Abstract
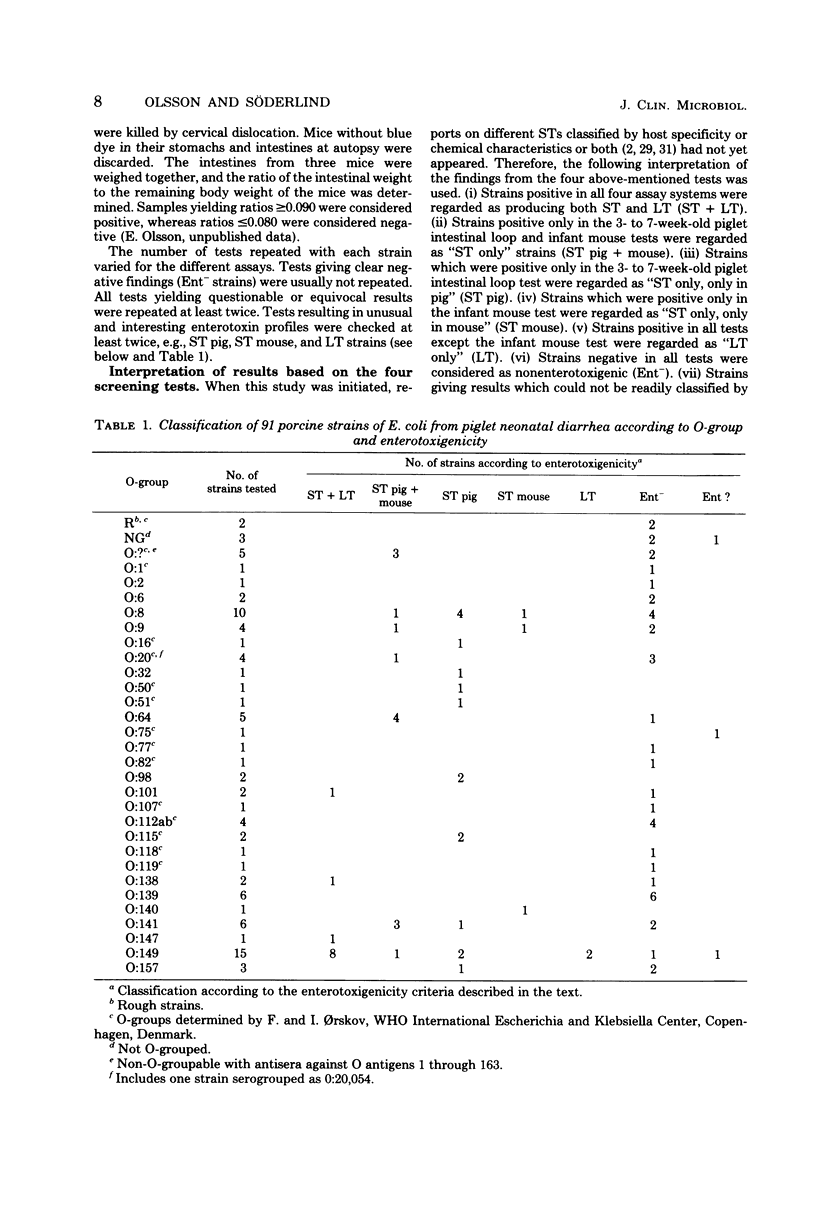
Ninety-one Escherichia coli strains isolated from porcine neonatal diarrhea, representing 28 O-groups and rough and non-O-groupable strains, were examined for enterotoxigenicity (heat stable [ST] or heat labile [LT]) by using bacterial suspensions in intestinal loop tests in 3- to 7-week-old piglets and culture supernatant fluids in the Y1 adrenal cell test, the 18-h rabbit intestinal loop test, and the infant mouse test. Eleven strains in O-groups 101, 138, 147, and 149 were positive in all four assay systems and were designated ST + LT. Fourteen strains within O-groups 8, 9, 20, 64, 141, and 149 and non-O-groupable were positive only in the 3- to 7-week-old piglet loop test and the infant mouse test and were designated ST pig + mouse. Sixteen strains distributed among O-groups 8, 16, 32, 50, 51, 98, 115, 141, 149, and 157 were positive only in the piglet intestinal loop test and were designated ST pig. Three strains of O-groups 8, 9, and 140 were positive only in the infant mouse assay and were designated ST mouse. Two strains of O-group 149 were positive in all tests except the infant mouse test and were designated LT. A total of 42 strains were negative in all four tests (Ent-), and 3 strains could not be categorized by the enterotoxigenicity criteria used. All K88-positive isolates, 17 strains of O-groups 8, 32, 147, and 149, were positive in at least one enterotoxigenicity test. ST pig and ST mouse strains gave positive intestinal loop tests as bacterial suspensions in 4- to 10-day-old piglets. A 6-h piglet intestinal loop test performed with heat-inactivated culture supernatants was preferable to an 18- to 20-h test for determination of ST production by strains of diverse O-groups. ST production by the two strains designated LT was detected by the 6-h test. The infant mouse test, although highly reproducible and convenient, appears to possess considerable limitations in routine screening of E. coli of porcine origin for ST production.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Robertson D. C. Nutrition and enterotoxin synthesis by enterotoxigenic strains of Escherichia coli: defined medium for production of heat-stable enterotoxin. Infect Immun. 1977 Mar;15(3):781–788. doi: 10.1128/iai.15.3.781-788.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantey J. R., Blake R. K. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977 Mar;135(3):454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- DE S. N., CHATTERJE D. N. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Moon H. W., Whipp S. C. Detection of heat-labile Escherichia coli enterotoxin with the use of adrenal cells in tissue culture. Science. 1974 Jan 25;183(4122):334–336. doi: 10.1126/science.183.4122.334. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Gorbach S. L. Identification of enterotoxigenic Escherichia coli and serum antitoxin activity by the vascular permeability factor assay. Infect Immun. 1973 Nov;8(5):731–735. doi: 10.1128/iai.8.5.731-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Pierce N. F. Differences in the response of rabbit small intestine to heat-labile and heat-stable enterotoxins of Escherichia coli. Infect Immun. 1973 Jun;7(6):873–880. doi: 10.1128/iai.7.6.873-880.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Chen L. C., Curlin G. T., Evans D. G. Stimulation of adenyl cyclase by Escherichia coli enterotoxin. Nat New Biol. 1972 Apr 5;236(66):137–138. doi: 10.1038/newbio236137a0. [DOI] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Drake K. W. Effect of purified Escherichia coli heat-stable enterotoxin on intestinal cyclic nucleotide metabolism and fluid secretion. Infect Immun. 1979 Apr;24(1):19–23. doi: 10.1128/iai.24.1.19-23.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun. 1976 Jul;14(1):95–99. doi: 10.1128/iai.14.1.95-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Brunton L. L., Schnaitman T. C., Rebhun L. I., Gilman A. G. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect Immun. 1974 Aug;10(2):320–327. doi: 10.1128/iai.10.2.320-327.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Ganguly U., Casper A. G., Moore E. J., Pierce N. F., Carpenter C. C. Effect of Escherichia coli on fluid transport across canine small bowel. Mechanism and time-course with enterotoxin and whole bacterial cells. J Clin Invest. 1973 Jul;52(7):1707–1714. doi: 10.1172/JCI107352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Moore R. A., Kirschenfeld P. M., Sande M. A. Role of toxigenic and invasive bacteria in acute diarrhea of childhood. N Engl J Med. 1975 Sep 18;293(12):567–572. doi: 10.1056/NEJM197509182931201. [DOI] [PubMed] [Google Scholar]
- Guinée P. A., Agterberg C. M., Jansen W. H., Frik J. F. Serological identification of pig enterotoxigenic Escherichia coli strains not belonging to the classical serotypes. Infect Immun. 1977 Feb;15(2):549–555. doi: 10.1128/iai.15.2.549-555.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H. Detection of enterotoxigenicity and attachment factors in Escherichia coli strains of human, porcine and bovine origin; a comparative study. Zentralbl Bakteriol Orig A. 1979 Apr;243(2-3):245–257. [PubMed] [Google Scholar]
- Gyles C. L., Barnum D. A. A heat-labile enterotoxin from strains of Eschericha coli enteropathogenic for pigs. J Infect Dis. 1969 Oct;120(4):419–426. doi: 10.1093/infdis/120.4.419. [DOI] [PubMed] [Google Scholar]
- Gyles C. L., Barnum D. A. Escherichia coli in ligated segments of pig intestine. J Pathol Bacteriol. 1967 Jul;94(1):189–194. doi: 10.1002/path.1700940124. [DOI] [PubMed] [Google Scholar]
- Gyles C. L., Stevens J. B., Craven J. A. A study of Escherichia coli strains isolated from pigs with gastro-intestinal disease. Can J Comp Med. 1971 Jul;35(3):258–266. [PMC free article] [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Klipstein F. A., Lee C. S., Engert R. F. Assay of Escherichia coli enterotoxins by in vivo perfusion in the rat jejunum. Infect Immun. 1976 Oct;14(4):1004–1010. doi: 10.1128/iai.14.4.1004-1010.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Rowe B., Engert R. F., Short H. B., Gross R. J. Enterotoxigenicity of enteropathogenic serotypes of Escherichia coli isolated from infants with epidemic diarrhea. Infect Immun. 1978 Jul;21(1):171–178. doi: 10.1128/iai.21.1.171-178.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Bergquist E. J., Nalin D. R., Waterman D. H., Hornick R. B., Young C. R., Sotman S. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978 May 27;1(8074):1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Fung P. Y., Whipp S. C., Isaacson R. E. Effects of age and ambient temperature on the responses of infant mice to heat-stable enterotoxin of Escherichia coli: assay modifications. Infect Immun. 1978 Apr;20(1):36–39. doi: 10.1128/iai.20.1.36-39.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C. Development of resistance with age by swine intestine to effects of enteropathogenic Escherichia coli. J Infect Dis. 1970 Sep;122(3):220–223. doi: 10.1093/infdis/122.3.220. [DOI] [PubMed] [Google Scholar]
- Morris G. K., Merson M. H., Sack D. A., Wells J. G., Martin W. T., Dewitt W. E., Feeley J. C., Sack R. B., Bessudo D. M. Laboratory investigation of diarrhea in travelers to Mexico: evaluation of methods for detecting enterotoxigenic Echerichia coli. J Clin Microbiol. 1976 May;3(5):486–495. doi: 10.1128/jcm.3.5.486-495.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan N. A., Burgess M. N., Newsome P. M. Characterization of a partially purified methanol-soluble heat-stable Escherichia coli enterotoxin in infant mice. Infect Immun. 1978 Mar;19(3):779–784. doi: 10.1128/iai.19.3.779-784.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalin D. R., Bhattacharjee A. K., Richardson S. H. Cholera-like toxic effect of culture filtrates of Escherichia coli. J Infect Dis. 1974 Dec;130(6):595–607. doi: 10.1093/infdis/130.6.595. [DOI] [PubMed] [Google Scholar]
- Nalin D. R., Levine M. M., Young C. R., Bergquist E. J., McLaughlin J. C. Increased Escherichia coli enterotoxin detection after concentrating culture supernatants: possible new enterotoxin detectable in dogs but not in infant mice. J Clin Microbiol. 1978 Dec;8(6):700–703. doi: 10.1128/jcm.8.6.700-703.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome P. M., Burgess M. N., Mullan N. A. Effect of Escherichia coli heat-stable enterotoxin on cyclic GMP levels in mouse intestine. Infect Immun. 1978 Oct;22(1):290–291. doi: 10.1128/iai.22.1.290-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaswamy G., Gyles C. L. The prevalence of enterotoxigenic Escherichia coli in the feces of calves with diarrhea. Can J Comp Med. 1976 Jul;40(3):241–246. [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Gyles C. L. The relationship between two apparently different enterotoxins produced by enteropathogenic strains of Escherichia coli of porcine origin. J Med Microbiol. 1970 Aug;3(3):387–401. doi: 10.1099/00222615-3-3-387. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Halls S. Observations by the ligated intestinal segment and oral inoculation methods on Escherichia coli infections in pigs, calves, lambs and rabbits. J Pathol Bacteriol. 1967 Apr;93(2):499–529. doi: 10.1002/path.1700930211. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Halls S. Studies on Escherichia coli enterotoxin. J Pathol Bacteriol. 1967 Apr;93(2):531–543. doi: 10.1002/path.1700930212. [DOI] [PubMed] [Google Scholar]
- Speirs J. I., Stavric S., Konowalchuk J. Assay of Escherichia coli heat-labile enterotoxin with vero cells. Infect Immun. 1977 May;16(2):617–622. doi: 10.1128/iai.16.2.617-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlind O., Möllby R. Enterotoxins, O-groups, and K88 antigen in Escherichia coli from neonatal piglets with and without diarrhea. Infect Immun. 1979 Jun;24(3):611–616. doi: 10.1128/iai.24.3.611-616.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



