Abstract
Background: The aim of this study was to compare the extent of pathologic response in patients with HER2-positive (HER2+) breast cancer treated with standard neoadjuvant chemotherapy, with or without trastuzumab (H), according to hormone receptor (HR) status.
Patients and methods: We included 199 patients with HER2+ breast cancer from three successive cohorts of neo-adjuvant chemotherapy on the basis of paclitaxel (Taxol) (P) administered weekly (w) or three weekly (3-w), followed by 5-fluorouracil (F), doxorubicin (A) or epirubicin (E), and cyclophosphamide (C). Residual cancer burden (RCB) was determined from pathologic review of the primary tumor and lymph nodes and was classified as pathologic complete response (pCR) or minimal (RCB-I), moderate (RCB-II), or extensive (RCB-III) residual disease.
Results: In HR-positive (HR+) cancers, a higher rate of pathologic response (pCR/RCB-I) was observed with concurrent H + 3-wP/FEC (73%) than with 3-wP/FEC (34%, P = 0.002) or wP/FAC (47%; P = 0.02) chemotherapy alone. In HR-negative (HR−) cancers, there were no significant differences in the rate of pathologic response (pCR/RCB-I) from 3-wP/FAC (50%), wP/FAC (68%), or concurrent H + 3-wP/FEC (72%).
Conclusions: Patients with HR+/HER2+ breast cancer obtained significant benefit from addition of trastuzumab to P/FEC chemotherapy; pathologic response rate was similar to that seen in HR−/HER2+ breast cancers.
Keywords: breast cancer, HER2, hormone receptor status, neoadjuvant chemotherapy, trastuzumab
introduction
Patients with HER2-positive (HER2+) breast cancer are eligible to receive trastuzumab, a recombinant mAb against HER2 (Herceptin; Genentech Inc, South San Francisco, CA). Trastuzumab (H) increases time to progression and improves survival when it is administered in combination with a standard chemotherapy for metastatic disease [1, 2]. Concurrent trastuzumab with paclitaxel/fluorouracil, epirubicin, and cyclophosphamide (P/FEC) chemotherapy as neoadjuvant treatment improved the rate of pathologic complete response (pCR), compared with P/FEC chemotherapy alone in a randomized trial that required only 42 patients to meet statistical significance [3]. The high rate of pCR was maintained in a subsequent cohort of patients who were treated with concurrent H + three weekly (3-wP)/FEC [4]. Furthermore, multicenter phase III trials have now proved that the combination of trastuzumab with a standard adjuvant chemotherapy regimen significantly reduces the risk of relapse [5–7].
It has been demonstrated that 12 weekly cycles of paclitaxel at lower dose is superior to four three-weekly cycles of paclitaxel when sequentially administered with anthracycline-based chemotherapy. In a prospective randomized trial of neoadjuvant P/fluorouracil, doxorubicin, and cyclophosphamide (FAC) chemotherapy, the rate of pCR was significantly higher for wP/FAC, compared with 3-wP/FAC (28.2% versus 15.7%, P = 0.02) [8].
Residual cancer burden (RCB) is a measurement of the extent of residual disease (RD) that takes into account both primary tumor bed features and axillary lymph node features [9]. RCB is a surrogate for distant relapse-free survival and has more prognostic power than the usual dichotomized response categories of pCR and RD or the American Joint Committee on Cancer staging system following neoadjuvant chemotherapy [9]. Furthermore, RCB identifies a subset of patients with minimal RD (RCB-I) whose prognosis is the same as for those who achieve pCR. This extends the definition of excellent pathologic response to include those with pCR or RCB-I.
The aim of this study was to compare pathologic responses from three successive neoadjuvant treatment regimens for patients with HER2+ breast cancer such as 3-wP/FAC, wP/FAC, and concurrent H + 3-wP/FAC.
patients and methods
patients
From 2001 to 2005, patients with stages I–III breast cancer that was positive as indicated by 3+ staining intensity on immunohistochemical staining for HER2 protein or amplification of the HER2 gene by FISH were treated in two clinical trials of neoadjuvant chemotherapy at The University of Texas M. D. Anderson Cancer Center. Fifty-five patients were treated in a nontrastuzumab-containing trial comparing 3-wP/FAC with wP/FAC [8], and subsequently an additional 63 patients were treated outside of protocol with wP/FAC. Forty-two patients were treated in a trial of concurrent H + 3-wP/FEC versus 3-wP/FEC alone [3], and subsequently an additional 34 patients were treated outside of this protocol with concurrent H + 3-wP/FEC. The institutional review board approved both trials, and all patients gave informed consent. For the purpose of this study, we considered the 3-wP/FAC and the 3-wP/FEC regimens to be the same and have grouped these as 3-wP/FAC. A total of 199 patients were included in this study after exclusion of 16 patients with missing pathological records due to surgery at an outside institution. Overall, we evaluated RCB in 47 patients who completed 3-wP/FAC, 63 who received wP/FAC, and 89 who received concurrent H + 3-wP/FEC. The diagnosis of invasive cancer, HER2 status, and hormone receptor (HR) status was defined from the pretreatment core needle biopsy.
treatment
In the wP/FAC regimen, patients received 12 weekly cycles of paclitaxel (P), with the dose on the basis of the nodal status (80 mg/m2 for node-negative and 150 mg/m2 for node-positive patients), followed by four cycles of fluorouracil (500 mg/m2), doxorubicin (50 mg/m2), and cyclophosphamide (500 mg/m2) (FAC). In the 3-wP/FAC regimen, patients received paclitaxel (225 mg/m2) every 3 weeks for four cycles, followed by four cycles of FAC. In the other protocol, patients were randomly assigned to receive 3-wP/FEC chemotherapy alone, consisting of four cycles of paclitaxel (225 mg/m2 once every 3 weeks for four cycles) followed by four cycles of fluorouracil (500 mg/m2), epirubicin (75 mg/m2), and cyclophosphamide (500 mg/m2) or concurrent weekly trastuzumab (4 mg/kg on day 1 and subsequent infusions at a dose of 2 mg/kg) with 3-wP/FEC chemotherapy. The full details of both trials have previously been reported elsewhere [3, 8].
Physical examination, mammography, and sonography were carried out at diagnosis after four cycles of chemotherapy and before surgery (after completion of FAC or FEC). In patients with clinical response to neo-adjuvant chemotherapy, metallic markers were placed in the tumor bed under ultrasound guidance to enable identification of the primary tumor site at the time of surgery. After completion of neoadjuvant chemotherapy, a multidisciplinary team determined what type of definitive surgery should be carried out (breast-conserving surgery or mastectomy with sentinel node biopsy or levels I and II axillary lymph node dissection). The treatment protocols called for postoperative adjuvant external-beam radiotherapy to be given to all patients who underwent breast-conserving surgery. Radiotherapy was delivered to the chest wall in patients with stage III disease or four or more positive axillary lymph nodes and in selected patients with one to three positive lymph nodes. All patients with HR-positive (HR+) tumors received adjuvant endocrine therapy.
pathologic assessment
The hematoxylin and eosin-stained slides from the postchemotherapy surgical resection specimen were reviewed microscopically by WFS and FP and used to calculate RCB as outlined at http://www.mdanderson.org/breastcancer_rcb [9]. On the basis of their RCB score, patients were assigned to one of four response categories: pCR (no residual invasive carcinoma in the breast or the axillary lymph nodes) or minimal (RCB-I), moderate (RCB-II), or extensive (RCB-III) RD. Patients who progressed during neoadjuvant treatment or whose disease was inoperable after treatment had response defined as RCB-III. Excellent pathologic response was defined as pCR or RCB-I on the basis of similarly excellent prognosis in HR-negative (HR−) and HR+ disease [9]. HER2 positivity was indicated by 3+ staining intensity on immunohistochemical staining for HER2 protein or amplification of the HER2 gene by FISH. HR positivity was defined by positive staining for estrogen- and/or progesterone-positive receptors in at least 10% of cancer cell nuclei.
statistical analysis
Statistical comparisons between groups were carried out using the chi-square (χ2) test and comparisons among groups using the equal variance t-test. One-way analysis of variance was used for the comparison of means according to the RCB categories, and two-sample t-tests with equal variances were used to calculate differences among these groups. All tests were two tailed, and a P value of <0.05 was considered significant. The SPSS 12.0 software package (SPSS Inc., Chicago, IL) was used for statistical analyses.
results
Of the 199 patients with HER2+ breast cancer in this study, 47 were treated with 3-wP/FAC or P/FEC, 63 were treated with wP/FAC, and 89 were treated with weekly H + 3-wP/FEC.
Patient and tumor characteristics are shown in Table 1. Advanced disease (stage IIIB or IIIC) was less common in patients treated with 3-wP/FAC than in patients treated with the other regimens (P = 0.02). Overall, 51% of patients had HR+ and 49% had HR− cancer. For 24 tumors, HER2+ status was determined by immunohistochemical staining only, for 84 tumors by FISH only, and for 91 tumors HER2+ status was assessed by both techniques.
Table 1.
Patient and tumor characteristicsa
| Characteristic | 3-wP/FAC (or FEC), n = 47 | wP/FAC, n = 63 | H + 3-wP/FEC, n = 89 | P |
| Age, years | ||||
| Median | 48 years | 51 years | 50 years | |
| Range | 25–77 years | 26–79 years | 21–81 years | |
| Ethnicity | 0.35 | |||
| White | 33 (70.2) | 47 (74.6) | 54 (60.7) | |
| Black | 6 (12.8) | 4 (6.3) | 8 (9) | |
| Hispanic | 5 (10.6) | 10 (15.9) | 18 (20.2) | |
| Asian | 3 (6.4) | 2 (3.2) | 9 (10.1) | |
| Clinical tumor category | 0.33 | |||
| T1 | 8 (17) | 3 (4.8) | 11 (12.5) | |
| T2 | 29 (61.7) | 38 (60.3) | 51 (58) | |
| T3 | 6 (12.8) | 12 (19.0) | 19 (21.6) | |
| T4 | 4 (8.5) | 10 (15.9) | 7 (8) | |
| Nodal status | 0.04 | |||
| N0 | 17 (36.2) | 17 (27.0) | 27 (30.3) | |
| N1 | 26 (55.3) | 29 (46.0) | 48 (53.9) | |
| N2 | 4 (8.5) | 4 (6.3) | 2 (2.2) | |
| N3 | 0 (0) | 13 (20.7) | 12 (13.5) | |
| Initial clinical stage | 0.03 | |||
| I | 3 (6) | 2 (3) | 2 (2) | |
| IIA | 17 (36) | 14 (22) | 24 (27) | |
| IIB | 16 (34) | 15 (24) | 33 (37) | |
| IIIA | 7 (15) | 11 (17) | 15 (17) | |
| IIIB | 4 (9) | 8 (13) | 3 (3) | |
| IIIC | 0 (0) | 13 (21) | 12 (14) | |
| Histologic subtype | 0.23 | |||
| IDC | 44 (93.6) | 61 (96.8) | 86 (96.6) | |
| ILC | 1 (2.1) | 0 (0) | 3 (3.4) | |
| Mucinous | 2 (4.3) | 2 (3.2) | 0 (0) | |
| Nuclear grade | 0.79 | |||
| 1 or 2 (well or moderately differentiated) | 16 (34) | 21 (33.3) | 26 (29.2) | |
| 3 (Poorly differentiated) | 31 (66) | 42 (66.7) | 63 (70.8) | |
| Unknown | 1 (2) | 0 (0) | 0 (0) | |
| Hormone receptor statusb | 0.59 | |||
| Positive | 27 (57.4) | 32 (50.8) | 43 (48.3) | |
| Negative | 20 (42.6) | 31 (49.2) | 46 (51.7) |
Values in table are no. (%) of patients unless otherwise indicated.
Estrogen receptor and/or progesterone receptor.
3-w, 3-weekly; P, paclitaxel; FAC, fluorouracil, doxorubicin, and cyclophosphamide; FEC, fluorouracil, epirubicin, and cyclophosphamide; H, trastuzumab; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
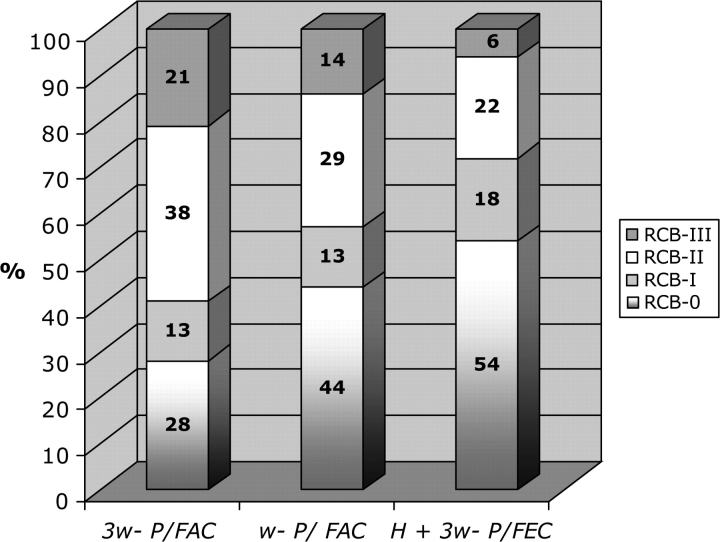
Frequencies of RCB categories of pathologic response after each regimen (H + 3-wP/FEC, wP/FAC, and 3-wP/FAC) were as follows: pCR (54%, 44%, and 28%), RCB-I (18%, 13%, and 13%), RCB-II (22%, 29%, and 38%), and RCB-III (6%, 14%, and 21%), respectively (Figure 1). The proportion of patients with extensive RD (indicating treatment resistance) was only 6% following H + 3-wP/FEC, significantly lower than after 3-wP/FAC alone (P = 0.008), and with a near significant trend toward lower following H + 3-wP/FEC than after wP/FAC (P = 0.06).
Figure 1.
Distribution of residual cancer burden in the neoadjuvant chemotherapy regimens.
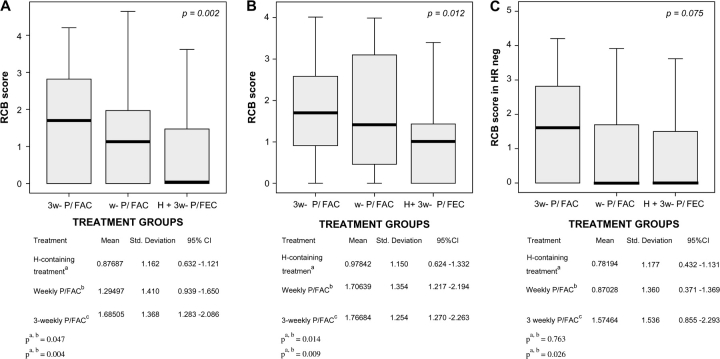
Considering RCB as a continuous parameter, we observed that mean RCB scores were lower following H + 3-wP/FEC than after wP/FAC (P = 0.07) or 3-wP/FAC chemotherapy (P = 0.0004) (Figure 2A). Among patients with HR+/HER2+ breast cancer, mean RCB score was significantly lower following H + 3-wP/FEC than after wP/FAC (P = 0.01) or 3-wP/FAC (P = 0.009) (Figure 2B). In contrast, among patients with HR−/HER2+ breast cancer there was no significant difference in the mean RCB scores for the three treatment regimens (Figure 2C).
Figure 2.
(A) Distribution of residual cancer burden (RCB) scores (means). (B) RCB scores in hormone receptor-positive status. (C) RCB scores in hormone receptor-negative status.
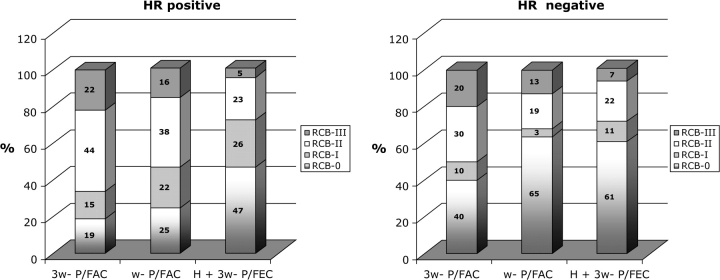
Among patients with HR+/HER2+ breast cancer, pCR was more common following H + 3-wP/FEC than after wP/FAC (P = 0.04) or 3-wP/FAC (P = 0.01). Among HR−/HER2+ breast cancer, there were no significant differences in pCR rates between treatment groups; pCR was equally common after H + 3-wP/FEC (61%) or wP/FAC (63%) (Table 2). The frequency of RCB-II in HR+ and HR− breast cancers treated with H + 3-wP/FEC was similar (23% and 22%, respectively), as was the frequency of RCB-III (5% and 7%, respectively) (Figure 3).
Table 2.
Comparison of pCR rates, with 95% confidence intervals (lower panel), among the regimens according to hormone receptor status
| Regimen | Overall | P | HR+ | P | HR− | P |
| 3-wP/FAC versus wP/FAC | 28% versus 44% | 0.05 | 19% versus 25% | 0.39 | 40% versus 65% | 0.07 |
| 3-wP/FAC versus H + 3-wP/FEC | 28% versus 54% | 0.003 | 19% versus 47% | 0.01 | 40% versus 61% | 0.09 |
| wP/FAC versus H + 3-wP/FEC | 44% versus 54% | 0.24 | 25% versus 47% | 0.04 | 65% versus 61% | 0.46 |
| Overall |
HR+ |
HR− |
|||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||||
| Treatment groups | |||||||||
| H + 3-wP/FEC | 0.539 | 0.436 | 0.643 | 0.465 | 0.316 | 0.614 | 0.609 | 0.468 | 0.750 |
| wP/FAC | 0.444 | 0.322 | 0.567 | 0.250 | 0.100 | 0.400 | 0.645 | 0.477 | 0.814 |
| 3-wP/FAC | 0.277 | 0.149 | 0.404 | 0.185 | 0.039 | 0.332 | 0.400 | 0.185 | 0.615 |
| Grand total | 0.447 | 0.378 | 0.516 | 0.324 | 0.233 | 0.414 | 0.577 | 0.479 | 0.676 |
pCR, pathologic complete response; HR, hormone receptor (estrogen or progesterone receptor); 3-w, 3-weekly; P, paclitaxel; FAC, fluorouracil, doxorubicin, and cyclophosphamide; FEC, fluorouracil, epirubicin, and cyclophosphamide; H, trastuzumab; CI, confidence interval.
Figure 3.
Distribution of residual cancer burden in (A) hormone receptor-positive and (B) hormone receptor-negative cases.
Among patients with HR+/HER2+ breast cancer, excellent pathologic response (pCR/RCB-I) was more common following H + 3-wP/FEC than after wP/FAC (P = 0.02) or 3-w/P/FAC (P = 0.002) (Table 3). Among HR−/HER2+ breast cancer, there was a near significant trend toward more frequent excellent pathologic response (pCR/RCB-I) following H + 3-wP/FEC, compared with 3-wP/FAC alone (P = 0.07), but no difference between H + 3-wP/FEC and wP/FAC chemotherapy (Table 3). There was an observed trend toward decreasing rates of RCB-III from 3-wP/FAC to wP/FAC and to H + 3-wP/FEC for HR+ breast cancer (22%, 16%, and 5% in Figure 3A) and for HR− breast cancers (20%, 13%, and 7% in Figure 3B).
Table 3.
Comparison of rates of pCR/RCB-I (pCR plus near-pCR), with 95% confidence intervals (lower panel), among the regimens according to hormone receptor status
| Regimen | Overall | P | HR+ | P | HR− | P |
| 3-wP/FAC versus wP/FAC | 41% versus 57% | 0.08 | 34% versus 47% | 0.21 | 50% versus 68% | 0.16 |
| 3-wP/FAC versus H + 3-wP/FEC | 41% versus 72% | <0.0001 | 34% versus 73% | 0.002 | 50% versus 72% | 0.07 |
| wP/FAC versus H + 3-wP/FEC | 57% versus 72% | 0.03 | 47% versus 73% | 0.02 | 68% versus 72% | 0.44 |
| Overall |
HR+ |
HR− |
|||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||||
| Treatment groups | |||||||||
| H + 3wP/FEC (0) | 0.719 | 0.626 | 0.812 | 0.721 | 0.587 | 0.855 | 0.717 | 0.587 | 0.848 |
| wP/FAC (1) | 0.571 | 0.449 | 0.694 | 0.469 | 0.296 | 0.642 | 0.677 | 0.513 | 0.842 |
| 3-wP/FAC (2) | 0.404 | 0.264 | 0.545 | 0.333 | 0.156 | 0.511 | 0.500 | 0.281 | 0.719 |
| Grand total | 0.598 | 0.530 | 0.666 | 0.539 | 0.442 | 0.636 | 0.660 | 0.566 | 0.754 |
pCR, pathologic complete response; HR, hormone receptor (estrogen or progesterone receptor); 3-w, 3-weekly; P, paclitaxel; FAC, fluorouracil, doxorubicin, and cyclophosphamide; H, trastuzumab; FEC, fluorouracil, epirubicin, and cyclophosphamide; CI, confidence interval.
discussion
In this study, we found that patients with HER2+ breast cancers treated with concurrent trastuzumab plus standard neo-adjuvant chemotherapy (H + 3-wP/FEC) had less RD than patients treated with standard neoadjuvant chemotherapy alone (3-wP/FAC).
In particular, we found that patients with HR+/HER2+ breast cancer were significantly more likely to achieve pCR or excellent pathologic response (pCR/RCB-I) with the trastuzumab-containing regimen than with either chemotherapy regimen alone. However, patients with HR−/HER2+ breast cancer tumors had similar rates of pCR or excellent pathologic response (pCR/RCB-I) with the trastuzumab-containing regimen (H + 3-wP/FEC) or with the weekly paclitaxel regimen (wP/FAC).
Trastuzumab as a single agent induces apoptosis in the neo-adjuvant setting [10]; it is most likely synergistic with standard chemotherapy drugs since standard chemotherapy also induces apoptosis [11]. This effect is evident in the current study. We were not able to examine whether the addition of trastuzumab to weekly (as opposed to three weekly) paclitaxel could further increase pCR rates in HR+ as well as HR− tumors. This is an interesting question, particularly because the timing of apoptotic response after a dose of paclitaxel appears to favor a weekly paclitaxel schedule [11]. A number of phase II trials have examined the addition of concurrent trastuzumab to weekly or three-weekly taxanes [12, 13] or other drugs and drug combinations [14–16], but those trials showed lower pCR rates than were seen in the current analysis. The longer duration of chemotherapy, incorporation of anthracyclines in a concurrent regimen, and inclusion of patients with operable breast cancer could each contribute to the higher pCR rates that we observed.
We have assessed RD after neoadjuvant chemotherapy, with or without trastuzumab, using three different methods: (i) the pCR rate (traditional end point), (ii) RCB scores, and (iii) RCB categories. However, it should be noted that the three treatment cohorts do not represent randomized treatment arms, and so direct comparisons of pathologic response should be interpreted with caution. Nevertheless, we identified that patients with HR+/HER2+ breast cancer appear to have the most improvement in pathologic response from the addition of trastuzumab to chemotherapy. On one hand, the results indicate that HR−/HER2+ breast cancers are more sensitive than HR+/HER2+ breast cancers to regimens that use dose density. On the other hand, the results also indicate that HR+/HER2+ breast cancers have less intrinsic chemosensitivity than HR−/HER2+ breast cancers, but that this can be overcome by synergy between chemotherapy and an appropriate targeted therapy. This is an interesting hypothesis to consider in the context of other HR+/HER2− breast cancers, if there is potential to improve chemosensitivity through combination with an appropriate targeted therapy.
Our study demonstrates that patients with HR+/HER2+ tumors derive a great benefit from inclusion of trastuzumab in their chemotherapy. This is an important observation because these patients do not have particularly high rates of excellent pathologic response after treatment with chemotherapy alone. These results indicate that it is possible to increase chemotherapy activity by using biologically targeted agents in a group of patients who are traditionally considered not particularly chemotherapy sensitive. Regarding trastuzumab benefit in patients with HR−/HER+ tumors, we cannot conclude that this group of patients do not benefit from inclusion of trastuzumab. Some further improvements in excellent pathologic response rates are possible even if we could not detect it with statistical significance. Since pCR rates are quite high in this patient subset, this study is probably underpowered to detect with confidence modest further improvement in response rate. However, our data suggest that large increase in pCR does not occur in this patient population as it does in the HR+/HER+ group.
In conclusion, we suggest that HR+/HER2+ breast cancers have considerable potential to achieve improved pathologic response from concurrent trastuzumab with neoadjuvant chemotherapy. A randomized phase III trial is currently active (National Surgical Adjuvant Breast and Bowel Project B41/American College of Surgeons Oncology Group Z1041) to compare a neoadjuvant regimen of FEC followed by weekly paclitaxel plus trastuzumab with a neoadjuvant regimen of weekly paclitaxel plus trastuzumab followed by FEC plus trastuzumab in patients with palpable and operable breast cancer. The results of this trial will provide further results of pathologic response and safety from concurrent trastuzumab with anthracycline treatment in HER2+ breast cancer. That study should also allow comparison of pCR and RCB responses according to HR status and will provide new results from the combination of trastuzumab with a regimen that uses weekly paclitaxel.
funding
Department of Defense Breast Cancer Research Program (DAMD17-02-1-0458 01) to WFS; Nellie B. Connally Breast Cancer Research Fund.
References
- 1.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 2.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 3.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 4.Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–233. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 5.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 6.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 7.Slamon D, Eiermann W, Robert N. Second interim analysis phase III trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC-T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC-TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2neu positive early breast cancer patients. San Antonio Breast Cancer Symposium 2006 (Abstr 52), http://www.abstracts2view.com/sabcs06. [Google Scholar]
- 8.Green MC, Buzdar AU, Smith T, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;23:5983–5992. doi: 10.1200/JCO.2005.06.232. [DOI] [PubMed] [Google Scholar]
- 9.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 10.Mohsin SK, Weiss HL, Gutierrez MC, et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J Clin Oncol. 2005;23:2460–2468. doi: 10.1200/JCO.2005.00.661. [DOI] [PubMed] [Google Scholar]
- 11.Symmans WF, Volm MD, Shapiro RL, et al. Paclitaxel-induced apoptosis and mitotic arrest assessed by serial fine-needle aspiration: implications for early prediction of breast cancer response to neoadjuvant treatment. Clin Cancer Res. 2000;6:4610–4617. [PubMed] [Google Scholar]
- 12.Bines J, Murad A, Lago S, et al. Weekly docetaxel and trastuzumab as primary therapy in stage III, HER-2 overexpressing breast cancer—a Brazilian multicenter study. Breast Cancer Res Treat. 2003;82:S56. (Abstr 243) [Google Scholar]
- 13.Burstein HJ, Harris LN, Gelman R, et al. Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: a pilot study. J Clin Oncol. 2003;21:46–53. doi: 10.1200/JCO.2003.03.124. [DOI] [PubMed] [Google Scholar]
- 14.Harris L, Burstein HJ, Gelman R, et al. Preoperative trastuzumab and vinorelbine (HN) is a highly active, well-tolerated regimen for HER2 3+/FISH+ Stage II/III breast cancer. Proc Am Soc Clin Oncol. 2003;22:22. (Abstr 86) [Google Scholar]
- 15.Hurley J, Doliny P, De Zarraga F, et al. Platinum salts and docetaxel as primary therapy of locally advanced and inflammatory breast cancer: the final report of three sequential studies. Breast Cancer Res Treat. 2003;82:S54. (Abstr 238) [Google Scholar]
- 16.Wenzel C, Hussian D, Bartsch R, et al. Preoperative therapy with epidoxorubicin and docetaxel plus trastuzumab in patients with primary breast cancer: a pilot study. J Cancer Res Clin Oncol. 2004;130:400–404. doi: 10.1007/s00432-004-0559-6. [DOI] [PubMed] [Google Scholar]