index of cardiac function than LVEF, left ventricular end-systolic dimension or the diastolic times. Studies have also demonstrated that the normal range for the MPI is narrower, and that the degree of intergroup overlap is smaller. The MPI has been shown to have incremental prognostic value in addition to LVEF in a variety of cardiac disorders, and also to be more independent of heart rate and loading conditions than other systolic or diastolic parameters. The MPI is the preferred method for assessing global cardiac function because of a narrower range in normal persons and its better discriminative activity.
Although both conventional PWD and TD of the mitral annulus measure the same parameters, ie, systolic and diastolic time intervals to calculate the MPI of the left ventricle, MPI-TD has several advantages. All of the data required for calculating the MPI are derived in a single cardiac cycle with TD. Thus, the problem of variability due to heart rate and posture are eliminated. It is also possible to assess regional myocardial function by separately measuring medial and lateral mitral annular velocities using TD, which is not the case with conventional PWD. This may be especially useful in settings such as postmyocardial infarction and in rare cardiomyopathies, which variably involve the different walls of the left ventricle. The weight of evidence thus supports that MPI-TD correlates well with the more extensively studied MPI-PW. Considering this and its technical superiority, TD is a preferable method for derivation of the MPI compared with the conventional PWD.
CONCLUSIONS
The findings of our study show that LGMD patients have sub-clinical cardiac dysfunction, as evidenced by a relatively abnormal MPI, compared with normal persons of similar age and sex. Long-term follow-up of these patients will show whether there is progression of left ventricular dysfunction in these patients.
REFERENCES
- 1.Melacini P, Vianello A, Villanova C, et al. Cardiac and respiratory involvement in advanced stage Duchenne muscular dystrophy. Neuromuscul Disord. 1996;6:367–76. doi: 10.1016/0960-8966(96)00357-4. [DOI] [PubMed] [Google Scholar]
- 2.Melacini P, Fanin M, Danieli GA, et al. Cardiac involvement in Becker muscular dystrophy. J Am Coll Cardiol. 1993;22:1927–34. doi: 10.1016/0735-1097(93)90781-u. [DOI] [PubMed] [Google Scholar]
- 3.Dincer P, Leturcq F, Richard I, et al. A biochemical, genetic, and clinical survey of autosomal recessive limb girdle muscular dystrophies in Turkey. Ann Neurol. 1997;42:222–9. doi: 10.1002/ana.410420214. [DOI] [PubMed] [Google Scholar]
- 4.Eymard B, Romero NB, Leturcq F, et al. Primary adhalinopathy (alpha-sarcoglycanopathy): Clinical, pathologic, and genetic correlation in 20 patients with autosomal recessive muscular dystrophy. Neurology. 1997;48:1227–34. doi: 10.1212/wnl.48.5.1227. [DOI] [PubMed] [Google Scholar]
- 5.Van der Kooi AJ, de Voogt WG, Barth PG, et al. The heart in limb girdle muscular dystrophy. Heart. 1998;79:73–7. doi: 10.1136/hrt.79.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stubgen JP. Limb girdle muscular dystrophy: A non-invasive cardiac evaluation. Cardiology. 1993;83:324–30. doi: 10.1159/000175988. [DOI] [PubMed] [Google Scholar]
- 7.Melacini P, Fanin M, Duggan DJ, et al. Heart involvement in muscular dystrophies due to sarcoglycan gene mutations. Muscle Nerve. 1999;22:473–9. doi: 10.1002/(sici)1097-4598(199904)22:4<473::aid-mus8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Piccolo F, Roberds SL, Jeanpierre M, et al. Primary adhalinopathy: A common cause of autosomal recessive muscular dystrophy of variable severity. Nat Genet. 1995;10:243–5. doi: 10.1038/ng0695-243. [DOI] [PubMed] [Google Scholar]
- 9.Fadic R, Sunada Y, Waclawik AJ, et al. Brief report: Deficiency of a dystrophin-associated glycoprotein (adhalin) in a patient with muscular dystrophy and cardiomyopathy. N Engl J Med. 1996;334:362–6. doi: 10.1056/NEJM199602083340604. [DOI] [PubMed] [Google Scholar]
- 10.Mascarenhas DA, Spodick DH, Chad DA, et al. Cardiomyopathy of limb-girdle muscular dystrophy. J Am Coll Cardiol. 1994;24:1328–33. doi: 10.1016/0735-1097(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 11.Tei C, Ling LH, Hodge DO, et al. New index of combined systolic and diastolic myocardial performance: A simple and reproducible measure of cardiac function – a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26:357–66. [PubMed] [Google Scholar]
- 12.Tei C, Nishimura RA, Seward JB, et al. Noninvasive Doppler-derived myocardial performance index: Correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr. 1997;10:169–78. doi: 10.1016/s0894-7317(97)70090-7. [DOI] [PubMed] [Google Scholar]
- 13.Poulsen SH, Nielsen JC, Andersen HR. The influence of heart rate on the Doppler-derived myocardial performance index. J Am Soc Echocardiogr. 2000;13:379–84. doi: 10.1016/s0894-7317(00)70007-1. [DOI] [PubMed] [Google Scholar]
- 14.Poulsen SH, Jensen SE, Tei C, et al. Value of the Doppler index of myocardial performance in the early phase of acute myocardial infarction. J Am Soc Echocardiogr. 2000;13:723–30. doi: 10.1067/mje.2000.105174. [DOI] [PubMed] [Google Scholar]
- 15.Tei C, Dujardin KS, Hodge DO, et al. Doppler index combining systolic and diastolic myocardial performance: Clinical value in cardiac amyloidosis. J Am Coll Cardiol. 1996;28:658–64. doi: 10.1016/0735-1097(96)00202-1. [DOI] [PubMed] [Google Scholar]
- 16.Dujardin KS, Tei C, Yeo TC, et al. Prognostic value of a Doppler index combining systolic and diastolic performance in idiopathic-dilated cardiomyopathy. Am J Cardiol. 1998;82:1071–6. doi: 10.1016/s0002-9149(98)00559-1. [DOI] [PubMed] [Google Scholar]
- 17.Bruch C, Schmermund A, Marin D, et al. Tei-index in patients with mild-to-moderate congestive heart failure. Eur Heart J. 2000;21:1888–95. doi: 10.1053/euhj.2000.2246. [DOI] [PubMed] [Google Scholar]
- 18.Tekten A, Aon T, Ceyhan C, et al. Novel approach to measure myocardial performance index: Pulsed-wave tissue Doppler echocardiography. Echocardiography. 2003;20:503–10. doi: 10.1046/j.1540-8175.2003.03086.x. [DOI] [PubMed] [Google Scholar]
- 19.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two dimensional echocardiography. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 20.Quinones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: A report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–84. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 21.Walton JN, Nattrass FJ. On the classification, natural history and treatment of the myopathies. Brain. 1954:77169–231. doi: 10.1093/brain/77.2.169. [DOI] [PubMed] [Google Scholar]
- 22.Emery AE. The muscle dystrophies. Lancet. 2002;359:687–95. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 23.Groh WJ, Zipes DP. Neurological disorders and cardiovascular disease. In: Zipes DP, Libby P, Bonow RO, Braunwald E, editors. Heart Disease A Textbook of Cardiovascular Medicine. 7th edn. Philadelphia: Elsevier Saunders; 2005. pp. 2145–60. [Google Scholar]
- 24.Gaibazzi N, Petrucci N, Ziacchi V. Left ventricle myocardial performance index derived either by conventional method or mitral annulus tissue-Doppler: A comparison study in healthy subjects and subjects with heart failure. J Am Soc Echocardiogr. 2005;18:1270–6. doi: 10.1016/j.echo.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Tekten T, Onbasili AO, Ceyhan C, et al. Value of measuring myocardial performance index by tissue Doppler echocardiography in normal and diseased heart. Jpn Heart J. 2003;44:403–16. doi: 10.1536/jhj.44.403. [DOI] [PubMed] [Google Scholar]
- 26.Voon WC, Su HM, Yen HW, et al. Left ventricular Tei index: Comparison between flow and tissue Doppler analyses. Echocardiography. 2005;22:730–5. doi: 10.1111/j.1540-8175.2005.00126.x. [DOI] [PubMed] [Google Scholar]