Abstract
PURPOSE
Postoperative intimal hyperplasia, the most common cause of vein graft occlusion, is initiated by endothelial injury. In the present study, the mechanism by which the free radical scavenger edaravone (Radicut, Mitsubishi Tanabe Pharma Co, Japan) protects against endothelial injury in postoperative intimal hyperplasia was investigated.
METHODS
In 18 male Lewis rats, a right epigastric vein graft was interposed into the common femoral artery. Nine rats received a pre-operative intraperitoneal administration of edaravone (3.0 mg/kg, edaravone group) and the other nine rats received an equal volume of saline (saline group). After 1 h, five vein grafts from each group were treated with Verhoeff-van Gieson elastica stain and subjected to a histological examination. The other four vein grafts from each group were examined with an S-800 Hitachi scanning electron microscope (SEM) (Hitachi High-Technologies Co, Japan) at ×1000 magnification, as were three unoperated right epigastric veins (unoperated vein group). The endothelial areas of the vein grafts were measured using computerized planimetry of the SEM images (ImageJ version 1.37, National Institutes of Health, USA). The mean endothelial areas (%) were compared between the two groups.
RESULTS
Verhoeff-van Gieson elastica stain revealed no significant differences between the two groups. SEM showed that endothelial cells in the unoperated epigastric vein had a cobblestone-like appearance. In the saline group, the endothelial cells were comb-shaped and had adherent monocytes. In the edaravone group, however, the cobblestone-like appearance of endothelial cells was well preserved, with little monocyte adhesion. Moreover, the mean (± standard error of the mean) endothelial area was significantly higher in vein grafts from the edaravone group than in those from the saline group (74±1.8% versus 56±4.3%, P<0.05), and was similar to those in the unoperated epigastric veins (72±1.9%).
CONCLUSION
These findings show that endothelial injury is present soon after placement of the interposition graft. The authors believe that edaravone suppresses postoperative intimal hyperplasia by alleviating endothelial injury.
Keywords: Edaravone (Radicut), Endothelial injury, Postoperative intimal hyperplasia, Scanning electron microscope
Endothelial injury is considered to be the first step toward postoperative intimal hyperplasia. Among Japanese patients who underwent coronary artery bypass surgery with either an internal thoracic artery graft or a saphenous vein graft, the 10-year patency rate was higher for the internal thoracic artery graft than the saphenous vein graft (90% versus 67%, P<0.001) (1). The free radical scavenger edaravone (3-methyl-1-phenyl-2-pyrazoline-5- one, C10H10N2O, MW=174.2; Radicut, Mitsubishi Tanabe Pharma Co, Japan) has been commercially available in Japan for acute cerebral infarction since June 2001 (2). At low doses, it suppresses endothelial injury in vitro (3). Following this, we found that edaravone appeared to suppress postoperative intimal hyperplasia of a vein graft in a rat model (4). However, edaravone’s mechanism of action was not elucidated at that time.
To investigate the mechanism of this protective effect, we directly examined the endothelial injury of vein grafts in rat models, using a scanning electron microscope (SEM).
METHODS
Rat model
Eighteen male rats with a mean weight (± standard error of the mean) of 497±26 g were divided into two groups. Nine rats received preoperative intraperitoneal administration of 3.0 mg/kg edaravone (edaravone group) and the other nine rats received an equal volume of saline (saline group).
At the same time, complete general anesthesia was induced with the intraperitoneal administration of 30 mg/kg of sodium pentobarbital (Nembutal, Abbott Laboratories, USA). With the operative microscope (OME-J&N J73507, Olympus, Japan), a right epigastric vein graft was interposed into the common femoral artery with 10-0 interrupted sutures, as previously described (4,5).
The present study protocol was approved by the Hyogo College of Medicine Animal Research Committee (No 235).
Histological study
One hour after placement of the interposition graft, all rats were subjected to either histological or SEM study. Five vein grafts from each group were cut into 5 μm sections and stained with hematoxylin and eosin, and Verhoeff-van Gieson elastica stain, and examined at ×40 magnification.
SEM study
The four remaining vein grafts from each group were subjected to SEM study. Three unoperated right epigastric veins were also examined as the control (unoperated vein group).
The specimens were prefixed in 2.5% glutaraldehyde and postfixed in 1% osmium tetroxide. After dehydration in ethanol, they were dried at the critical point (HCP-1, Hitachi High-Technologies Co, Japan), placed on copper grids and sputter-coated with a platinum-palladium alloy. The specimens were then examined with a S-800 Hitachi SEM (Hitachi High-Technologies Co, Japan) at ×1000 magnification. Three SEM images of each specimen were obtained. Therefore, the total number of specimens in each group became nine in the unoperated vein group (three images of each of the three specimens), and 12 in both the edaravone and saline groups (three images of each of the four specimens subjected to SEM).
To quantify the SEM images, the percentage endothelial area was defined as the endothelial surface area divided by the total surface area. The endothelial areas of SEM images were measured using computerized planimetry (ImageJ version 1.37, National Institutes of Health, USA).
Statistical analysis
All results were expressed as the mean ± standard error of the mean. Statistically significant differences were determined by unpaired t tests using StatView J-4.5 for Macintosh (Abacus Concepts, USA). A P value of less than 0.05 was considered significant.
RESULTS
Histological study
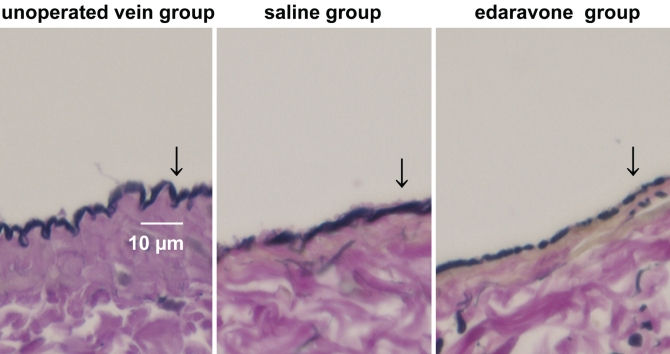
The mean ischemic time was 65±9 min, and the initial patency rate was 89%. Representative 5 μm sections treated with Verhoeff-van Gieson elastica stain are shown in Figure 1. No significant differences among endothelial cell treatment groups were observed.
Figure 1.
Comparison of postoperative intimal hyperplasia in vein grafts (Verhoeff-van Gieson elastica stain; original magnification ×40). Arrows indicate the endothelial cells. There are no significant differences among endothelial cell treatment groups
SEM study
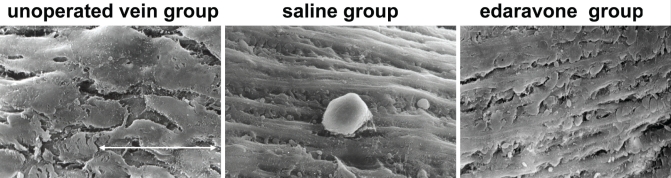
The results of the SEM study are shown in Figure 2. The examinations by SEM showed that endothelial cells in unoperated epigastric veins had a cobblestone-like appearance. Only 1 h after the bypass, the endothelial cells in the saline group were comb-shaped and had many adherent monocytes. In the edaravone group, however, the cobblestone-like appearance of the endothelial cells was well preserved, with little monocyte adhesion.
Figure 2.
Comparison of postoperative intimal hyperplasia in vein grafts (scanning electron microscope; original magnification ×1000). Endothelial cells in the unoperated vein group have a cobblestone-like appearance. The endothelial cells in the saline group are comb-shaped and have adherent monocytes. The cobblestone-like endothelial cells in the edaravone group are well preserved. White arrow represents 30 μm
Endothelial area
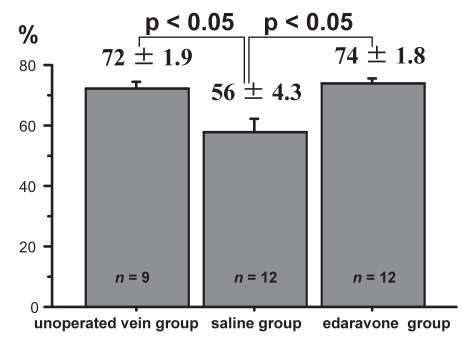
A comparison of mean endothelial area is shown in Figure 3. The mean endothelial area in the unoperated vein group was 72±1.9%. The mean endothelial area in the saline group was significantly decreased (56±4.3%, P<0.05) and the mean endothelial area in the edaravone group was well preserved (74±1.8%). There was a significant difference in area between the edaravone group and the saline group (P<0.05).
Figure 3.
Comparison of the percentage endothelial area. The mean endothelial area is significantly higher in vein grafts from the edaravone group than in those from the saline group (74±1.8% versus 56±4.3%, P<0.05), and is similar to those in the unoperated vein group (72±1.9%)
DISCUSION
Endothelial injury
In 1999, Ross (6) stated that “atherosclerosis is an inflammatory disease”. Similar to atherosclerosis, postoperative intimal hyperplasia is now also thought to be an inflammatory disease (7,8). In a dog model of postoperative intimal hyperplasia, electron microscopic study by Shiokawa et al (9) showed that endothelial cells were denuded one day postoperative. In an electron microscopic analysis of a rat model, Stark et al (10) also suggested that the regeneration of damaged endothelial cells is the first step toward postoperative intimal hyperplasia.
In the present study, we found that endothelial cells in epigastric vein grafts were damaged only 1 h after interpositional grafting to the common femoral artery. The shear stress is another cause of endothelial injury. Given the difference in internal blood pressure between the epigastric vein and the femoral artery, the shear stress may cause endothelial injury and may be the first step in the development of postoperative intimal hyperplasia.
Edaravone
We previously reported that edaravone may suppress postoperative intimal hyperplasia four weeks after the interposition bypass (4). At that time, it was not clear that how the edaravone could exert this effect for such a long time, given the serum half life of only 2.3 h. Our SEM results now show that endothelial cells in the edaravone group were well preserved at 1 h after the interposition bypass. Therefore, we hypothesize that edaravone suppresses postoperative intimal hyperplasia by alleviating endothelial injury in vivo. Consistent with our findings, edaravone may suppress not only the development of postoperative intimal hyperplasia, but also atherosclerosis (11). However, additional clinical research will be necessary to confirm the mechanism of how edaravone alleviates endothelial injury in vivo.
ACKNOWLEDGEMENTS
The authors thank Atsuhiko Kihara (The Institute of Experimental Sciences, Hyogo College of Medicine), Ritsuko Fujimoto and Hirotsugu Kubo (Joint-Use Research Facilities, Hyogo College of Medicine) for their technical support. The authors also thank Stephen Ordway BA (University of California San Francisco) for his editorial assistance.
Footnotes
DISCLOSURE: This study was presented at the 49th Annual World Congress, International College of Angiology on July 22, 2007, in Vancouver, British Columbia, Canada. This study is partially supported by Grant-in-Aid for Researchers, Hyogo College of Medicine, 2006-2007.
REFERENCES
- 1.Kitamura S, Kawachi K, Taniguchi S, et al. Long-term benefits of internal thoracic artery-coronary artery bypass in Japanese patients. Jpn J Thorac Cardiovasc Surg. 1998;46:1–10. doi: 10.1007/BF03217715. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe K, Watanabe K, Hayase T. Radical scavennging mechanism of MCI-186. Jpn Pharmacol Ther. 1997;25:S1699–707. (Abst in English) [Google Scholar]
- 3.Watanabe T, Morita I, Nishi H, Murota S. Preventive effect of MCI-186 on 15-HPETE induced vascular endothelial cell injury in vitro. Prostaglandins Leukot Essent Fatty Acids. 1988;33:81–7. doi: 10.1016/0952-3278(88)90127-5. [DOI] [PubMed] [Google Scholar]
- 4.Yamamura M, Miyamoto Y, Mitsuno M, et al. Suppression of rat lower extremity postoperative reperfusion injury with edaravone. Int J Angiol. 2006;15:34–6. doi: 10.1055/s-0031-1278238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamura M, Miyamoto T, Hoch JR. Tumor necrosis factor-alpha expression in rat models of intimal hyperplasia. Int J Angiol. 2001;10:77–9. [Google Scholar]
- 6.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 7.Yamamura M, Miyamoto T, Yao H. Serum monocyte chemoattractant protein-1 levels in rat models of intimal hyperplasia. Int J Angiol. 2002;11:80–2. [Google Scholar]
- 8.Yamamura M, Miyamoto Y. Postoperative intimal hyperplasia. J Jpn Coll Angiol. 2007;47:421–7. doi: 10.1055/s-0031-1278269. (Abst in English) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiokawa Y, Rahman MF, Ishii Y, Sueishi K. The rate of re-endothelialization correlates inversely with the degree of the following intimal thickening in vein grafts. Electron microscopic and immunohistochemical studies. Virchows Arch A Pathol Anat Histopathol. 1989;415:225–35. doi: 10.1007/BF00724909. [DOI] [PubMed] [Google Scholar]
- 10.Stark VK, Warner TF, Hoch JR. An ultrastructural study of progressive intima hyperplasia in rat vein grafts. J Vasc Surg. 1997;26:94–103. doi: 10.1016/s0741-5214(97)70152-6. [DOI] [PubMed] [Google Scholar]
- 11.Okabe TA, Kishimoto C, Shimada K, Murayama T, Yokode M, Kita T. Effects of MCI-186 (edaravone), a novel free radical scavenger, upon experimental atherosclerosis in apolipoprotein E-deficient mice. Circ J. 2006;70:1216–9. doi: 10.1253/circj.70.1216. [DOI] [PubMed] [Google Scholar]