Abstract
Background: Adding oral clodronate to postoperative adjuvant breast cancer therapy significantly improves disease-free survival (DFS) and overall survival (OS). Long-term follow-up data from the prospective, randomized, controlled study are reported.
Patients and methods: Patients with primary breast cancer received clodronate 1600 mg/day for 2 years or no treatment along with standard adjuvant breast cancer treatment.
Results: Analysis of 290 of 302 patients demonstrated that a significant improvement in OS was maintained in the clodronate group at a median follow-up of 103 ± 12 months; 20.4% of patients in the clodronate group versus 40.7% of control group patients (P = 0.04) died during the 8.5 years following primary surgical therapy. Significant reductions in the incidence of bony and visceral metastases and improvement in duration of DFS at 36- and 55-month follow-up periods were no longer seen with clodronate.
Conclusion: These long-term survival data extend the survival advantage reported in previous studies with oral clodronate in breast cancer.
Keywords: adjuvant therapy, bisphosphonate, breast neoplasm, clodronate
introduction
Breast cancer is the second most common cancer in the world, affecting one in eight women (∼400 000 overall) in the western world [1, 2]. In these patients, survival rates are directly correlated with the stage of breast cancer. For example, 5-year survival rates are much higher in patients with localized (stages I and II) disease (97%) than in patients with regional (stage III, 79%) or metastasized disease (stage IV, 23%) [1]. Approximately 70% of patients with progressive disease will eventually develop bone metastases, with bone seen as the initial site of metastasis in 30% of patients [3, 4]. Thus, the prevention of bone metastases has the potential to decrease tumor burden and ultimately improve survival in patients with breast cancer.
Because of their beneficial effects on bone turnover, bisphosphonates have been evaluated for the treatment and prevention of bone metastases in women with breast cancer [5]. Clodronate is an oral bisphosphonate that accumulates on bone surfaces after oral or i.v. administration, where it inhibits osteoclast activity, induces direct apoptotic effects on cancer cells, and inhibits tumor adhesion to bone. Together, these effects reduce the development of new bone metastases and inhibit the progression of existing lesions while preserving bone structure and metabolism [6, 7].
Notably, clodronate is the first and only oral bisphosphonate shown to significantly improve overall survival (OS) and reduce the occurrence of bone metastases when used as an adjunctive therapy in women with primary (stages I–III) breast cancer [8]. In a large-scale, randomized, placebo-controlled trial of 1069 patients with stages I–III breast cancer who received clodronate 1600 mg or placebo daily for 2 years, adjuvant clodronate treatment reduced the risk of death by 23% at 5 years (P = 0.048) and reduced the risk of bone metastases by 31% over the same time period (P = 0.043). These differences were more pronounced in the higher-risk subgroup of stages II and III patients: the mortality risk was reduced by 26% (P = 0.041), while the risk of bone metastasis was reduced by 41% (P = 0.009).
Like Powles and colleagues, we have previously reported that postoperative treatment with oral clodronate (Ostac®, Boehringer Mannheim, Mannheim, Germany/Roche Pharma, Basel, Switzerland) in conjunction with adjuvant breast cancer therapy significantly improves disease-free survival (DFS) and OS compared with that seen in patients receiving standard systemic adjuvant breast cancer therapy alone [9, 10]. Here, we report the long-term follow-up data from the same prospective, randomized, controlled study, now with a median of 8.5 years of follow-up.
patients and methods
patients
This study population has been described previously. Briefly, all patients (N = 302) had primary breast cancer classified as stage T1, T2, T3, or T4 and histologically classified as N0, N1 or N2 (i.e. tumor size ranged from <2 to >5 cm, with or without ipsilateral lymph node involvement). All patients had immunocytochemical evidence of at least one tumor cell per million cells in the bone marrow. Patients with confirmed distant metastasis, prior or simultaneous secondary malignant disease, skeletal disease, hepatic or renal dysfunction, pregnancy, or a history of neo-adjuvant chemotherapy or hormonal therapy were excluded from the study. In accordance with the Declarations of Helsinki, all patients provided written informed consent before study participation.
study design
This prospective, randomized, controlled study recruited patients at the University Hospital Heidelberg from 1990 to 1995. Primary surgical breast cancer treatment consisted of either mastectomy or breast-conserving surgery (lumpectomy or segmental resection plus 50 Gy of radiotherapy to the breast), and all patients underwent iliac crest bone marrow aspiration for immunocytochemical assessment of the presence of tumor cells within the bone marrow.
Patients were randomly assigned to receive either clodronate 1600 mg/day for 2 years (treatment group) or standard follow-up (control group). All patients in both groups received standard surgical treatment and customary adjuvant endocrine therapy or chemotherapy ± radiotherapy. Patients who developed confirmed metastases during the study received endocrine therapy, with chemotherapy in the event of rapid progression or extensive metastasis. If patients developed bone metastases during the study, clodronate therapy was continued in those patients in the clodronate group or was initiated in those patients in the control group. Osteolytic lesions were irradiated in the event of bone pain or the risk of pathologic fracture, while patients with hypercalcemia were treated with a 2-h i.v. infusion of clodronate 1500 mg.
Follow-up examinations were carried out every 3–4 months during the 2-year treatment period. At each visit, history and physical examination were carried out. Chest radiographs, bone scans, ultrasound examination of the liver, and mammography were carried out annually. If evidence of bone metastasis was present, additional radiographs were taken of the affected areas and the pattern of metastasis was analyzed by two independent radiologists at the end of the study. Although skeletal complications were recorded as events, they were not included in the statistical analysis plan.
study end points
The primary study end points included the incidence and number of new bone and visceral metastases, as well as the length of time to their appearance and OS. These end points have previously been analyzed at a median of 36 and 55 months of follow-up after primary surgical therapy. The current intend to treat analysis represents a median of 103 months (8.5 years) of follow-up.
statistical methods
The initial statistical projection was that, after 36 months of follow-up, a difference of 10% would be seen between treatment groups in the rate of bone metastasis. This assumption was on the basis of earlier studies of tumor cell detection. The planned sample size was 300 patients, and the data were last updated in February 2004.
The chi-square test was used to assess between-group differences in baseline characteristics and prognostic factors. Kaplan–Meier analyses were used to assess the differences in metastasis-free survival and OS. All P values are two sided. The statistical analyses were carried out using SAS software (SAS Institute Inc, Cary, NC) and Systat software (Systat, Evanston, IL).
results
A total of 302 patients were enrolled and randomly assigned to treatment with oral clodronate (n = 157) or to the control group (n = 145). The median patient age was 51 years (range 24–78 years), and the treatment groups were well balanced with respect to baseline disease characteristics and prognostic factors (Table 1). In both the oral clodronate and control groups, most patients were estrogen receptor positive (75% and 71%, respectively), progesterone receptor positive (62% and 63%, respectively), postmenopausal (63% and 61%, respectively), and had a tumor stage of T1 or T2 (83% for both groups). Of the 302 patients enrolled in the study, 246 (81%) received adjuvant systemic treatment with chemotherapy, hormonal therapy, or both. As shown in Table 2, there were no significant differences between treatment groups in the proportion of patients receiving adjuvant therapy [9]. Approximately one-third of patients in both groups received tamoxifen, which was the most common adjuvant systemic therapy in each group.
Table 1.
Baseline clinical characteristics
| Characteristic | Patients, n (%) |
|
| Oral clodronate (n = 157) | Control (n = 145) | |
| Tumor stage | ||
| T1 | 59 (38) | 54 (37) |
| T2 | 71 (45) | 67 (46) |
| T3–4 | 27 (17) | 24 (16) |
| Histologic grades I and II | 93 (68) | 92 (73) |
| Node-positive disease | 80 (51) | 79 (54) |
| Postmenopausal | 101 (64) | 88 (61) |
| Hormone receptor status | ||
| ER positive | 104 (75) | 84 (71) |
| PR positive | 85 (62) | 72 (63) |
| S phase <5% | 59 (50) | 52 (51) |
ER, estrogen receptor; PR, progesterone receptor.
Table 2.
Adjuvant systemic therapy
| Therapy | Patients, n (%) |
|
| Oral clodronate (n = 157) | Control (n = 145) | |
| CMF (standard) | 31 (20) | 32 (22) |
| EC or FEC | 8 (5) | 9 (6) |
| Goserelin | 16 (10) | 11 (8) |
| Tamoxifen | 49 (31) | 43 (30) |
| Combination therapya | 25 (16) | 22 (15) |
| No treatment | 28 (18) | 28 (19) |
Combination therapy consisted of tamoxifen plus standard CMF.
CMF, cyclophosphamide, methotrexate, fluorouracil; EC, epirubicin, cyclophosphamide; FEC, fluorouracil, epirubicin, cyclophosphamide.
In earlier analyses of the current study population, patients treated with clodronate 1600 mg/day for 2 years demonstrated significantly better outcomes than those in the control group [9, 10]. At the 36- and 55-month follow-up, the incidence of bone metastases was significantly reduced with clodronate treatment (P = 0.003 and P = 0.044, respectively; Table 3). Moreover, patients treated with clodronate had significantly increased DFS and OS compared with controls (P < 0.001) [9, 10].
Table 3.
Incidence of metastatic disease and death
| Outcome | Patients, n (%) |
||
| Oral clodronate (n = 157) | Control (n = 145) | P value | |
| Median 36-month follow-up [9] | |||
| Distant metastases | 21 (13.4) | 42 (29.0) | <0.001 |
| Bone metastases | 12 (7.6) | 25 (17.2) | 0.003 |
| Visceral metastases | 13 (8.3) | 27 (18.6) | 0.003 |
| Deaths | 6 (3.8) | 22 (15.2) | 0.001 |
| Median 55-month follow-up [10] | |||
| Distant metastases | 32 (20.4) | 51 (35.2) | 0.022 |
| Bone metastases | 20 (12.7) | 34 (23.4) | 0.044 |
| Visceral metastases | 24 (15.3) | 37 (25.5) | 0.091 |
| Deaths | 13 (8.3) | 32 (22.1) | 0.002 |
| Median 103-month (8.5-year) follow-up | |||
| Distant metastases | 61 (38.9) | 57 (39.3) | 0.816 |
| Bone metastases | 37 (23.6) | 38 (26.2) | 0.770 |
| Visceral metastases | 33 (21.0) | 32 (22.1) | 0.222 |
| Deaths | 32 (20.4) | 59 (40.7) | 0.049 |
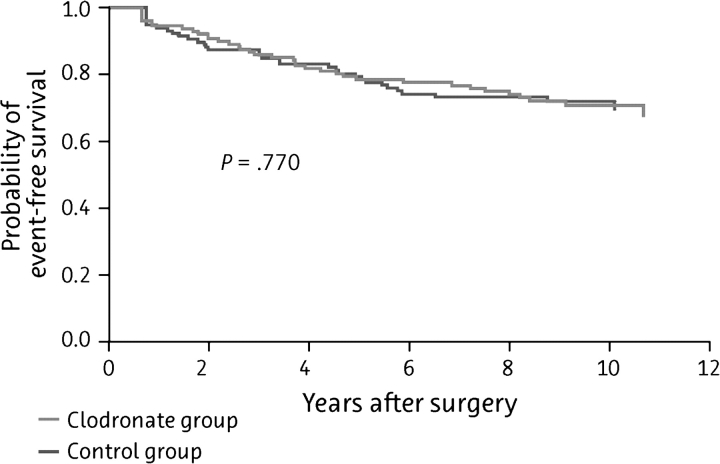
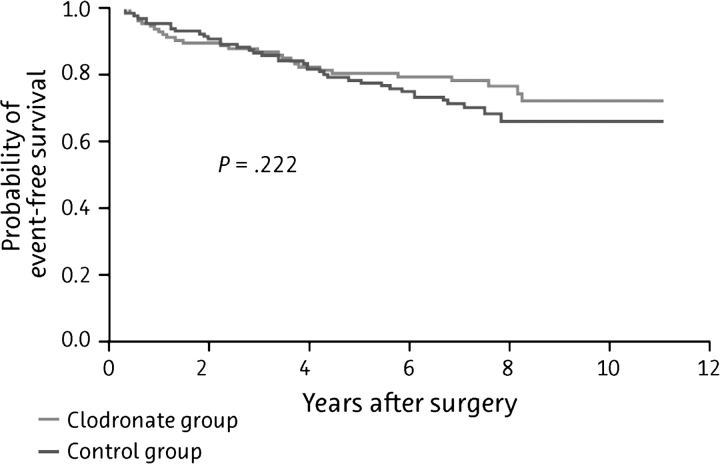
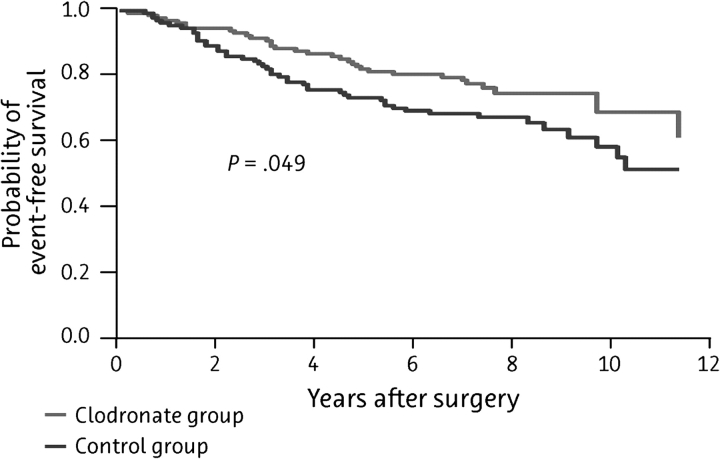
The current analysis includes a total of 290 of the original 302 patients enrolled in the study, with a median follow-up time of 103 ± 12 months; patient outcomes (distant metastases, bone metastases, visceral metastases, and deaths) are summarized in Table 3. Although the differences in the incidence of bony and visceral metastases and the duration of DFS were no longer significant by this time (Figure 1 and Figure 2, respectively), the significant improvement in OS was maintained in the clodronate group, with death occurring in 20.4% of clodronate-treated patients and 40.7% of controls (P = 0.049; Figure 3).
Figure 1.
Kaplan–Meier curve of bone metastasis-free survival among patients treated with oral clodronate compared with standard follow-up therapy (N = 209).
Figure 2.
Kaplan–Meier curve of visceral metastasis-free survival among patients treated with oral clodronate compared with standard follow-up therapy (N = 209).
Figure 3.
Kaplan–Meier curve of overall survival among patients treated with oral clodronate compared with standard follow-up therapy (N = 209).
discussion
In this bone metastasis prevention study of oral clodronate 1600 mg/day as adjuvant therapy in patients with primary breast cancer, a significant reduction in mortality was demonstrated at a median long-term follow-up of 109 months. The incidence of bone and visceral metastases was significantly lower in patients receiving clodronate than in the control group at earlier follow-up time points (P = 0.003 for both); however, this difference was not seen at the median 109 month follow-up.
Similar results were seen in a larger, randomized, placebo-controlled study, although inclusion criteria were slightly different in the two studies [8, 11]. The study by Powles and colleagues, in which patients with stages I–III breast cancer received oral clodronate or placebo for 2 years, reported a better OS rate in the treatment group over the 5-year study period. Similar to treatment with endocrine therapy, a longer treatment period may be necessary to achieve more favorable results; however, further study is warranted to test this hypothesis. The study by Powles and colleagues did not require patients to have evidence of tumor cells in the bone marrow; however, it demonstrated that patients with a high risk of recurrence experience the most benefit from adjuvant bisphosphonate therapy. Their study showed that with the exclusion of low-risk patients with stage I disease, patients with stages II and III disease who received oral clodronate showed significantly improved bone metastasis-free survival and OS at 2 and 5 years [8, 11]. In contrast, the present study included only patients with immunohistochemical confirmation of at least one tumor cell in the bone marrow and the presence of which is a confirmed risk factor for distant metastases.[10, 12–14]. The increased mortality rate in the control group compared with the oral clodronate group (40% versus 20%) suggests that prophylactic treatment with oral clodronate leads to risk reduction in patients with high-risk breast cancer in an unselected group of patients with breast cancer [15].
In contrast, another randomized study of oral clodronate in patients with primary node-positive breast cancer (n = 299) demonstrated increased bone metastases and decreased OS in the oral clodronate group compared with the control group [16]. In a 10-year follow-up study, Saarto et al. [17] reported no difference in the incidence of bone metastases between the two groups, but reported a higher number of visceral metastases and deaths in the oral clodronate group. The negative effect of oral clodronate reported in those studies may be attributed to a significant imbalance in the hormone receptor status among patients in the two treatment groups. More patients who received oral clodronate were hormone receptor negative compared with the control group (25 patients versus 10 patients; P = 0.03); thus, those patients did not receive the standard of care. In addition, protocol violations led to the exclusion of an additional 15 patients (5%) with distant metastases who were incorrectly classified as being metastases free. Therefore, the number of assessable patients was 282. It should be noted that the study by Saarto et al. [16, 17] is the only report of an elevated death rate in a placebo-controlled trial of bisphosphonates in patients with metastatic breast cancer, including those studies with long-term use.
The efficacy of bisphosphonates in the prophylaxis of bone metastases can be explained by two hypotheses: first, bisphosphonates normalize bone metabolism and inhibit the production of locally released growth factors from the skeleton, thus reducing the growth stimulus for tumor cells [18]. Secondly, perhaps more importantly, bisphosphonates have a direct apoptotic and antiadhesive effect on tumor cells [19], which was first described with oral clodronate [6, 7, 20–22], and has recently been described for other bisphosphonates [23].
Because bisphosphonates accumulate on the surface of bone tissue, it seems reasonable to assume that their prophylactic effects would be limited to bone metastases. However, evidence from the current study has shown that visceral metastases are also reduced in patients receiving clodronate, suggesting that the prevention of bone metastases may have protective effects elsewhere in the body, perhaps by eliminating the origin of a secondary metastasis. Further investigation of this phenomenon is warranted.
Oral clodronate is the first and only oral bisphosphonate reported to significantly reduce the occurrence of bone metastases and significantly prolong survival in women with primary breast cancer [8, 9, 24], although the optimal duration of treatment remains to be defined. Although the studies conducted to date have utilized a 2-year treatment period, it is possible that a longer duration of treatment would provide greater and more sustained benefits in terms of the prevention of later metastases and ultimately survival. Because oral clodronate is safe and well tolerated, with a low incidence of serious side-effects that have been observed in studies of aminobisphosphonates (i.e. renal insufficiency and osteonecrosis of the jaw), a longer treatment period should not be problematic and would have the added benefit of protecting patients against cancer treatment-induced bone loss.
Numerous studies of adjuvant bisphosphonate treatment in patients with breast cancer are recruiting patients or have completed patient recruitment and await data analysis. A study conducted by the National Surgical Adjuvant Breast and Bowel Project (NSABP B34) has recruited 3200 North American patients with primary stages I and II breast cancer to compare the efficacy of oral clodronate with or without chemotherapy and/or hormone therapy in the prevention of bone metastases. A Southwest Oncology Group study (SWOG SO 307) is currently recruiting patients (expected accrual of 6000 patients) to compare the efficacy of oral clodronate, ibandronate, and zoledronic acid in preventing bone metastases in patients who have undergone surgery for stages I–III breast cancer. The AZURE trial, which has recruited 3356 patients, was designed to determine whether adjuvant treatment with zoledronic acid plus (neo)adjuvant chemotherapy and/or (neo)adjuvant endocrine therapy is superior to (neo)adjuvant chemotherapy and/or (neo)adjuvant endocrine therapy alone in improving the DFS and bone metastasis-free survival of patients with stages II and III breast cancer. Furthermore, the longer intervals of treatment in the AZURE trial may indicate whether a normalization of bone metabolism leads to a reduction of bone metastases and whether an i.v. application is as effective as an oral one.
funding
Boehringer Mannheim (Roche Pharma).
References
- 1.American Cancer Society. Breast Cancer Facts and Figures, 2003–2004. American Cancer Society Inc.; 2003. . American Cancer Society, Atlanta, GA, USA. [Google Scholar]
- 2.Ferlay J, Bray P, Pisani P, Parkin DM. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. IARC CancerBase No. 5. Version 2.0. IARC Press; 2004. [Google Scholar]
- 3.Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCloskey EV, Guest JF, Kanis JA. The clinical and cost considerations of bisphosphonates in preventing bone complications in patients with metastatic breast cancer or multiple myeloma. Drugs. 2001;61:1253–1274. doi: 10.2165/00003495-200161090-00003. [DOI] [PubMed] [Google Scholar]
- 5.Paterson AH. The role of adjuvant therapy with bisphosphonates in cancer. Am J Cancer. 2004;3:25–39. [Google Scholar]
- 6.Mundy G. Preclinical models of bone metastases. Semin Oncol. 2001;28:2–8. doi: 10.1016/s0093-7754(01)90225-8. [DOI] [PubMed] [Google Scholar]
- 7.Yoneda T, Michigami T, Yi B, et al. Actions of bisphosphonate on bone metastasis in animal models of breast carcinoma. Cancer. 2000;88:2979–2988. doi: 10.1002/1097-0142(20000615)88:12+<2979::aid-cncr13>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Powles TJ, Paterson AHG, Kanis JA, et al. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol. 2002;20:3219–3224. doi: 10.1200/JCO.2002.11.080. [DOI] [PubMed] [Google Scholar]
- 9.Diel IJ, Solomayer EF, Costa SD, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339:357–363. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 10.Diel IJ. Bisphosphonates in the prevention of bone metastases: current evidence. Semin Oncol. 2001;28:75–80. doi: 10.1016/s0093-7754(01)90237-4. [DOI] [PubMed] [Google Scholar]
- 11.Powles T, Paterson A, McCloskey E, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026] Breast Cancer Res. 2006;8:R13. doi: 10.1186/bcr1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diel IJ, Kaufmann M, Goerner R, et al. Detection of tumor cells in bone marrow of patients with primary breast cancer: a prognostic factor for distant metastasis. J Clin Oncol. 1992;10:1534–1539. doi: 10.1200/JCO.1992.10.10.1534. [DOI] [PubMed] [Google Scholar]
- 13.Diel IJ, Kaufmann M, Costa SD, et al. Micrometastatic breast cancer cells in bone marrow at primary surgery: prognostic value in comparison with nodal status. J Natl Cancer Inst. 1996;88:1652–1658. doi: 10.1093/jnci/88.22.1652. [DOI] [PubMed] [Google Scholar]
- 14.Janni W, Rack B, Sommer H, et al. Intra-mammary tumor location does not influence prognosis but influences the prevalence of axillary lymph-node metastases. J Cancer Res Clin Oncol. 2003;129:503–510. doi: 10.1007/s00432-003-0465-3. [DOI] [PubMed] [Google Scholar]
- 15.American Cancer Society. Breast Cancer Facts and Figures, 2005–2006. Atlanta, GA: American Cancer Society Inc.; 2005. [Google Scholar]
- 16.Saarto T, Blomqvist C, Virkkunen P, Elomaa I. Adjuvant clodronate treatment does not reduce the frequency of skeletal metastases in node-positive breast cancer patients: 5-year results of a randomized controlled trial. J Clin Oncol. 2001;19:10–17. doi: 10.1200/JCO.2001.19.1.10. [DOI] [PubMed] [Google Scholar]
- 17.Saarto T, Vehmanen L, Virkkunen P, Blomqvist C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 2004;43:650–656. doi: 10.1080/02841860410032885. [DOI] [PubMed] [Google Scholar]
- 18.Mundy GR. Realising the potential of anti-host cell therapy. Cancer Today. 2004;(Suppl 1):6–8. [Google Scholar]
- 19.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 20.Boissier S, Ferreras M, Peyruchaud O, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000;60:2949–2954. [PubMed] [Google Scholar]
- 21.Fromigue O, Lagneaux L, Body JJ. Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res. 2000;15:2211–2221. doi: 10.1359/jbmr.2000.15.11.2211. [DOI] [PubMed] [Google Scholar]
- 22.Fromigue O, Kheddoumi N, Body JJ. Bisphosphonates antagonise bone growth factors’ effects on human breast cancer cells survival. Br J Cancer. 2003;89:178–184. doi: 10.1038/sj.bjc.6601009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rack BK, Janni W, Schindlbeck C, et al. Effect of zoledronate on persisting isolated tumor cells (ITC) in the bone marrow (BM) of patients without recurrence of early breast cancer. Proc Am Soc Clin Oncol. 2004;23 (Abstr 9515) [Google Scholar]
- 24.Kanis JA, Powles T, Paterson AH, et al. Clodronate decreases the frequency of skeletal metastases in women with breast cancer. Bone. 1996;19:663–667. doi: 10.1016/s8756-3282(96)00285-2. [DOI] [PubMed] [Google Scholar]