Abstract
Background: Not all patients with locally advanced rectal cancer (LARC) respond equally to neo-adjuvant radiochemotherapy (RCT). Patients with highly apoptotic less advanced rectal cancers do not benefit from short-term radiotherapy. This study investigates whether this is also the case in the setting of RCT for LARC.
Patients and methods: Tissue microarrays were constructed of biopsy and resection specimens of 201 LARC patients. Apoptosis (M30) and several apoptosis-regulating proteins [p53, Bcl-2, Bax, cyclooxygenase-2 (Cox-2) and mamma serine protease inhibitor (maspin)] were studied with immunohistochemistry. Subsequently, predictive values for local recurrence (LR), overall survival (OS) and histological tumour regression were analysed.
Results: Apoptotic levels, quantified as the number of apoptotic cells/mm2 tumour epithelium, were higher in posttherapy tissues compared with biopsies (P < 0.001). Biopsies from clinical T4 stage tumours demonstrated significantly higher levels of apoptosis than clinical T3 stage tumours (P = 0.020). Therapy-induced apoptosis was higher when the interval between the last day of irradiation and surgery increased (P < 0.001, correlation coefficient = 0.355). Pre- and posttherapy apoptosis, p53, Bcl-2, Bax and Cox-2 were not associated with LR, OS or tumour regression. Intense pretherapy cytoplasmatic staining of maspin indicated a higher risk on LR (P = 0.009) only.
Conclusion: Combined RCT is also successful in highly apoptotic tumours and is therefore independent of intrinsic apoptosis.
Keywords: apotosis, locally advanced rectal carcinoma, radiochemotherapy
introduction
Patients with locally advanced rectal cancer (LARC) are at high risk of local failure. Therefore, surgery alone is often not curative and neo-adjuvant radiochemotherapy (RCT) is required in order to improve local control and achieve a radical resection. Tumour response to RCT varies considerably and various stages of histological tumour regression can be observed in the surgical resection specimens. Markers that can predict therapy outcome would permit adaptation of the therapeutic strategy and improve treatment of LARC; e.g. poor responders could be offered a different therapy regimen or could be operated much sooner with more extensive surgery.
In order to prevent local recurrences (LRs) and improve local control, patients with mobile rectal cancer (T1–T3) currently receive short-term neo-adjuvant radiotherapy (RT) as standardised treatment for rectal cancer in The Netherlands. However, not all patients benefit equally; some might experience side-effects [1]. Therefore, we would like to predict tumour response. de Bruin et al. [2] showed that levels of apoptosis (assessed with M30) can attribute to the selection of patients with a high risk on local failure since RT was effective in patients with low levels of intrinsic apoptosis, but patients who had high levels of intrinsic apoptosis in their primary tumour and did not receive RT had the same risk on developing a LR, as patients that did receive RT [2]. It is, however, unclear what the prognostic implications of intrinsic and therapy-induced apoptosis are in patients with LARC in the neo-adjuvant setting.
RT and chemotherapy can induce apoptosis in malignancies of the gastrointestinal tract. However, the complexity of the apoptotic pathway enables tumour cells to escape from apoptosis inducing therapy resistance and affecting patient's outcome. At the cellular level, the regulation of apoptosis depends on a very complex balance between pro- and anti-apoptotic proteins. Among them are p53, Bcl-2, Bax, cyclooxygenase-2 (Cox-2) and mamma serine protease inhibitor (maspin). The extensively described oncogene p53 is commonly inactivated in colorectal cancer and is a cell cycle regulator and a potent inducer of apoptosis as a response to DNA damage [3–5]. Bcl-2 and Bax act as antagonists and are members of the same family of proteins. While Bcl-2 is an important inhibitor of apoptosis, overexpression of Bax induces programmed cell death [6, 7]. During irradiation, Cox-2 has been described to act as a survival factor by inhibiting apoptosis [8]. Maspin is a multifaceted protein that has been described to promote apoptosis [9].
In the present study, we evaluated the prognostic value of intrinsic and induced apoptosis (M30) and five different apoptosis-regulating proteins: p53, Bcl-2, Bax, Cox-2 and maspin in patients with LARC who were treated with RCT. LR and overall survival (OS) were the main outcome parameters. In addition, the association between these markers and histological tumour regression was investigated.
patients and methods
patient selection
The patient population consisted of a consecutive series of 201 LARC patients with biopsy-proven adenocarcinoma as described previously [10]. All patients received multimodality treatment at the Catharina hospital from 1994 to 2005 [11]. Treatment decision was on the basis of the best regimen according to the national guidelines of that moment in time.
histological assessment of therapy-induced tumour regression
Histological therapy-induced tumour regression was assessed according to the regression grading system described by Rodel et al.[12]. The degree of tumour was estimated and subdivided into the following categories: (i) no regression or <25% of tumour mass, (ii) 25% to >50% tumour regression and (iii) complete regression. The degree of tumour regression was determined semiquantitatively by an experienced pathologist (JHJMvK) who was unfamiliar with patients' clinical outcome. The investigations of the clinical value of therapy-induced tumour regression assessment in this patient population have been extensively described in a previous study [10].
tissue microarray construction
Tissue microarrays (TMA) were constructed from formalin-fixed, paraffin-embedded biopsy tissues (three 0.6-mm punches) and resection specimens (three 2-mm punches) using an Alphelys TMA booster (Westburg, Leusden, The Netherlands). Three punches have been previously described to be sufficient to overcome tumour heterogeneity [13]. Moreover, most studies validating the use of TMA for studying markers with immunohistochemistry (IHC) advise to use three to four punches of 0.6 mm [14–16]. We used an equivalent of 10 tumour punches of 0.6 mm which is well above the advised number of 0.6-mm punches. In case normal tissue was available, one punch was also taken from this area in order to construct a TMA containing normal tissues only. Preferably, normal tissue adjacent to the tumor tissue was used (55%). If this was not present, normal tissue was obtained from another paraffin block (45%).
immunohistochemistry
Immunohistochemical stainings were carried out on 4-μm TMA sections using the PowerVision plus method (ImmunoVision, Brisbane, CA). Visualisation involved incubation with PowerDAB (ImmunoVision) for 5 min. Methods for antigen retrieval and the dilutions of the antibodies used can be found in Table 1. Slides were incubated in a humidity chamber with the primary antibody for 20 h (overnight) at 4°C. Negative controls involved incubation with phosphate-buffered saline with 2% goat serum and 0.1% sodium azide only. Slides were counterstained with haematoxylin. Staining patterns of p53, Bcl-2, Bax, Cox-2 and maspin were all evaluated semiquantitatively (Table 1) by two independent observers who were unfamiliar with patients' outcome. In case of disagreement, a consensus score was obtained which was used for further analysis.
Table 1.
Immunohistochemical specifications of the antibodies used
| Antibody (company) | Clone | Dilution | Antigen retrieval | IHC assessment |
| M30 CytoDEATH (Roche, Roche Applied science, Indiapolis, IN) | M30 specific for human cytokeratine 18 | 1:500 | Incubation with a 10 mM sodium citrate buffer (pH 6.0) for 30 min at 98°C | Number of apoptotic cells/mm2 tumour epithelial was quantified |
| P53 (BioGenex, San Ramon, CA) | BP53-12 | 1:10 000 | Intensity of nuclear staining (0–3) and the proportion of negative cells: 10%–30%, 30%–60% or 60%–90%a | |
| Bcl-2 Oncoprotein (Dako, Glostrup, Denmark) | 124 | 1:500 | Intensity of cytoplasmic staining (0–3)a | |
| Bax (Santa Cruz Biotechnology, Santa Cruz, CA) | B-9 | 1:1000 | Intensity of cytoplasmic staining (0–3)a | |
| Cox-2 (Cayman Chemicals, Ann Arbor, MI) | CX229 | 1:100 | Combination of intensity and proportion of cytoplasmic staininga [17, 18] | |
| Maspin (Becton Dickinson, Pharmingen, Franklin Lakes, NJ) | G167-70 | 1:20 000 | Intensity of cytoplasmic and nuclear staining (0–3)a |
Staining patterns were assessed by two independent observers.
IHC, immunohistochemistry; Cox-2, cyclooxygenase-2; Maspin, mamma serine protease inhibitor.
quantification of apoptosis
In case of M30 IHC, the number of apoptotic tumour cells/mm2 tumour epithelium was assessed for each punch. In order to establish the area of tumour epithelium for each punch, TMA were stained with the pancytokeratin MAK-6 (1:10, protease antigen retrieval, Zymed Laboratories, Invitrogen, Carlsbad, CA). Microscopic images measuring 0.74 mm2 were digitised using an RGB CCD camera (AxioCam MRc, Zeiss, Germany) resulting in a specimen level pixel size of 1.3 × 1.3 um2. Quantitative measurements of the tumour area or area of normal epithelium were carried out using a digital image analysis system (KS400, Carl Zeiss, Germany). Punches of 0.6-mm diameter were analyzed using a ×10 objective and 2-mm punches using an objective of ×2.5. Digitised images were corrected for unequal illumination using a stored image of an empty microscopic field. The immunopositive area (in mm2) was calculated automatically. Subsequently, the number of apoptotic cells was manually counted to calculate the apoptotic level defined as the number of apoptotic cells/mm2 tumour epithelium. Values that exceeded the standard deviation two times were considered outliers and were therefore excluded from further analysis.
statistics
Data were analyzed with the SPSS package (Statistical Product and Service Solutions 11.0 for Windows, SPSS Inc., Chicago, IL). Interobserver variability was calculated by κ statistic as described by Cohen [19, 20]. κ values of 0.2–0.4 indicate ‘fair’, 0.4–0.6 ‘moderate’ and values of >0.6 ‘excellent’ agreements [21]. Correlations between apoptosis and the interval between the last day of irradiation and surgery were analyzed according to the Spearman's rank correlation test. Univariate survival analyses of time to death or LR were carried out using the Kaplan–Meier method and log-rank testing with the time of surgery as the entry date. Patient outcome parameters were LR and OS. P values of ≤0.05 were considered as statistically significant.
Multiple variable analyses involved Cox proportional hazards regression (enter method), logistic regression (enter method) and building of decision trees using the DTREG software for predictive modelling and forecasting (www.dtreg.com). Single-tree models for classification using the Gini splitting algorithm in which the variables were equally weighted were constructed. Pruning and validation of the tree model was carried out with the V-Fold cross validation in order to determine the statistically optimal tree size.
results
patient and tumour characteristics
Patient, treatment and tumour characteristics are summarised in Table 2. The majority (59%) of the patient population initially presented with a tumour invading into other adjacent organs or structures [clinical T4 stage (cT4)]. Complete histological tumour regression was observed in 21 (11%) patients. Poor (Rödel 0) and moderate (Rödel 1) tumour regression grades were observed in 50% and 39% patients, respectively.
Table 2.
Patient, treatment and tumour characteristics
| Factor | Category | n (%) |
| Median age (range) | 63 (35–86) | |
| Sex | Male | 122 (61) |
| Female | 79 (39) | |
| cT stage | cT3 | 83 (41) |
| cT4 | 118 (59) | |
| Neo-adjuvant therapy | RT | 74 (37) |
| RCT interrupted | 102 (51) | |
| RCT continuous | 25 (12) | |
| Median interval between last day RT and surgery | 7.8 weeks | |
| CRM | Positive | 43 (21) |
| Negative | 158 (79) | |
| ypT stage | ypT0 | 20 (10) |
| ypTis | 1 (0.5) | |
| ypT1 | 2 (1) | |
| ypT2 | 15 (7) | |
| ypT3 | 132 (66) | |
| ypT4 | 30 (15) | |
| not assessable | 1 (0.5) | |
| ypN stage | N0 | 131 (65) |
| N1 | 47 (24) | |
| N2 | 23 (11) | |
| Histologic regression | 0 | 101 (50) |
| 1 | 79 (39) | |
| 2 | 21 (11) | |
| Pretreatment apoptosis (mean of cells) | 0 | |
| Posttreatment apoptosis (mean of cells) | 10.39 |
cT3, clinical T3 stage, cT4: clinical T4 stage; RT, radiotherapy; RCT, radiochemotherapy; CRM, circumferential margin.
apoptosis; prognostic significance and correlations with clinicopathological factors
Representative staining patterns observed after M30 IHC are depicted in Figure 1A. The apoptotic levels in neither the posttherapy resection specimens nor the pretreatment biopsies were associated with LR or OS. The amount of apoptotic cells was significantly higher in posttreatment resection specimens compared with pretreatment biopsies (median 10.4 versus 0, P = <0.001, Wilcoxon test). M30 IHC was also carried out on TMA-containing normal tissues. This revealed that the amount of apoptosis in normal epithelium was significantly lower in patients who received the capecitabine-containing continuous schedule (7.2 apoptotic cells/mm2 epithelium) compared with patients who received the interrupted RCT (15.3 apoptotic cells/mm2 epithelium) schedule (P = 0.018). Apoptotic levels did not differ between normal tissues obtained adjacent to the tumour from another paraffin block (P = 0.163). The amount of apoptotic tumour cells did not significantly differ between the different therapy regimens (Table 3).
Figure 1.
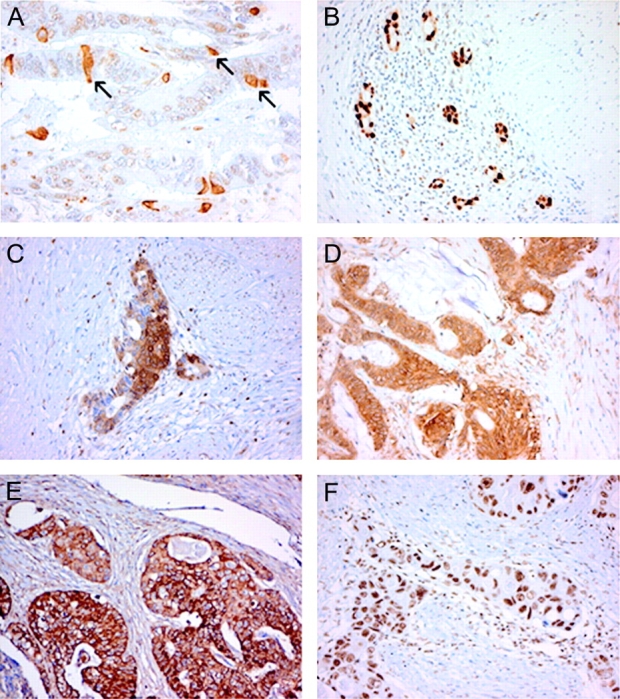
Examples of immunohistochemical staining patterns observed after staining with (A) M30 CytoDEATH, (B) p53, (C) Bcl-2, (D) Bax, (E) cyclooxygenase-2 and (F) mamma serine protease inhibitor. The arrows in panel (A) depict examples of apoptotic tumour cells that demonstrate intense staining with the M30 antibody. Original magnifications (B)–(F): 200×, (A): 400×.
Table 3.
Correlation between apoptosis and clinicopathological factors
| Factor | Category | Pretreatment apoptosis (median/mean) | P | Posttreatment apoptosis (median/mean) | P |
| cT stage | cT3 | 0/5.89 | 0.020a | 11.37/21.12 | 0.57a |
| cT4 | 3.65/11.71 | 9.78/17.95 | |||
| Neo-adjuvant therapy | RT: | 0/9.51 | 0.12b | 11.45/19.23 | 0.97b |
| RCT: interrupted | 2.47/9.43 | 9.68/20.11 | |||
| RCT: continuous | 6.03/2.47 | 10.71/18.08 | |||
| Interval between last day RT and surgery | P = 0.34c, cc = 0.084 | P = 0.001c, cc = 0.328 | |||
| ypT stage | ypT0, ypTis, ypT1, ypT2 | 0/8.40 | 0.93a | 9.28/21.39 | 0.75a |
| ypT3, ypT4 | 0/9.52 | 10.39/18.84 | |||
| ypN stage | N0 | 0/9.45 | 0.23b | 9.48/19.91 | 0.87b |
| N1 | 3.32/10.62 | 17.14/18.79 | |||
| N2 | 0/5.56 | 11.45/16.33 | |||
| CRM | Positive | 0/9.34 | 0.96 | 11.45/18.47 | 0.811 |
| Negative | 0/9.28 | 9.48/19.50 | |||
| Histologic regression | 0 | 0/8.94 | 0.041b | 12.40/22.11 | 0.003a |
| 1 | 5.42/10.80 | 3.9410.68 | |||
| 2 | 1.24/5.94 | n.a. | |||
Mann–Whitney's U test.
Kruskal–Wallis test.
Pearson correlation.
cT3, clinical T3 stage, cT4: clinical T4 stage; RT, radiotherapy; RCT, radiochemotherapy; cc, correlation coefficient; CRM, circumferential margin; n.a., not assessed.
Significant P values are printed bold.
Associations between apoptosis and the different clinicopathological factors can be found in Table 3. Tumours with a cT4 were found to have higher levels of intrinsic apoptosis than clinical T3 stage tumours (P = 0.020). In addition, M30 IHC revealed that a longer interval between last day of RT and surgery resulted in an increased number of apoptotic cells (P = 0.001, Pearson correlation coefficient = 0.328), indicating a large effect of this parameter on the posttreatment apoptosis. Apoptotic levels were found to be significantly higher in resection specimens with limited tumour regression (Rödel 0) than in specimens with 25% to >50% tumour regression (Rödel 0, P = 0.003). Intrinsic apoptosis was not predictive for histological tumour regression since comparable levels of apoptosis were observed in patients with a poor and complete response (no residual tumor cells) (Table 3).
p53, Bcl-2, Bax, Cox-2 and maspin; prognostic significance
Representative staining patterns after staining for p53, Bcl-2, Bax, Cox-2 and maspin are depicted in panel B, C, D, E and F, respectively, of Figure 1. As indicated in Table 1, IHC staining patterns for p53, Bcl-2, Bax, Cox-2 and maspin were assessed by two independent observers. In the case of the resection specimens, the calculated mean κ values of the three tumours containing punches were found to be 0.61, 0.79, 0.79, 0.71 and 0.78, respectively, for p53, Bcl-2, Bax, Cox-2 and maspin. For pretreatment biopsies, these values were, respectively, 0.71, 0.85, 0.85, 0.58 and 0.86. These κ values mainly indicate excellent degrees of agreement. Negative IHC controls showed no staining.
Staining patterns and intensities of p53, Bcl-2, Bax, Cox-2 and maspin that were assessed in the biopsies and resection specimens did not correlate with histological tumour regression. Pre- and posttherapy levels of p53, Bcl-2, Bax and Cox-2 did not influence LR or OS rates. Stratification for the different treatment regimens resulted in similar findings.
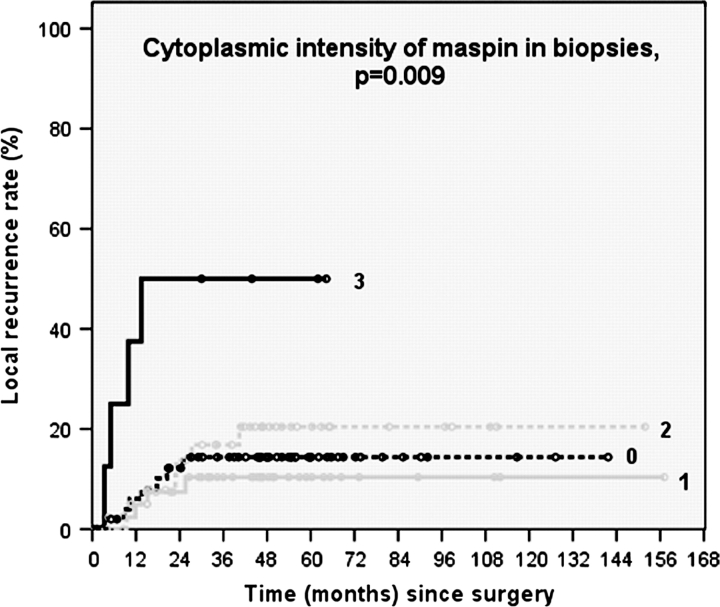
A strong cytoplasmic staining of maspin in the biopsy tissues was associated with a higher risk on developing a LR (Figure 2). This association, however, was not observed after separate analysis of each treatment regimen.
Figure 2.
Kaplan–Meier curves depicting the correlation between cytoplasmic staining patterns of maspin in the pre-therapy biopsies and local recurrence.
multiple variable analysis for prediction of patients' prognosis and therapy response
Investigation on the associations between apoptosis-regulating proteins and apoptotic levels showed that increased nuclear staining of maspin in tumour biopsies was associated with increased levels of apoptosis in biopsy specimens (P = 0.019). Increased Cox-2 staining intensities in the resection specimens were significantly associated with increased levels of apoptosis (P = 0.028). This, however, is not in consistency with the anti-apoptotic effect of Cox-2 that has been described in literature [8, 17].
Cox proportional hazards regression indicated that different combinations of apoptosis and the different apoptosis-regulating proteins were not predictive for LR or OS. In order to study whether combinations of apoptosis and the different apoptosis-regulating proteins are predictive for tumour regression, logistic regression was carried out and decision tree models were built. In the case of both logistic regression analysis and the CART analysis, histological tumour regression was dichotomised in two different ways: Rödel 0 and 1 versus Rödel 2 and Rödel 0 versus Rödel 1 and 2. This analysis revealed that none of the variables or variable combinations was predictive for tumour regression.
discussion
Pretherapy (intrinsic) or posttherapy (induced) levels of apoptosis, p53, Bcl-2, Bax and Cox-2 in tumour cells, were not found to be associated with LR or OS in patients with LARC. Maspin was found to be correlated to LR only (Figure 2). These data implicate that success of neo-adjuvant RCT is independent of these factors. In addition, we found that apoptosis of tumour cells was not predictive for the degree of tumour response in these patients.
de Bruin et al. [2] also studied the prognostic value of apoptosis in TMA with M30 IHC which is similar to our study design. In concordance with that study, we also found that the posttherapy apoptotic levels did not influence patients' prognosis. However, the study reported by Bruin et al. was a randomised trial in which the surgery-only arm acted as a control, while we did have access to pretreatment biopsies. Tannapfel et al. [22] investigated intrinsic- and therapy-induced levels of apoptosis in LARC patients who received RCT and also did not find a correlation between apoptosis and LR, OS or tumour regression after RCT which is consistent with our findings. Other studies, however, found that intrinsic levels of apoptosis were related to the degree of tumour regression and patients' outcome [23, 24] after long-term neo-adjuvant RCT. These differences in literature indicate that the prognostic implications of apoptosis in patients with rectal cancer are not established and need further research in order to determine the clinical usefulness (reviewed in Ref. [25]) Since RT is the main therapy used in the present population, it would also be interesting to study the clinical consequences of apoptosis in the setting of systemic treatment with CT.
Our finding that apoptotic levels were significantly increased in the surgical resection specimens is in consistency with other reports [22, 26]. On the basis of the apoptosis-inducing potential of RT and CT, this was not an unexpected finding. Analysis of apoptotic levels in normal epithelial cells yielded the surprising result that the number of apoptotic cells significantly decreased in case of the continuous RCT schedule in comparison to the interrupted RCT schedule. This might be explained by the use of capecitabine in the continuous schedule which, is in contrast to 5-fluorouracil (5-FU), converted into the active metabolite in cells with a higher metabolism delivering the 5-FU predominately to the tumour cells [27, 28]. Another, interesting finding was that when the interval between the last day of irradiation and surgery was longer, the posttherapy apoptotic levels increased. It is tempting to speculate that this observation could partially explain the positive effect of a prolonged interval between RCT and surgery as indicated by a French trial [29]. Several studies have shown that long course RT with concurrent 5-FU-based CT contributed to tumour downstaging and increased local control [30, 31]. However, the optimal doses of RT and CT of this multimodality therapy and the type of 5-FU administration and combination with other cytotoxic agents can still be improved.
Our study design has several limitations. First, investigations on posttherapy resection specimens have the general limitation that no tumour can be assessed after a complete response, especially after neo-adjuvant RCT. An added problem of TMA evaluation is that after a high degree of tumour regression, tumour sampling can be problematic. This can obscure the evaluation of the IHC staining patterns in the posttherapy specimens. Secondly, the complex interactions between the different apoptosis-regulating proteins interfere with the analysis of each individual marker. We, however, tried to overcome this problem by adding a multiple variable statistical approach. Numerous reports studying the predictive and prognostic role of apoptosis controlling proteins can be found. The clinical implications of these markers, however, remain elusive due to conflicting data. Our data show that the regulating proteins did not have prognostic implications and, more importantly, the downstream effect of these markers (i.e. apoptosis of tumour cells) was also clinically irrelevant. This indicates that the discussion about the individual contributions of the regulation proteins is less relevant.
Finally, evaluation of apoptosis with the M30 CytoDEATH antibody detects only apoptotic events in tumour cells or normal epithelial cells [32]; caspase-independent apoptosis, mitotic catastrophe and apoptosis of stroma cells are not detected. Other reports, for example, used the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphatase biotin nick-end labelling method [12, 33, 34] or measured caspase activity [35, 36]. Evaluation of caspase-3 activity, measuring caspase-dependent apoptosis in tumour and stroma cells, in patients with rectal cancer indicated that low caspase activity in biopsies decreased the risk on LR [35]. These findings could indicate that apoptosis in stroma cells could be of substantial importance as previously described by others [37]. Fresh frozen tissues, however, are required for this assays which were unfortunately not available in the present patient population.
In the present study, we investigated the effect of apoptosis and five apoptosis-regulating proteins on prognosis and tumour regression in patients with LARC.
We studied the effect of apoptosis in patients with LARC by quantifying M30-positive tumour cells and semiquantitatively analysing p53, Bcl-2, Bax, Cox-2 and maspin. Subsequently, we analyzed these data in both pre- and posttherapy tumour and normal tissues in a multiple variable fashion in a well-characterised population of 201 patients with LARC. The data show that apoptosis of tumour cells does not predict local control or tumour regression in LARC patients. This indicates that, in contrary to short-term RT, the success of long-term RCT does not depend on this parameter since RCT is also effective in tumours with high intrinsic levels of apoptosis. This could suggest that the multimodality RCT regimen can adequately manage heterogeneity with respect to intrinsic levels of apoptosis.
References
- 1.Marijnen CA, van de Velde CJ, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005;23:1847–1858. doi: 10.1200/JCO.2005.05.256. [DOI] [PubMed] [Google Scholar]
- 2.de Bruin EC, van de Velde CJ, van de PS, et al. Prognostic value of apoptosis in rectal cancer patients of the dutch total mesorectal excision trial: radiotherapy is redundant in intrinsically high-apoptotic tumors. Clin Cancer Res. 2006;12:6432–6436. doi: 10.1158/1078-0432.CCR-06-0231. [DOI] [PubMed] [Google Scholar]
- 3.Hollstein M, Sidransky D, Vogelstein B, et al. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 4.Merritt AJ, Potten CS, Kemp CJ, et al. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994;54:614–617. [PubMed] [Google Scholar]
- 5.Lowe SW, Schmitt EM, Smith SW, et al. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 6.Scopa CD, Vagianos C, Kardamakis D, et al. bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with rectal cancer. Appl Immunohistochem Mol Morphol. 2001;9:329–334. doi: 10.1097/00129039-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 8.Milas L, Hanson WR. Eicosanoids and radiation. Eur J Cancer. 1995;31A:1580–1585. doi: 10.1016/0959-8049(95)00327-f. [DOI] [PubMed] [Google Scholar]
- 9.Shao ZM, Nguyen M, Alpaugh ML, et al. The human myoepithelial cell exerts antiproliferative effects on breast carcinoma cells characterized by p21WAF1/CIP1 induction, G2/M arrest, and apoptosis. Exp Cell Res. 1998;241:394–403. doi: 10.1006/excr.1998.4066. [DOI] [PubMed] [Google Scholar]
- 10.Gosens MJ, Klaassen RA, Tan-Go I, et al. Circumferential margin involvement is the crucial prognostic factor after multimodality treatment in patients with locally advanced rectal carcinoma. Clin Cancer Res. 2007;13:6617–6623. doi: 10.1158/1078-0432.CCR-07-1197. [DOI] [PubMed] [Google Scholar]
- 11.Mannaerts GH, Martijn H, Crommelin MA, et al. Feasibility and first results of multimodality treatment, combining EBRT, extensive surgery, and IOERT in locally advanced primary rectal cancer. Int J Radiat Oncol Biol Phys. 2000;47:425–433. doi: 10.1016/s0360-3016(99)00492-7. [DOI] [PubMed] [Google Scholar]
- 12.Rodel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 13.Marijnen CA, Nagtegaal ID, Mulder-Stapel AA, et al. High intrinsic apoptosis, but not radiation-induced apoptosis, predicts better survival in rectal carcinoma patients. Int J Radiat Oncol Biol Phys. 2003;57:434–443. doi: 10.1016/s0360-3016(03)00580-7. [DOI] [PubMed] [Google Scholar]
- 14.Fernebro E, Dictor M, Bendahl PO, et al. Evaluation of the tissue microarray technique for immunohistochemical analysis in rectal cancer. Arch Pathol Lab Med. 2002;126:702–705. doi: 10.5858/2002-126-0702-EOTTMT. [DOI] [PubMed] [Google Scholar]
- 15.Rubin MA, Dunn R, Strawderman M, et al. Tissue microarray sampling strategy for prostate cancer biomarker analysis. Am J Surg Pathol. 2002;26:312–319. doi: 10.1097/00000478-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Su Y, Shrubsole MJ, Ness RM, et al. Immunohistochemical expressions of Ki-67, cyclin D1, beta-catenin, cyclooxygenase-2, and epidermal growth factor receptor in human colorectal adenoma: a validation study of tissue microarrays. Cancer Epidemiol Biomarkers Prev. 2006;15:1719–1726. doi: 10.1158/1055-9965.EPI-05-0946. [DOI] [PubMed] [Google Scholar]
- 17.de Heer P, Gosens MJ, de Bruin EC, et al. Cyclooxygenase 2 expression in rectal cancer is of prognostic significance in patients receiving preoperative radiotherapy. Clin Cancer Res. 2007;13:2955–2960. doi: 10.1158/1078-0432.CCR-06-2042. [DOI] [PubMed] [Google Scholar]
- 18.Buskens CJ, Van Rees BP, Sivula A, et al. Prognostic significance of elevated cyclooxygenase-2 expression in patients with adenocarcinoma of the esophagus. Gastroenterology. 2002;122:1800–1807. doi: 10.1053/gast.2002.33580. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 20.Cohen J. Weighted kappa: nominal scale agreement with provision for scale disagreement or partial credit. Psychol Bull. 1968;70:213–230. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 21.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363–374. [PubMed] [Google Scholar]
- 22.Tannapfel A, Nusslein S, Fietkau R, et al. Apoptosis, proliferation, bax, bcl-2 and p53 status prior to and after preoperative radiochemotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 1998;41:585–591. doi: 10.1016/s0360-3016(98)00076-5. [DOI] [PubMed] [Google Scholar]
- 23.Rodel C, Grabenbauer GG, Papadopoulos T, et al. Apoptosis as a cellular predictor for histopathologic response to neoadjuvant radiochemotherapy in patients with rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52:294–303. doi: 10.1016/s0360-3016(01)02643-8. [DOI] [PubMed] [Google Scholar]
- 24.Rodel F, Hoffmann J, Grabenbauer GG, et al. High survivin expression is associated with reduced apoptosis in rectal cancer and may predict disease-free survival after preoperative radiochemotherapy and surgical resection. Strahlenther Onkol. 2002;178:426–435. doi: 10.1007/s00066-002-1003-y. [DOI] [PubMed] [Google Scholar]
- 25.Smith FM, Reynolds JV, Miller N, et al. Pathological and molecular predictors of the response of rectal cancer to neoadjuvant radiochemotherapy. Eur J Surg Oncol. 2006;32:55–64. doi: 10.1016/j.ejso.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Tannapfel A, Nusslein S, Katalinic A, et al. Proliferation and apoptosis before and after preoperative simultaneous radiochemotherapy of rectal carcinomas. Strahlenther Onkol. 1998;174:295–299. doi: 10.1007/BF03038542. [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa T, Utoh M, Sawada N, et al. Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol. 1998;55:1091–1097. doi: 10.1016/s0006-2952(97)00682-5. [DOI] [PubMed] [Google Scholar]
- 28.Miwa M, Ura M, Nishida M, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34:1274–1281. doi: 10.1016/s0959-8049(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 29.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 30.Minsky BD, Cohen AM, Kemeny N, et al. Enhancement of radiation-induced downstaging of rectal cancer by fluorouracil and high-dose leucovorin chemotherapy. J Clin Oncol. 1992;10:79–84. doi: 10.1200/JCO.1992.10.1.79. [DOI] [PubMed] [Google Scholar]
- 31.Rodel C, Grabenbauer GG, Schick C, et al. Preoperative radiation with concurrent 5-fluorouracil for locally advanced T4-primary rectal cancer. Strahlenther Onkol. 2000;176:161–167. doi: 10.1007/s000660050051. [DOI] [PubMed] [Google Scholar]
- 32.Koornstra JJ, Rijcken FE, De Jong S, et al. Assessment of apoptosis by M30 immunoreactivity and the correlation with morphological criteria in normal colorectal mucosa, adenomas and carcinomas. Histopathology. 2004;44:9–17. doi: 10.1111/j.1365-2559.2004.01739.x. [DOI] [PubMed] [Google Scholar]
- 33.Schwandner O, Schiedeck TH, Bruch HP, et al. Apoptosis in rectal cancer: prognostic significance in comparison with clinical histopathologic, and immunohistochemical variables. Dis Colon Rectum. 2000;43:1227–1236. doi: 10.1007/BF02237426. [DOI] [PubMed] [Google Scholar]
- 34.Adell GC, Zhang H, Evertsson S, et al. Apoptosis in rectal carcinoma: prognosis and recurrence after preoperative radiotherapy. Cancer. 2001;91:1870–1875. doi: 10.1002/1097-0142(20010515)91:10<1870::aid-cncr1208>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 35.de Heer P, de Bruin EC, Klein-Kranenbarg E, et al. Caspase-3 activity predicts local recurrence in rectal cancer. Clin Cancer Res. 2007;13:5810–5815. doi: 10.1158/1078-0432.CCR-07-0343. [DOI] [PubMed] [Google Scholar]
- 36.de Oca J, Azuara D, Sanchez-Santos R, et al. Caspase-3 activity, response to chemotherapy and clinical outcome in patients with colon cancer. Int J Colorectal Dis. 2008;23:21–27. doi: 10.1007/s00384-007-0362-3. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]