Abstract
Background
Risk-taking behavior is a major cause of morbidity and mortality in adolescence. In the context of decision theory and motivated (goal-directed) behavior, risk-taking reflects a pattern of decision-making that favors the selection of courses of action with uncertain and possibly harmful consequences. We present a triadic, neuroscience systems-based model of adolescent decision-making.
Method
We review the functional role and neurodevelopmental findings of three key structures in the control of motivated behavior, i.e. amygdala, nucleus accumbens, and medial/ventral prefrontal cortex. We adopt a cognitive neuroscience approach to motivated behavior that uses a temporal fragmentation of a generic motivated action. Predictions about the relative contributions of the triadic nodes to the three stages of a motivated action during adolescence are proposed.
Results
The propensity during adolescence for reward/novelty seeking in the face of uncertainty or potential harm might be explained by a strong reward system (nucleus accumbens), a weak harm-avoidant system (amygdala), and/or an inefficient supervisory system (medial/ventral prefrontal cortex). Perturbations in these systems may contribute to the expression of psychopathology, illustrated here with depression and anxiety.
Conclusions
A triadic model, integrated in a temporally organized map of motivated behavior, can provide a helpful framework that suggests specific hypotheses of neural bases of typical and atypical adolescent behavior.
INTRODUCTION
Adolescence is the transition period from childhood to adulthood, a ‘rite of passage’, through which adolescents acquire the physical and psychological tools to assume the roles and responsibilities of adults (Dahl, 2004). Independence, the foremost goal of this developmental period, is achieved through separation, and individuation. A wealth of work, most notably by Erik Erikson, summarizes psychological transitions that typify this period (Erikson, 1950, 1968). The advent of cognitive neuroscience and functional neuroimaging has brought unprecedented new opportunities to study the neurobiology of these processes. Here, we focus on motivated action (i.e. goal-directed action), which embodies drastic changes that take place throughout adolescence.
This review is divided into four sections. First, we define adolescence from a behavioral perspective. Second, we propose a triadic model underlying the neural substrates of adolescent motivated behavior. Third, we describe a cognitive neuroscience approach to the study of motivated behavior, and we integrate the triadic model with this approach. Fourth, we demonstrate the relevance of this work to psychopathology. We conclude by offering future directions.
DEFINITION OF ADOLESCENCE
Adolescence is defined as the developmental period during which physical (e.g. growth spurt, change in body mass, sexual maturation), psychological (e.g. affective intensity and lability, romantic and idealistic aspirations, sense of invulnerability, abstract thinking), and social (e.g. distancing from adults and children, primacy of peer relationships, romantic involvement) milestones are being reached. The two most conspicuous changes are physical growth and sexual maturation, which define ‘puberty’.
Whereas puberty is part of adolescence, it does not encompass all the changes marking this period. Pubertal changes depend on developmental alterations in the function of the hypothalamo-pituitary-gonadal (HPG) axis (Romeo, 2003; Sisk & Foster, 2004). These alterations, as well as other biological processes (e.g. prefrontal synaptic pruning, increased cortical dopaminergic projections) evidenced in the primate brain occur in parallel or serially. An ‘internal clock’, a predetermined genetic program that leads to a cascade of neurochemical changes, triggers the onset of these processes (Sisk & Foster, 2004). The scope of this paper does not allow for coverage of these biochemical events. Readers are referred to a recent issue of the Annals of the New York Academy of Science, which is devoted to behavioral and biological characteristics of adolescence (Cameron, 2004; Dahl, 2004).
It is important to note that the functional relationships among these neurochemical events remain poorly understood. For example, we do not know to what extent the maturation of brain structures, such as the prefrontal cortex, depends on the increased release of sexual or growth hormones. Indirect evidence suggests that specific cognitive functions (e.g. abstract thinking, self-regulation) mature independently of sexual maturation. This conclusion is based on clinical observations of individuals with delayed or premature sexual maturation. Furthermore, the chronology of these events varies among individuals. A better understanding of the behavioral significance of the different trajectories of biological maturation can aid in the development of neurobiological models that may ultimately predict healthy and pathological outcomes.
The purpose of the present work is to present such a model. As with all models, the proposed conceptualization is schematic and addresses restricted aspects of adolescent development. Yet, this approach can lead to the formulation of more sophisticated and comprehensive models that can be tested in future studies.
ADOLESCENT TRIADIC MODEL OF MOTIVATED BEHAVIOR
Definition of the triadic model
The passage through adolescence is characterized by typical patterns of motivated behavior, namely risk-taking, sensation/novelty/reward-seeking, and impulsivity. Although there is wide inter-individual variability in the degree of risk-taking, generic changes in decision-making during adolescence have been acknowledged throughout human history (Hall, 1904) and across species (Spear, 2000), and are recognized as primary sources of morbidity and mortality in adolescents (Dahl, 2004).
The triadic model is based on the assumption that motivated behavior results from the balanced engagement of three behavioral/neural systems: (1) approach (reward-driven); (2) avoidance (harm-avoidant); and (3) regulatory. The concept of two separate neurobehavioral systems underlying responses to reward (approach) and responses to punishment (avoidance) has been formalized by Jeffery A. Gray (1972), and is used extensively in research on temperament and personality (Pickering & Gray, 2001). Generically, rewards are stimuli which individuals strive to approach, and punishments are stimuli which individuals strive to avoid. The approach behavioral system underlies goal-seeking behavior in response to cues of reward, and is typically associated with positively valenced emotions. The avoidant behavioral system underlies withdrawal from aversive cues and is typically associated with negatively valenced emotion.
Neural correlates of these two basic systems have been proposed, suggesting a role of the dorsolateral prefrontal cortex, ventral striatum (particularly the nucleus accumbens), and dopamine in the approach system, and a role of the amygdala, temporal pole, and serotonin in the avoidant system (Davidson, 1998). The novelty of the present model lies in the integration of these two behavioral systems into a neurodevelopmental framework, including the addition of a third regulatory system, and the dynamic functional interactions of the underlying neural circuits across development, in a manner that explains the distinct behaviors of adolescents.
The triadic model (depicted in Fig. 1) involves three functionally distinct sets of distributed neural circuits. The respective functions that these neural circuits play in the triadic model are specific to the context of a goal direct action, and should not be viewed as exclusive of other roles supported by these structures (see below ‘Boundaries of the triadic model’). The ventral striatum circuits, particularly the nucleus accumbens, support reward processes and approach behavior (Wise et al. 1992; Di Chiara, 2002). The amygdala circuits have been described as the ‘behavioral brake’ to protect organisms from potential harm (Amaral, 2002; Zald, 2003), and are a key mediator of avoidant behavior (LeDoux, 2000). Finally, circuits of the prefrontal cortex, owing to their widely accepted role in cognitive control (Miller, 1999, 2000), help to orchestrate the relative contribution of the approach and avoidant behavioral systems, thus providing a supervisory or modulatory control of behavior. As discussed later, only specific aspects of the more complex functions of these circuits are highlighted in the triadic model.
Fig. 1.
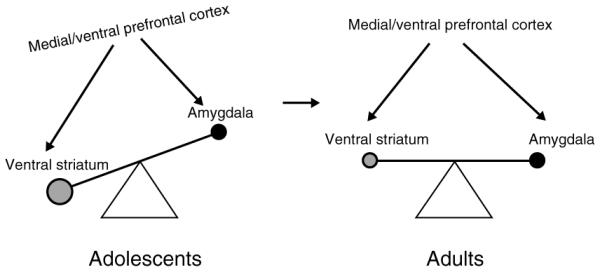
Triadic model of motivated behavior. The balance between reward-driven and harm-avoidant behavior is tilted toward reward driven in adolescents compared to adults. This pattern may be the results of a stronger reward-related system, weaker harm-avoidant system, and/or poor regulatory controls. Distinct distributed neural circuits are associated with these systems, ventral striatum, amygdala and medial/ventral prefrontal cortex.
These specialized circuits are first discussed in isolation, although they are functionally interconnected through substantial direct and indirect projections (e.g. McDonald, 1991; Carmichael & Price, 1995; Fuster, 2001). As such, the triadic model raises the question of the exact contribution to adolescent behavior of the maturation of each node separately, and in relationship with each other. Alteration in any of these circuits or their connectivity could account for characteristics of adolescent behavior.
The triadic model is mainly concerned with the translation of the representations of stimuli (e.g. cues, events, situations) into behavior. Developmental changes in the formation and maintenance of these representations (i.e. specific attributes including physical, autonomic, emotional, spatial, and computational aspects), particularly within somatosensory, insula, orbital frontal, and parietal cortices (Dehaene et al. 1999; Ernst et al. 2003; Paulus et al. 2003, 2005; Bechara, 2004; McCoy & Platt, 2005; Nieder, 2005), can also be critical to the distinct features of adolescent behavior. Evidence suggests that these regions have roles that extend beyond the coding and maintenance of representations of specific attributes of stimuli (e.g. Romo & Salinas, 2001; Romo et al. 2002; Paulus et al. 2003). In the first iteration of the triadic model, this area of research will not be considered. Similarly, neurochemical changes during neurodevelopment will not be addressed despite a number of studies indicating significant age-related alterations of neurotransmitter activity (e.g. Andersen et al. 1997, 2001). These neurochemical changes are an essential part of the functional maturation of the neural circuits described here. They will need to be integrated into this model in the future.
Boundaries of the triadic model
The triadic model is based on a parsimonious account of the dominant role of critical structures in the coding of behavior. Mainly, the attribution of avoidant behavior (in response to aversive stimuli) selectively to amygdala circuits and of approach behavior (in response to appetitive stimuli) selectively to ventral striatal circuits is an oversimplification of the functions of these structures. Although a voluminous literature attributes a specialized role for harm avoidance to the amygdala circuits (see review,LeDoux, 2000) and for reward processing to the nucleus accumbens (see reviews, Wise et al. 1992; Di Chiara, 2002), these structures support a number of additional functions, such as associative learning (Baxter & Murray, 2002; Cardinal et al. 2002b; Salamone & Correa, 2002; Gabriel et al. 2003) and attention filtering (Pessoa & Ungerleider, 2004), which cut across both appetitive and aversive processing. The literature supporting these specialized functions is based on research both in animals, including rodents and non-human primates, and humans.
The amygdala has been shown to mediate not only aversive, but also appetitive, associative learning in rodents, non-human primates, and humans (Baxter & Murray, 2002; Cardinal et al. 2002a; Gottfried et al. 2002, 2003; Gabriel et al. 2003). Current formulations of the role of the amygdala based on the animal literature consider two separate associative learning models: a reward model and an aversion model (see review,Gabriel et al. 2003). These models invoke anatomically distinct circuits, including different amygdala nuclei. The reward model implicates the central nucleus of the amygdala, which mediates the ability of an appetitive conditioned stimulus to drive operant behavior by the modulation of the nucleus accumbens (Holland & Gallagher, 1999; Baxter & Murray, 2002; Everitt et al. 2003). The aversion model relies on the lateral and basolateral amygdala nuclei (LeDoux, 2000). These nuclei process simple sensory and contextual conditioned information respectively. This integrated information is sent to the central nucleus of the amygdala where it is dispatched to effector centers, such as the hypothalamus and brainstem structures, to produce autonomic and motor responses (Amaral et al. 1992). The reward model involves the nucleus accumbens, whereas the aversion model does not, at least not directly. Human lesion (e.g. Aggleton, 2000; Bechara et al. 2003) and functional neuroimaging studies (e.g. Dolan, 2000) support a role of the amygdala for both appetitive and aversive coding, although its role in aversive processing seems to predominate. The triadic model focuses on the role of the amygdala and associated circuits in avoidant behavior.
Similarly to the mixed role of the amygdala, the ventral striatum (particularly the nucleus accumbens) has been shown in rat studies to be involved not only in appetitive, but also aversive, associative learning (Salamone, 1994; Salamone & Correa, 2002; Schoenbaum & Setlow, 2003). The nucleus accumbens dopaminergic system is thought to code for the intensity (salience) of stimuli and to adjust the strength of the link between stimuli and outcome in both appetitive and aversive contexts (see review, Horvitz, 2000). The triadic model postulates that, in addition to this general behavioral facilitation, the nucleus accumbens may play a specialized role in mediating responses to appetitive stimuli. This seems to be true in primates, as evidenced by the difficulty in evoking mesolimbic dopamine activity in response to aversive stimuli in monkeys (Amaral et al. 1992; Joseph et al. 2003), and the weaker response of ventral striatum to aversive stimuli relative to appetitive stimuli in human functional neuroimaging studies (Breiter et al. 2001; Knutson et al. 2001a, b; Reuter et al. 2005). Here, the triadic model concentrates on the reward-related function of the ventral striatum.
The prefrontal cortex supports executive functions, which are required for the planning and execution of complex behavioral sequences (Krawczyk, 2002). Executive functions cover a variety of processes, including attention selection, planning, monitoring, behavioral inhibition, action switching, and working memory. Efforts have been made to map these processes onto distinct prefrontal neural networks (see review Goldman-Rakic, 1996). Based on this functional diversity, the regional specificity of behavioral modulation may differ as a function of cognitive and emotional contexts and demands. For example, behavioral responses to stimuli may rely on abstract rule representation (Bunge et al. 2003), or change in rule as in task shifting or response reversal (Blair, 2001; Deco & Rolls, 2005). Various levels of attention, working memory, or computation can be engaged in behavioral responses. Hence, the nature of the prefrontal circuits that help to balance approach versus avoidant systems is complex, and whether a discrete core site is dedicated to this function is unknown. However, likely candidates are the medial prefrontal cortices, including the anterior cingulate, and the ventral prefrontal cortex, including the orbital frontal cortex. These regions play important roles in the control of motivated behavior, such asconflict/error monitoring for the anterior cingulate (Carter et al. 1998; Bush et al. 2000, 2002; Krawczyk, 2002), behavioral adaptation to changes in stimuli value as in response reversal for the orbital frontal cortex (see review, Fuster, 1993; Blair, 2004), and self-monitoring for the medial prefrontal cortex (see review, Northoff & Bermpohl, 2004).
A more elaborate rendition of the triadic model will be possible as a better understanding of how the main functions of the amygdala, ventral striatum, and medial and ventral prefrontal cortices mature and contribute both in isolation and collaboratively to behavior throughout development. Fostering new developmental adolescent research based on a simple framework is the main goal for the proposed triadic model.
Behavioral support for the triadic model
We propose that adolescence is the period during which the activity of the reward system prevails over that of the avoidant system while the still immature regulatory system fails to adaptively balance these two behavioral controllers. Indirect evidence in animal models and humans supports this theory.
Before considering this evidence, it is important to note that the extrapolation of animal data to human subjects has limitations (Spear, 2004). Drawbacks of this translation include the relatively poorly defined temporal boundaries of this transition period in animals (e.g. most commonly agreed upon: rats, post-natal days 28-42; non-human primates, 2-4 years of age), the species differences in the developmental trajectories of neural structures and functions, and the difficulty in mapping the complexity of human behavior onto other species.
Adolescence, across species, seems to be characterized by a uniquely high sensitivity to reward (see review, Chambers et al. 2003; Laviola et al. 2003). In humans, the increased susceptibility to drugs of abuse (Chambers et al. 2003) and the greater vulnerability to developing substance dependence (e.g. Kandel et al. 1992) in adolescents compared to adults suggest a hypersensitive reward system. Consistent with these observations in humans, findings in animal models of adolescence concur with the notion that adolescence represents a unique period in the development of reward systems. This conclusion is supported by distinct responses to substances of abuse (Spear, 2000; Andersen et al. 2002; Laviola et al. 2003). For example, adolescent rodents show greater locomotor sensitivity to cocaine (Schramm-Sapyta et al. 2004) and reduced signs of nicotine withdrawal (O’Dell et al. 2004) relative to adult animals.
With respect to avoidance behavior, adolescents, as described above, are less sensitive to risks in the context of goal-directed actions (Arnett, 1992; Wills et al. 1994; Maggs et al. 1995; Steinberg, 2004), suggesting that the coding of potential harm and response to warning signals is altered in adolescence. Furthermore, this implies that the amygdala and related structures that process warning signals are less sensitive to potentially harmful stimuli in adolescents than in adults.
Neural maturation and connectivity in support of the triadic model
Empirical reports support delayed maturation of the behavioral inhibitory systems (Casey et al. 2000; Luna & Sweeney, 2004). The medial and ventral prefrontal cortices, involved in behavioral inhibition and error monitoring, have been found to exhibit diffierent pattern of activation in youth than in adults. A common finding is a more diffuse pattern of prefrontal activation during performance inhibition in youth compared to adults (Casey et al. 2000; Luna & Sweeney, 2001, 2004). In addition, in support of these neuroimaging findings, performance on tasks probing motor inhibition, such as Stroop, Go-No go, or antisaccade eye-movement tasks, has consistently been found to be worse in youth than in adults (Costantini & Hoving, 1973; Casey et al. 2000; Leon-Carrion et al. 2004). Morphometric age-related changes also support continued maturation of this region throughout adolescence (e.g. Giedd, 2004). The triadic model postulates an immature supervisory role for the medial/ventral prefrontal cortex in modulating the respective contributions of ventral striatum (approach behavior) and amygdala (avoidant behavior) responses to stimuli. It is not clear whether the loci of maturational lag lay within these specialized circuits themselves, or in the functional connectivity among these structures, or in both.
Relatively recent work in animals suggests that structural and functional connectivity among these neural systems evolve during adolescence (Cunningham et al. 2002; see reviews, Lewis, 1997; Lewis et al. 2004). For example, amygdalo-cortical fibers become denser throughout adolescence in the rodent, perhaps reflecting the development of better regulatory controls with respect to harm-avoidant behavior (Cunningham et al. 2002). At the same time, preliminary findings in the non-human primate indicate reduction in dendritic branching in the medial amygdala in adolescence (J. L. Zehr, unpublished observations). Findings in adolescent monkeys show marked changes of pre- and post-synaptic markers of GABA neurotransmission in the prefrontal cortex during adolescence, suggesting continued maturation of inhibitory controls (Lewis et al. 2004).
Connectivity among amygdala and nucleus accumbens has been explored in adult animals. Early evidence suggested an inhibitory control of amygdala over nucleus accumbens activity in the rodent (Simon et al. 1988; Yim & Mogenson, 1989). Recent electrophysiological work in adult rats, however, describes opposite effects of amygdala activation on dopamine efflux in the nucleus accumbens as a function of the site of stimulation, i.e. the basolateral amygdala nucleus having a direct excitatory effects, and the central amygdaloid nucleus having an indirect inhibitory effect on the nucleus accumbens (Phillips et al. 2003). Reciprocal direct and indirect connections link the prefrontal cortex to the nucleus accumbens and to the amygdala (Jackson & Moghaddam, 2001; see review, Morgane et al. 2005). More needs to be learned about the functional relationships of these three neural circuits across development.
In its present form, the triadic model does not specify the nature of the developmental processes that affect these functional connections. Nor does it identify the exact neural and molecular developmental mechanisms that result in an imbalanced function of the amygdala, ventral striatum, and medial/ventral prefrontal circuits. These questions warrant additional research. However, in this initial version, the model can be applied to the examination of a motivated action using a neurocognitive framework.
In the next section, we describe the strategy used to study motivated behavior from a cognitive neuroscience perspective. This strategy allows for the testing of predictions based on the triadic model.
COGNITIVE/AFFECTIVE NEUROSCIENTIFIC APPROACH: SPIRAL OF MOTIVATED ACTION
Spiral of motivated action (Fig. 2)
Fig. 2.
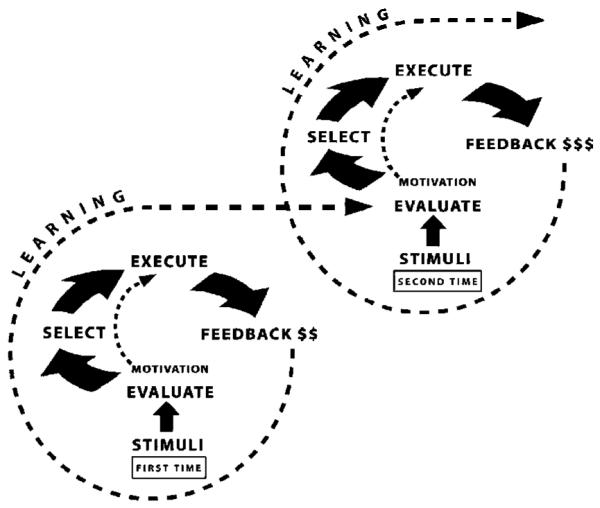
Spiral of motivated action. This graph depicts the progression of processes that take place in a simple and completed motivated action. Individuals are first exposed to stimuli, which represent options from which one needs to be selected. Upon exposure, individuals evaluate the stimuli options, and form a preference (stage 1). Based on preference, they select a course of action (stage 2), and execute the action (stage 3). If the result of their action occurs with a delay, subjects anticipate the outcome to their action, and finally experience the feedback (stage 4). The experience of feedback will inform the value of the option that they selected in the first stage of this motivated action, which occurs through learning. Motivation, a psychological state that modulates behavior, is most influential on the first three stages of motivated behavior, formation of preference, selection and execution. (Graphic designed by Cynthia Friedman.)
Motivated, or goal-directed, behavior has historically been approached from a number of perspectives including economics, sociology, psychology, neurology, physiology and neuroscience, each employing its own terminology and theories (see review, Ernst & Paulus, in press). For example, terms like ‘directed action’, ‘intentional behavior’, ‘conscious behavior’, and ‘decision-making’ have often been used interchangeably, resulting in possible confusions. In addition, a host of models have been proposed to describe components of motivated action based on their suitability for study within a particular field of research. Advances in understanding the multifaceted processes of motivated action have been most successful through efforts to integrate theories from various frameworks.
Most relevant to the present work are the Somatic Marker Theory (Damasio, 1996; Bechara, 2004) and the dopamine error signal model (Schultz, 2002). The Somatic Marker Theory was elaborated by Damasio and colleagues on the basis of work with patients suffering from brain lesions (Damasio, 1996; Bechara et al. 1999; Bechara, 2004). This theory pertains to the emotional appraisal of stimuli, which contributes to decision-making and motivated behavior. Briefly, the Somatic Marker Theory proposes that decision-making is influenced by somatic markers, which are originally triggered by the amygdala for innately valenced stimuli, and the ventromedial prefrontal cortex for learned valence stimuli. These somatic markers are relayed to the brainstem (covert signaling), parietal cortices (insular/SI, SII), and cingulate cortex, where they are translated into feeling states (Damasio, 1998; Bechara, 2004). The prediction error model was proposed by Schultz and colleagues (Schultz et al. 1997; Schultz, 2002) on the basis of single cell recordings of dopaminergic neurons in non-human primates performing reward-related tasks. This model is a neurochemical rendition of processes that contribute to learning about the rewarding values of stimuli (Waelti et al. 2001; O’Doherty et al. 2004). The error model is based on the observation that firing of dopamine neurons increases in response to an unexpected or greater than expected reward, vanishes in response to an expected reward, and is reduced in response to a punishment (see review, Schultz, 2004).
In the current work, we adhere to a cognitive neuroscientific framework (see review, Ernst & Paulus, in press). The cognitive neuroscience approach is based on the parcellation of complex behaviors into smaller parts, each more easily accessible to scientific inquiry (Posner & DiGirolamo, 2000). Using this strategy, the elemental components of a motivated action are identified as: evaluation of options (situations, events, stimuli), formation of preference, execution of action, anticipation of outcome, and response to feedback. These processes define the consecutive stages that constitute a completed motivated action. They are functionally inter-dependent, present some degree of overlap, and always occur in this order. Therefore, these stages form a dynamic loop, which is better described as a spiral because each onset of the loop (stimuli evaluation) starts at a different point than the previous one (Fig. 2). Indeed, the experience of the outcome of a motivated action (the last stage of the loop) informs the value of the initially selected option, and contributes to the motivation to act (or to not act) on the selected option the next time it is presented (the first stage of the next loop). Thus, the forces that drive this spiral rest on two critical processes, learning and motivation. Basic cognitive functions, including attention and memory, are necessarily involved. Similarly, affective coding operates throughout the spiral, with different levels of influence at each stage.
This formulation of a motivated action constitutes a road map, along which various neural networks operate to successfully orchestrate a motivated action. We briefly describe the processes within the spiral of goal-directed action that engage the neural components of the triadic model, and the functional predictions based on the triadic model. Of note, anticipation of outcomes is not included in the following section. Anticipation is present in various degrees and forms throughout the stages of motivated action, and developmental changes in the pathways coding for this cognitive construct may be critically involved in driving adolescent behavior.
Integration of the triadic model with the spiral of motivated action
The pre-execution of action stage involves the evaluation of stimuli-options, the formation of preference, and the selection of a course of action. The amygdala and the ventral striatum are critical to the coding of affective and motivational information that guide the formation of preference (Salamone & Correa, 2002; Arana et al. 2003; Zald, 2003). In the context of the triadic model and with respect to the formation of preference, adolescents would show relatively higher impact of stimuli signaling reward on striatal activation and lesser impact of stimuli signaling punishment on amygdala activation compared to adults. This pattern would support predominant approach and risk-seeking behavior.
The execution of action stage involves preparatory and executory components. Both aspects are directed and energized by the motivation to act on the preferred option. During this stage, ventral striatum contributes to the motivation to act (Mogenson et al. 1980; Salamone et al. 2005), and medial prefrontal cortex, particularly anterior cingulate, to conflict and error monitoring (Carter et al. 1998; Bush et al. 2000). For similar levels of motivation-to-act in adults and adolescents, adolescents would show less activation of the ventral striatum than adults due to a lower threshold to act (approach behavior). In other words, adolescents would require less activation of the reward system relative to adults to generate similar approach behavior. In addition, adolescents would show a relatively weaker engagement of the avoidant system in aversive conditions in the context of a goal-directed action. Finally, adolescents would present greater activation of the anterior cingulate compared to adults due to the relative inefficiency of the neural systems to monitor errors.
The response to feedback evokes an affective response and, as a corollary, an error-signal (also referred to as a teaching signal) that reflects the difference between the expected value of the outcome and its actual value (Schultz et al. 1997). These affective and learning processes serve to inform the value of the stimulus-option associated with a particular feedback, which, in turn, contributes to the formation of preference the next time the stimulus options appear (i.e. reinforcement). These processes involve dopamine function, amygdala, ventral and dorsal striatum, orbitofrontal cortex, and medial prefrontal cortex: The error signal has been attributed to dopamine function (Waelti et al. 2001). The amygdala and ventral striatum are known to play an essential role in classical and instrumental learning (Salamone, 1994; Baxter & Murray, 2002; Cardinal et al. 2002a; Salamone & Correa, 2002; Gabriel et al. 2003; Schoenbaum & Setlow, 2003). The dorsal striatum (i.e. caudate and putamen) has often been shown to be engaged in response to feedback (Delgado et al. 2000; Martin-Soelch et al. 2003; O’Doherty et al. 2004). Since learning is predicated on the reliable affective representations of outcomes, the integrity of the orbitofrontal cortex, which harbors these representations (O’Doherty et al. 2003; Rolls, 2004), is critical to this stage. Finally, appraisal of outcome may also engage higher level representations of values, including self-referential processes carried out by medial prefrontal cortical regions (e.g. BA 32, 10) (Knutson et al. 2001b, 2003). Based on the triadic model, adolescents would show greater impact of a positive outcome on the ventral striatum, and lower impact of a negative outcome on the amygdala compared to adults in the context of goal-directed actions. The dopamine learning signal would be heightened and the medial prefrontal cortex would be more activated in adolescents relative to adults.
Age-related differences in associative learning function between adolescents and adults are difficult to predict. Learning processes are among the earliest to be in place from an ontological and evolutionary perspective. Associative learning is affected by the way in which feedback is processed, i.e. the representation of the value of the outcome that becomes linked through learning to the stimuli options. Although we postulate that feedback processes continue to mature through adolescence, the learning itself may already be fully developed by adolescence, and possibly much earlier.
Of note, investigators have also proposed the theory of a weaker reward system in adolescents as opposed to our position supporting a stronger reward system in this population. A weaker reward system would manifest itself as enhanced reward-seeking behavior to maintain a state of homeostasis (see in Bjork et al. 2004).
Initial studies probing the neural substrates of reward systems in adolescents
Some of the predictions outlined above are supported by three recent human studies using functional magnetic resonance imaging. Two of these studies used a direct comparison of adolescents and adults, and one study replicated with adolescents a previous work conducted with adults. In brief, Bjork et al. (2004) reported in adolescents less activation of the ventral striatum for a similar level of reward-related performance as adults during motivation to act. Although the authors interpreted this finding as a weaker reward system in adolescents, we ascribe it to a more sensitive reward system (see above). The latter interpretation is supported in a recent study (Ernst et al. in press), which showed greater impact of feedback on ventral striatum and less impact on amygdala in adolescents than in adults. Finally, compared to the adult study by Delgado et al. (2000), the adolescent study by May et al. (2004) suggested a weaker amygdala involvement in processes of motivated action in adolescents than in adults.
These studies represent the first attempts to unravel the precise nature of the neural substrates that underlie typical motivated behaviors in adolescence. More studies are needed, not only to understand the relative contribution of the functionally distinct neural circuits, particularly within the triadic model, but also their neurochemical modulation (e.g. catecholamines, serotonin), and the interaction of genetic and environmental influences on the functional development of these circuits and their connectivity. This knowledge is necessary to guide research in psychopathology, particularly since adolescence represents the most vulnerable period within the lifespan for the onset of psychiatric disorders.
IMPLICATION FOR PSYCHOPATHOLOGY
We will focus the application of the triadic model and the spiral of motivated behavior onto two highly prevalent psychopathologies in youth: depression and anxiety.
Depression
Considerable evidence indicates that adolescence is a period of peak vulnerability for the onset of depression (Costello et al. 2002).
Cognitive models of depression identify a number of processes that contribute to the etiology and maintenance of the disorder. In particular, biases to interpret information negatively (Gotlib et al. 2004), and to ruminate on these negative interpretations (Gur et al. 1992; Bouhuys et al. 1999) likely represent major cognitive vulnerabilities (Beck, 1967; Abramson et al. 1989; Nolen-Hoeksema, 2000). These deficits, translated at the level of the spiral of motivated behavior, are particularly relevant to the evaluative stages of pre-execution of action, and error monitoring during feedback. Motivation and learning are also affected, either secondarily to biases in evaluation or primarily as separate deficits.
Decision-making characteristics in depressed individuals may depend on symptom severity. One theory proposes that negative mood leads individuals to indulge immediate impulses as an attempt to improve affect, thus prioritizing short-term affect regulation over other self-regulatory goals (Tice et al. 2001). Alternatively, risk avoidance has been hypothesized to reflect greater severity of negative mood and lower levels of subjective experience of self-control, consistent with a gradient in risk avoidance as a function of severity of depression (Lerner & Keltner, 2000). Avoidance of risky choices can also result from failure to experience positive emotions (anhedonia), and loss of energy and motivation (Nelson & Charney, 1981; Ernst et al. 2004; Hasler et al. 2004).
In contrast to the relatively large literature in emotion processing, few clinical studies have examined decision-making processes in mood disorders, and most of this work pertains to adults. Application of the triadic model can help in mapping developmental trajectories of depression symptoms onto relevant neural systems. In addition, maturational changes at a neural systems level can contribute to age-related vulnerability (or resilience) to depression.
Abnormalities in amygdala, medial prefrontal and orbitofrontal cortices, and striatum have been reported in adult depression (see review, Drevets, 2003). Dopaminergic dysfunction may underlie anhedonia and amotivation (Drevets, 2003). Additionally, abnormally high levels of activation, both at baseline and in response to negative emotional stimuli, have consistently been found in the amygdala and medial prefrontal cortex of depressed adults (Davidson et al. 2002; Whalen et al. 2002; Drevets, 2003).
Recent magnetic resonance imaging studies indicate reduced amygdala volume (Rosso et al. 2005) and amygdala response to fearful faces in depressed adolescents relative to healthy adolescents (Thomas et al. 2001a). This is consistent with suggestions of amygdala dysfunction in depressed adolescents during evaluative and encoding processes of fearful faces (Pine et al. 2004), and in contributing to the increased rate of depression in adolescence.
Medial prefrontal cortex and striatal function in response to emotional or motivationally salient stimuli have yet to be examined in depressed adolescents. However, in view of their roles in adult depression (Drevets, 2003) and their developmental changes observed during adolescence (Giedd, 2004), these areas are also likely to contribute to the observed increase incidence rates of depression during adolescence.
Anterior cingulate dysfunction, inferred from error-monitoring deficits, has been observed in depressed adults during event-related potential studies examining the error-related negativity (ERN) (Tucker et al. 2003; Ruchsow et al. 2004). Evidence of development-related differences in ERN (Davies et al. 2004; Ladouceur et al. 2004) suggests that maturation of the anterior cingulate and related circuits can also affect vulnerability to depression in youth.
This brief review highlights the paucity of data in the neural development contributing to adolescent depression. We propose that the role of neural development in the expression of depression can be better understood through the use of developmentally based models of motivated behavior, and by systematic assessment of discrete behavioral components of motivated action.
Anxiety
Traditional (Gray, 1970) and contemporary (Davidson, 2002; Corr, 2004; McNaughton & Corr, 2004) conceptualizations of normal and pathological anxiety emphasize processes and neural systems involved in motivated behavior. Most of this work focuses on behavioral responses to potential threat or aversive stimuli, and the neural systems involved in withdrawal or harm avoidance.
Clinical anxiety is characterized by hyper-vigilance or exaggerated attention toward threat (Mogg & Bradley, 1998; Derryberry & Reed, 2002). At the level of the spiral of motivated action, these behavioral characteristics are expected to influence the pre-execution of action and the anticipation preceding feedback. Presented with emotional stimuli, adults with high levels of anxiety demonstrate an orienting bias toward threat, while adults with low or extremely high levels of anxiety tend to orient away from threat (Mogg & Bradley, 1998; Mogg et al. 2000). The processing of facial emotions, particularly negative emotions (e.g. anger, fear), are modulated by neural systems implicated in withdrawal motivation (Davidson, 2002) and threat processing (LeDoux, 2000). Specifically, the amygdala responds to the presentation of fearful faces (Morris et al. 1998; Whalen et al. 1998) and has been consistently implicated in the pathophysiology of anxiety disorders (Rauch et al. 2003).
Findings regarding threat biases in children and adolescents with anxiety are less consistent (e.g. Ehrenreich & Gross, 2002; Monk & Pine, 2004). For example, behavioral biases toward threat words have been reported for children and adolescents with generalized anxiety disorder (GAD), and post traumatic-stress disorder (PTSD) (Vasey et al. 1995; Taghavi et al. 1999; Dalgleish et al. 2003), while biases away from threat faces have also been reported in children and adolescents with PTSD (Pine et al. 2005). These discrepancies may result from differences in severity of anxiety among study samples (Ehrenreich & Gross, 2002) or in the neurodevelopment of the neural substrates underlying these biases.
Neuroimaging results also show inconsistencies. Amygdala activity in response to emotional faces has been found both increased (Monk et al. 2004) and decreased (Thomas et al. 2001b) in healthy adolescents compared to adults. Anxious children and adolescents (GAD, panic disorder) demonstrate abnormally high amygdala activity in response to fear faces (Thomas et al. 2001a).
Finally, decision-making and reward-related processes have scarcely been examined in conjunction with anxiety disorders. Risk avoidance, emotion interference, impulsive responses related to hyperarousal, and delay aversion are expected features of motivated behavior in anxious individuals.
SUMMARY
In conclusion, we propose a neuroscience systems-based developmental model of adolescent behavior that permits the framing of specific hypotheses regarding the regulation of the various components of a motivated action in health and disease. This model posits that the propensity for risk-/reward-seeking behavior of adolescents partly originates from predetermined ontogenic changes in three neural systems that support (1) reward-related (approach) behavior, (2) harm avoidance, and (3) regulation of both approach and avoidance systems. Key neural substrates include the ventral striatum (nucleus accumbens), the amygdala, and the medial/ventral prefrontal cortices. Refinement of this model depends on a finer delineation of these neural networks and their functional development, in isolation and collaboratively. The triadic model can facilitate the identification of specific behavioral and intermediate neural phenotypes to be used in molecular genetic studies, in health and disease.
Footnotes
DECLARATION OF INTEREST
None.
REFERENCES
- Abramson LY, Metalsky GI, Alloy LB. Hopelessness depression: a theory-based subtype of depression. Psychological Review. 1989;96:358–372. [Google Scholar]
- Aggleton JP. The Amygdala: A Functional Analysis. 2nd edn Oxford University Press; New York, USA: 2000. The functional effects of amygdala lesions in humans: a comparison with findings from monkeys; pp. 485–504. [Google Scholar]
- Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biological Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pikanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. Wiley-Liss; New York: 1992. pp. 1–66. [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nature Neuroscience. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (+/-)-7-OH-DPAT. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1997;356:173–181. doi: 10.1007/pl00005038. [DOI] [PubMed] [Google Scholar]
- Andersen SL, LeBlanc CJ, Lyss PJ. Maturational increases in c-fos expression in the ascending dopamine systems. Synapse. 2001;41:345–350. doi: 10.1002/syn.1091. [DOI] [PubMed] [Google Scholar]
- Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. Journal of Neuroscience. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ. Reckless behavior in adolescence: a developmental perspective. Developmental Review. 1992;12:339–373. [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature Reviews Neuroscience. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain and Cognition. 2004;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Annals of the New York Academy of Sciences. 2003;985:356–369. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. Depression: Clinical, Experimental, and Theoretical Aspects. Harper & Row; New York: 1967. [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. Neurocognitive models of aggression, the anti-social personality disorders, and psychopathy. Journal of Neurology, Neurosurgery & Psychiatry. 2001;71:727–731. doi: 10.1136/jnnp.71.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. The neurobiology of antisocial behavior and psychopathy. In: Easton A, Emery NJ, editors. Cognitive Neuroscience of Social Behaviour. Psychology Press; New York: 2004. pp. 291–324. [Google Scholar]
- Bouhuys AL, Geerts E, Gordijn MC. Depressed patients’ perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. Journal of Nervous and Mental Disease. 1999;187:595–602. doi: 10.1097/00005053-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. Journal of Neurophysiology. 2003;90:3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences USA. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JL. Interrelationships between hormones, behavior, and affect during adolescence: complex relationships exist between reproductive hormones, stress-related hormones, and the activity of neural systems that regulate behavioral affect. Comments on part III. Annals of the New York Academy of Sciences. 2004;1021:134–142. doi: 10.1196/annals.1308.015. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience & Biobehavioral Reviews. 2002a;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J, Morrison CH, Howes SR, Robbins TW, Everitt BJ. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behavioral Neuroscience. 2002b;116:553–567. doi: 10.1037//0735-7044.116.4.553. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr PJ. Reinforcement sensitivity theory and personality. Neuroscience & Biobehavioral Reviews. 2004;28:317–332. doi: 10.1016/j.neubiorev.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Costantini AF, Hoving KL. The relationship of cognitive and motor response inhibition to age and IQ. Journal of Genetical Psychology. 1973;123:309–319. doi: 10.1080/00221325.1973.10532690. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM, Biederman J, Goldsmith HH, Kaufman J, Lewinsohn PM, Hellander M, Hoagwood K, Koretz DS, Nelson CA, Leckman JF. Development and natural history of mood disorders. Biological Psychiatry. 2002;52:529–542. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. Journal of Comparative Neurology. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Taghavi R, Neshat-Doost H, Moradi A, Canterbury R, Yule W. Patterns of processing bias for emotional information across clinical disorders: a comparison of attention, memory, and prospective cognition in children and adolescents with depression, generalized anxiety, and posttraumatic stress disorder. Journal of Clinical Child and Adolescent Psychology. 2003;32:10–21. doi: 10.1207/S15374424JCCP3201_02. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London. B: Biological Sciences. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Emotion in the perspective of an integrated nervous system. Brain Research. Brain Research Reviews. 1998;26:83–86. doi: 10.1016/s0165-0173(97)00064-7. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective styles and affective disorders: perspectives from affective neuroscience. Cognition and Emotion. 1998;12:307–330. [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, Drevets WC, Farah MJ, Kagan J, McClelland JL, Nolen-Hoeksema S, Peterson BS. Neural and behavioral substrates of mood and mood regulation. Biological Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of error-monitoring event-related potentials in adolescents. Annals of the New York Academy of Sciences. 2004;1021:324–328. doi: 10.1196/annals.1308.039. [DOI] [PubMed] [Google Scholar]
- Deco G, Rolls ET. Synaptic and spiking dynamics underlying reward reversal in the orbitofrontal cortex. Cerebral Cortex. 2005;15:15–30. doi: 10.1093/cercor/bhh103. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science. 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural Brain Research. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. The Amygdala: A Functional Analysis. 2nd edn Oxford University Press; New York, USA: 2000. Functional neuroimaging of the human amygdala during emotional processing and learning; pp. 631–653. [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Annals of the New York Academy of Science. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Ehrenreich JT, Gross AM. Biased attentional behavior in childhood anxiety. A review of theory and current empirical investigation. Clinical Psychological Review. 2002;22:991–1008. doi: 10.1016/s0272-7358(01)00123-4. [DOI] [PubMed] [Google Scholar]
- Erikson EH. Childhood and Society. Norton; New York: 1950. [Google Scholar]
- Erikson EH. Identity, Youth, and Crisis. Norton; New York: 1968. [Google Scholar]
- Ernst M, Dickstein DP, Munson S, Eshel N, Pradella A, Jazbec S, Pine DS, Leibenluft E. Reward-related processes in pediatric bipolar disorder: a pilot study. Journal of Affective Disorders. 2004;82(Suppl. 1):S89–S101. doi: 10.1016/j.jad.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Ernst M, Jazbec S, McClure EB, Monk CS, Blair RJR, Leibenluft E, Pine DS. Neuroimage. Amygdala and nucleus accumbens activation in response to receipt and omission of gains in adults and adolescents. in press. [DOI] [PubMed] [Google Scholar]
- Ernst M, Kimes AS, London ED, Matochik JA, Eldreth D, Tata S, Contoreggi C, Leff M, Bolla K. Neural substrates of decision making in adults with attention deficit hyperactivity disorder. American Journal of Psychiatry. 2003;160:1061–1070. doi: 10.1176/appi.ajp.160.6.1061. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision-making: a selective review from a neurocognitive and clinical perspective. Biological Psychiatry. doi: 10.1016/j.biopsych.2005.06.004. in press. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Annals of the New York Academy of Sciences. 2003;985:233–250. [PubMed] [Google Scholar]
- Fuster JM. Frontal lobes. Current Opinion in Neurobiology. 1993;3:160–165. doi: 10.1016/0959-4388(93)90204-c. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex - an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Burhans L, Kashef A. Consideration of a unified model of amygdalar associative functions. Annals of the New York Academy of Sciences. 2003;985:206–217. doi: 10.1111/j.1749-6632.2003.tb07083.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Science. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philosophical Transactions of the Royal Society of London. B: Biological Sciences. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Kasch KL, Traill S, Joormann J, Arnow BA, Johnson SL. Coherence and specificity of information-processing biases in depression and social phobia. Journal of Abnormal Psychology. 2004;113:386–398. doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. Journal of Neuroscience. 2002;22:10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Gray JA. The psychophysiological basis of introversion-extraversion. Behaviour Research and Therapy. 1970;8:249–266. doi: 10.1016/0005-7967(70)90069-0. [DOI] [PubMed] [Google Scholar]
- Gray JA. The Psychophysiological Nature of Introversion-Extraversion: A Modification of Eysenck’s Theory. Academic Press; New York: 1972. [Google Scholar]
- Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Research. 1992;42:241–251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Hall GS. Adolescence: Its Psychology and Its Relations to Physiology, Anthropology, Sociology, Sex, Crime, Religion, and Education. Appleton; New York: 1904. [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends in Cognitive Science. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Moghaddam B. Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. Journal of Neuroscience. 2001;21:676–681. doi: 10.1523/JNEUROSCI.21-02-00676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph MH, Datla K, Young AM. The interpretation of the measurement of nucleus accumbens dopamine by in vivo dialysis: the kick, the craving or the cognition? Neuroscience & Biobehavioral Reviews. 2003;27:527–541. doi: 10.1016/j.neubiorev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. Journal of Studies on Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001a;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001b;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Journal of Comparative and Physiological Psychology. 2003;47:419–427. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neuroscience & Bio-behavioral Reviews. 2002;26:631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. ERP correlates of action monitoring in adolescence. Annals of the New York Academy of Sciences. 2004;1021:329–336. doi: 10.1196/annals.1308.040. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neuroscience & Biobehavioral Reviews. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leon-Carrion J, Garcia-Orza J, Perez-Santamaria FJ. Development of the inhibitory component of the executive functions in children and adolescents. International Journal of Neuroscience. 2004;114:1291–1311. doi: 10.1080/00207450490476066. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Keltner D. Beyond valence: toward a model of emotion-specific influences on judgment and choice. Cognition and Emotion. 2000;14:473–493. [Google Scholar]
- Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz D, Eggan S, Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Annals of the New York Academy of Sciences. 2004;1021:64–76. doi: 10.1196/annals.1308.008. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. Studies of brain and cognitive maturation through childhood and adolescence: a strategy for testing neurodevelopmental hypotheses. Schizophrenia Bulletin. 2001;27:443–455. doi: 10.1093/oxfordjournals.schbul.a006886. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Annals of the New York Academy of Sciences. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Maggs JL, Almeida DM, Galambos NL. Risky Business: the paradoxical meaning of problem behavior for young adolescents. Journal of Early Adolescence. 1995;15:344–362. [Google Scholar]
- Martin-Soelch C, Missimer J, Leenders KL, Schultz W. Neural activity related to the processing of increasing monetary reward in smokers and nonsmokers. European Journal of Neuroscience. 2003;18:680–688. doi: 10.1046/j.1460-9568.2003.02791.x. [DOI] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- McCoy AN, Platt ML. Expectations and outcomes: decision-making in the primate brain. Journal of Comparative Physiology. A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 2005;191:201–211. doi: 10.1007/s00359-004-0565-9. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neuroscience & Biobehavioral Reviews. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex: complex neural properties for complex behavior. Neuron. 1999;22:15–17. doi: 10.1016/s0896-6273(00)80673-x. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature Reviews Neuroscience. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Progress in Neurobiology. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour and Research Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. Journal of Abnormal Psychology. 2000;109:695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, Woldehawariat G, Montgomery LA, Zarahn E, McClure EB, Guyer AE, Leibenluft E, Charney DS, Ernst M, Pine DS. Experience-dependent plasticity for attention to threat: behavioral and neurophysiological evidence in humans. Biological Psychiatry. 2004;56:607–610. doi: 10.1016/j.biopsych.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Monk CS, Pine DS. Childhood anxiety disorders: a cognitive neurobiological perspective. In: Charney DS, Nestler E, editors. Neurobiology of Mental Illness. Oxford University Press; Oxford: 2004. [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Progress in Neurobiology. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(Pt 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Charney DS. The symptoms of major depressive illness. American Journal of Psychiatry. 1981;138:1–13. doi: 10.1176/ajp.138.1.1. [DOI] [PubMed] [Google Scholar]
- Nieder A. Counting on neurons: the neurobiology of numerical competence. Nature Reviews Neuroscience. 2005;6:177–190. doi: 10.1038/nrn1626. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109:504–511. [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Annals of the New York Academy of Sciences. 2004;1021:167–174. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. Journal of Neuroscience. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Leland D, Simmons AN. Superior temporal gyrus and insula provide response and outcome-dependent information during assessment and action selection in a decision-making situation. Neuroimage. 2005;25:607–615. doi: 10.1016/j.neuroimage.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider LG. Neuroimaging studies of attention and the processing of emotion-laden stimuli. Progress in Brain Research. 2004;144:171–182. doi: 10.1016/S0079-6123(03)14412-3. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Howland JG. Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neuroscience & Biobehavioral Reviews. 2003;27:543–554. doi: 10.1016/j.neubiorev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Pickering AD, Gray JA. The Neuroscience of personality. In: Pervin LA, John OP, editors. Handbook of Personality: Theory and Research. Guilford Press; New York, USA: 2001. pp. 277–299. [Google Scholar]
- Pine DS, Lissek S, Klein RG, Mannuzza S, Moulton JL, III, Guardino M, Woldehawariat G. Face-memory and emotion: associations with major depression in children and adolescents. Journal of Child Psychology and Psychiatry. 2004;45:1199–1208. doi: 10.1111/j.1469-7610.2004.00311.x. [DOI] [PubMed] [Google Scholar]
- Pine DS, Mogg K, Bradley BP, Montgomery L, Monk CS, McClure E, Guyer AE, Ernst M, Charney DS, Kaufman J. Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. American Journal of Psychiatry. 2005;162:291–296. doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ. Cognitive neuroscience: origins and promise. Psychological Bulletin. 2000;126:873–889. doi: 10.1037/0033-2909.126.6.873. [DOI] [PubMed] [Google Scholar]
- Rauch S, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Annals of the New York Academy of Sciences. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Glascher I, Buchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nature Neuroscience. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Convergence of sensory systems in the orbitofrontal cortex in primates and brain design for emotion. Anatomical Record. 2004;281A:1212–1225. doi: 10.1002/ar.a.20126. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development. Journal of Neuroendocrinology. 2003;15:1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Salinas E, Brody CD, Zainos A, Lemus L, de Lafuente V, Luna R. From sensation to action. Behavioural Brain Research. 2002;135:105–118. doi: 10.1016/s0166-4328(02)00161-4. [DOI] [PubMed] [Google Scholar]
- Romo R, Salinas E. Touch and go: decision-making mechanisms in somatosensation. Annual Review of Neuroscience. 2001;24:107–137. doi: 10.1146/annurev.neuro.24.1.107. [DOI] [PubMed] [Google Scholar]
- Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA. Amygdala and hippocampus volumes in pediatric major depression. Biological Psychiatry. 2005;57:21–26. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Herrnberger B, Wiesend C, Gron G, Spitzer M, Kiefer M. The effect of erroneous responses on response monitoring in patients with major depressive disorder: a study with event-related potentials. Psychophysiology. 2004;41:833–840. doi: 10.1111/j.1469-8986.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- Salamone JD. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behavioural Brain Research. 1994;61:117–133. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behavioural Brain Research. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Current Opinion in Pharmacology. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Lesions of nucleus accumbens disrupt learning about aversive outcomes. Journal of Neuroscience. 2003;23:9833–9841. doi: 10.1523/JNEUROSCI.23-30-09833.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Pratt AR, Winder DG. Effects of periadolescent versus adult cocaine exposure on cocaine conditioned place preference and motor sensitization in mice. Psychopharmacology (Berlin) 2004;173:41–48. doi: 10.1007/s00213-003-1696-3. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Current Opinion in Neurobiology. 2004;14:139–147. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Simon H, Taghzouti K, Gozlan H, Studler JM, Louilot A, Herve D, Glowinski J, Tassin JP, LeMoal M. Lesion of dopaminergic terminals in the amygdala produces enhanced locomotor response to D-amphetamine and opposite changes in dopaminergic activity in prefrontal cortex and nucleus accumbens. Brain Research. 1988;447:335–340. doi: 10.1016/0006-8993(88)91136-5. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent brain development and animal models. Annals of the New York Academy of Sciences. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Taghavi MR, Neshat-Doost HT, Moradi AR, Yule W, Dalgleish T. Biases in visual attention in children and adolescents with clinical anxiety and mixed anxiety-depression. Journal of Abnormal Child Psychology. 1999;27:215–223. doi: 10.1023/a:1021952407074. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001a;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001b;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Tice DM, Bratslavsky E, Baumeister RF. Emotional distress regulation takes precedence over impulse control: if you feel bad, do it! Journal of Personality and Social Psychology. 2001;80:53–67. [PubMed] [Google Scholar]
- Tucker DM, Luu P, Frishkoff G, Quiring J, Poulsen C. Frontolimbic response to negative feedback in clinical depression. Journal of Abnormal Psychology. 2003;112:667–678. doi: 10.1037/0021-843X.112.4.667. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Daleiden EL, Williams LL, Brown LM. Biased attention in childhood anxiety disorders: a preliminary study. Journal of Abnormal Psychology. 1995;23:267–279. doi: 10.1007/BF01447092. [DOI] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, Somerville LH, McLean AA, Kim H. Functional neuroimaging studies of the amygdala in depression. Seminars in Clinical Neuropsychiatry. 2002;7:234–242. doi: 10.1053/scnp.2002.35219. [DOI] [PubMed] [Google Scholar]
- Wills TA, Vaccaro D, McNamara G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: an application of Cloninger’s theory. Journal of Substance Abuse. 1994;6:1–20. doi: 10.1016/s0899-3289(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bauco P, Carlezon WA, Jr., Trojniar W. Self-stimulation and drug reward mechanisms. Annals of the New York Academy of Sciences. 1992;654:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Low doses of accumbens dopamine modulate amygdala suppression of spontaneous exploratory activity in rats. Brain Research. 1989;477:202–210. doi: 10.1016/0006-8993(89)91408-x. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research. Brain Research Reviews. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]


