Abstract
Adolescence is characterized by increased risk-taking and sensation-seeking, presumably brought about by developmental changes within reward-mediating brain circuits. A better understanding of the neural mechanisms underlying reward-seeking during adolescence can have critical implications for the development of strategies to enhance adolescent performance in potentially dangerous situations. Yet little research has investigated the influence of age on the modulation of behavior by incentives with neuroscience-based methods. A monetary reward antisaccade task (the RST) was used with 23 healthy adolescents and 30 healthy adults. Performance accuracy, latency and peak velocity of saccade responses (prosaccades and antisaccades) were analyzed. Performance accuracy across all groups was improved by incentives (obtain reward, avoid punishment) for both, prosaccades and antisaccades. However, modulation of antisaccade errors (direction errors) by incentives differed between groups: adolescents modulated saccade latency and peak velocity depending on contingencies, with incentives aligning their performance to that of adults; adults did not show a modulation by incentives. These findings suggest that incentives modulate a global measure of performance (percent direction errors) in adults and adolescents, and exert a more powerful influence on the control of incorrect motor responses in adolescents than in adults. These findings suggest that this task can be used in neuroimaging studies as a probe of the influence of incentives on cognitive control from a developmental perspective as well as in health and disease.
Keywords: Eyemovement, Cognitive control, Sensation seeking
Introduction
Adolescents show more risky behaviors compared to adults or children, especially when faced with situations holding some promise for instant satisfaction (Arnett 1992; Spear 2000). The negative consequences of such behaviors have inspired a wealth of research from various disciplines investigating the intrinsic (neural, genetic, psychological) and extrinsic (environmental) determinants of adolescent-like behavioral propensities. This research points toward an important role of developmental changes in reward-related function in mediating adolescent risk-taking proclivity (Spear 2000; Chambers et al. 2003; Bjork et al. 2004; May et al. 2004; Ernst et al. 2005).
Behaviorally, developmental changes in reward-related function during adolescence are reflected in enhanced sensitivity to novel and rewarding stimuli, and reduced sensitivity to aversive stimuli (Arnett 1992). At the brain level, structural and functional developmental changes in brain areas mediating reward-related behavior follow the trajectory of these behavioral alterations. Most striking is the extent of synaptic pruning and GABA-related reorganization occurring in the prefrontal cortex of adolescents (Huttenlocher 1979; Giedd et al. 1999; Casey et al. 2000; Luna and Sweeney 2004; Lewis et al. 2004). Less data exist in regard to the maturation of limbic circuits during adolescence, although a number of findings support the notion of a unique pattern of limbic function throughout this developmental period (Sowell and Jernigan 1998; Giedd et al. 1996; Yurgelun-Todd et al. 2003; Monk et al. 2003; Bjork et al. 2004; Ernst et al. 2005).
To address the behavioral and neural nature of these distinct maturational changes in adolescence, we developed a saccade task (the Reward Saccade Task; RST) in which performance is linked to a reward-schedule (Jazbec et al. 2005). The RST includes the mixed presentation of prosaccades (reflexively guided eye movement toward a suddenly appearing visual target) and antisaccades (eye movement toward the mirror position of a suddenly appearing target). Performance on this task requires the integrity of mechanisms supporting attention and, in the case of antisaccades, cognitive control (inhibition of the reflexive prosaccade to the target and programming of a saccade in absence of visual input).
Saccade tasks are uniquely well suited for study of the influence of reward-related changes on cognitive processes and their neural underpinnings during development for several reasons: (1) The sensory modality for the input and output processes is the same (visual), thus allowing for tight control of the operations occurring between input and output. (2) The neural mechanisms underlying saccadic eye movements have been exquisitely defined in non-human primates (Munoz and Everling 2004), providing a superb tool for translational work, and in humans by means of functional imaging (e.g., Rosano et al. 2002; Matsuda et al. 2004) and lesion studies (Gaymard et al. 1998). (3) Several saccadic eye movement paradigms, such as the prosaccade or antisaccade task paradigm, have been developed that allow separate examination of different cognitive processes engaged during eye movement control and their allocation to distinct neural circuits (Broerse et al. 2001; Leigh and Kennard 2004). (4) These eye movement paradigms have been used extensively to characterize psychopathology in adults (Trillenberg et al. 2004; Broerse et al. 2001; Sweeney et al. 2002; Everling and Fischer 1998) and children (Sweeney et al. 2004), and normal development in humans (e.g., Abel et al. 1983; Fischer et al. 1997a; Munoz et al. 1998; Fukushima et al. 2000; Klein and Foerster 2001; Luna et al. 2001; Klein et al. 2003). For example, developmental findings in antisaccade performance indicate shorter latency and enhanced accuracy with age, but no changes in peak velocity of antisaccades with age. (5) Finally, studies of reward processes using saccadic eye movements have already been conducted in non-human primates (e.g., Takikawa et al. 2002; Kawagoe et al. 1998; Amador et al. 2000) and humans (Duka and Lupp 1997; Jazbec et al. 2005), providing a solid basis for forming hypotheses and interpreting findings. These studies have shown that incentive manipulation does influence saccade performance parameters: In non-human primates, saccades to a rewarded location are initiated earlier (shorter latencies) and have faster peak velocities (Kawagoe et al. 1998); in humans, the number of correct antisaccades increases with incentives in adults (Duka and Lupp 1997) and adolescents (Jazbec et al. 2005). In our previous work with the RST, where prosaccades and antisaccades are presented in conjunction with incentives, reward also influenced dynamic performance parameters (latency and peak velocity) in healthy adolescents and adolescents with mood- and anxiety disorders.
However, despite this large body of research, to our knowledge no work has yet investigated the influence of age on the modulation of saccadic eye movements by incentives. The aim of the present study is to fill this gap. Here, we test the following hypotheses: (1) Incentives will improve performance on the RST (i.e., greater accuracy and shorter latencies in both adults and adolescents); (2) Adolescents will perform worse than adults, particularly during antisaccade trials which require fully mature inhibitory processes; and (3) the influence of incentives is stronger in adolescents than in adults, reducing the gap between adult and adolescent performance.
The directional effect of incentives on the metrics of direction errors in antisaccade trials is difficult to predict because of the various processes underlying these events (e.g., failure to inhibit a prepotent response, and/or failure to internally generate a goal-directed action). However, we predicted that any potential changes seen in adults would be accentuated in adolescents because of their higher sensitivity to incentives.
Methods
Participants
The sample consisted of 23 healthy adolescents (age: 15.7 ± 1.4 years, gender: 11 male, 12 female) and 30 healthy adults (age: 27.9 ± 5.7 years; gender: 17 male, 13 female). The Institutional Review Board of the National Institute of Mental Health approved the study. Adult volunteers and parents gave written informed consent, and adolescents gave written assent prior to participation, after the study was fully explained and all questions answered. Subjects were recruited through advertisements in local newspapers and word of mouth.
Inclusion and exclusion criteria were age between 13 and 18 years for adolescents and 19 and 40 years for adults, absence of acute or chronic medical problems, and of current or past psychiatric disorders. All participants were evaluated through semi-structured psychiatric interviews using the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS-PL) and the Structured Clinical Interview for DSM-IV (SCID). These evaluations were performed by experienced clinicians who each had demonstrated acceptable inter-rater reliability (κ > 0.75) for all relevant diagnoses. Reliability was ascertained based on scoring of videotaped interviews that senior investigators had performed (Kaufman et al. 1997). Other exclusion criteria comprised mental retardation (IQ < 70), use of any medication, and pregnancy. All participants were tested for IQ prior to entering the study with the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1999).
Procedures
Recordings were obtained in a room lit by standard overhead fluorescent lights. Following initial eye calibration,eye movements were measured with high-resolution infrared oculography (Applied Science Laboratories [ASL] Model 504, Boston). Calibration was repeated between runs as needed. Prior to performing the task, subjects were thoroughly trained to prevent any learning effect. They also were debriefed after the completion of the task.
Task
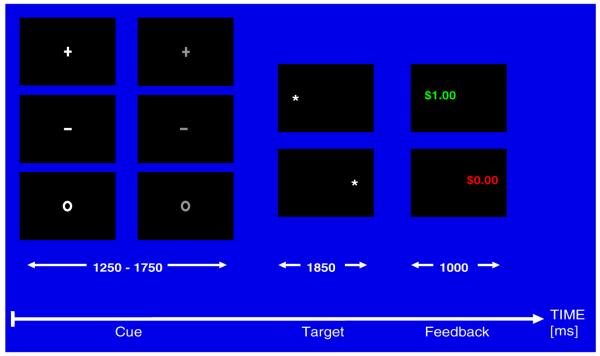
The task assessed eye movement responses in three contingency contexts: potential monetary gain (reward condition), potential monetary loss (punishment condition) and no incentive (neutral condition). It comprised three phases: (1) the initial cue phase (1,250-1,750 ms), which informed the subject about the type of trial (prosaccade or antisaccade; reward, punishment, or neutral); (2) the target phase or saccade phase (1,850 ms); (3) and the feedback phase (1,000 ms) (Fig. 1).
Fig. 1.
Reward Saccade Task paradigm: A cue (1,250 to 1,750 ms duration) is presented at the onset of each trial. The cue indicates the type of trial (gray for antisaccade and white for prosaccade) and the incentive condition of the trial (‘o’ = neutral, ‘ + ’ = gain, and ‘-’ = loss). As the cue disappears, a target appears on the right or left side of the screen (1,850 ms duration), until the feedback appears for 1,000 ms (only prosaccade feedback is shown in the Figure). As the feedback disappears, the next trial starts with appearance of the central fixation cue. In the illustration above, we represented prosaccade examples, where the feedback is presented at the location of the target
Each trial started with one of 6 cues displayed at the center of a black computer screen, and subtending approximately 0.5° visual angle. The cues included a plus sign (“ + ”), a minus sign (“-”), or a small circle (“o”), presented in either white or gray. The color of the cue indicated which type of eye movement was required in response to the subsequent appearance of the target: White cues signaled a prosaccade (i.e., an eye movement towards the target), and gray cues signaled an antisaccade (i.e., an eye movement to the mirror position of the target). The shape of the cue indicated the valence of the trial: a plus (+) sign meant a $1.00 monetary gain for a correct eye movement, or no gain for an incorrect eye movement (reward condition); a minus sign (-) meant a $1.00 monetary loss for an incorrect eye movement, or no loss for a correct eye movement (punishment condition); and a circle (o) meant the absence of monetary incentive (neutral condition).
After a variable period of 1,250-1,750 ms, the central cue was replaced by a lateral white target stimulus. The target, a white asterix subtending 0.5° visual angle, appeared at approximately 6.15° eccentricity on the horizontal meridian either to the left or the right of the centrally located cue position. To succeed on a trial, subjects had to fixate for at least 100 ms an area of 60 pixels radius around the correct location within 500 ms after target appearance. Subjects were asked to maintain fixation until they received feedback. Feedback (1,000 ms) was presented 1,850 ms after target onset, and subtended approximately 1.8° visual angle. Feedback consisted of dollar amounts (+ $1.00, - $1.00, $0.00) presented in green font for a correct response and red font for an incorrect response. The feedback appeared at the location where the subject was supposed to have gazed, replacing the target in the prosaccade trials, or appearing in the mirror location of the target in the antisaccade trials. In contrast to other tasks reported in the literature (e.g., Luna et al. 2001), only two target locations were used. While the restriction to two locations may have made the task slightly easier to perform, this facilitatory effect was most likely mitigated by the randomly distributed presentation of both prosaccades and antisaccades.
The task consisted of three runs of 4 min duration each. Each run comprised 48 trials, with four trials per side (right, left) and condition (antisaccade-reward, antisaccade-punishment, antisaccade-neutral, prosaccade-reward, prosaccade-punishment, and prosaccade-neutral). The task included a total of 144 trials (24 trials per condition). Subjects started with $0.00 and could win up to $48.00 per run. Adolescents won on average $24.8 ± 11.5, and adults won on average $32.4 ± 8.0. Participants were told that they would receive the dollar amount they won prior to performing the task, and were sent a check at the completion of the study.
Eye movement recording
Eye movements were measured with an ASL Model 504 eye tracker with remote pan/tilt optics, auto-focusing lens, and magnetic head tracker. This eye tracking system uses a corneal reflection method with bright pupil technology: the point-of-gaze is determined by relating the corneal reflection of a near infrared beam that is projected to the eye, to the center of the illuminated pupil rotating with each eye movement. Spatial accuracy of the eye tracker is 0.25° visual angle. The range within which valid data can be obtained is 50° (± 25°) horizontally and 35° (+ 25° to -10°) vertically. Sampling rate is 60 Hz.
Use of a magnetic head tracker and an auto-focusing lens minimized the possibility of artifacts due to head movements. Nevertheless, participants were instructed to remain still, and a chin rest was employed when necessary or desired by the subject. Differences in eye-screen distance emerging across subjects were corrected for in the off-line analysis of the raw data. The average distance to the screen was 66.2 ± 5.5 cm.
Eye movement analysis
The raw data were analyzed off-line with EYENAL software provided by ASL.
Saccadic eye movement parameters
The saccadic eye movement was characterized by a standard set of parameters including latency and peak velocity of the first saccade after target onset. EYENAL bases its calculation of performance parameters on the identification of stationary gazes, so called fixations. A fixation was defined as occurring when at least six consecutive data samples occur within a radius of less than .5°. The onset of a fixation was marked by the first sample point in the series of six or more to meet these criteria. The offset was marked by the last sample point in the series of six or more to meet these criteria. Assuming that a saccade is the event between two consecutive fixations, saccades were estimated from offset of the first gaze following target onset to the onset of the second gaze following target onset. Saccade latency was defined as the elapsed time period in ms between the onset of the target and the onset of the first saccade after target onset. In other words, saccade onset was defined by the end of a fixation (gaze offset), and saccade offset by the beginning of a fixation (gaze onset).
To reduce the possibility of including eye movements that do not qualify as saccades, we used the following criteria for inclusion in the analysis. (1) Saccades had to have a latency between 80 ms and 700 ms. Saccades with a latency of less than 80 ms are commonly considered to be anticipatory responses (e.g., Fischer and Weber 1992), whereas saccades with a latency of 700 ms or longer can be considered to be delayed responses (Klein et al. 2003). (2) Saccade durations had to be between 25 ms and 100 ms, and amplitude had to be greater than 3°, which decreased the risk of including other types of eye movements, such as square wave jerks.
Finally, we used the following strategy to extrapolate saccade metrics (i.e., peak velocity). Saccade amplitude was defined as the spatial distance between two consecutive fixations. A mathematical relationship between saccade amplitude and duration has been found for saccades of small to medium size ( < 10°) (Carpenter 1988), making it possible to use measures of amplitude to determine saccade duration (Joos et al. 2003). Furthermore, based on the notion of symmetrical velocity profile of small to medium size saccades, peak velocity was defied as occurring at around saccade mid-duration (Takagi et al. 1993). Accordingly, peak velocity was estimated as saccade amplitude divided by half saccade duration (saccade amplitude[vis ang°]/ 0.5 × saccade duration[s]). As a caveat, in contrast to prosaccades which are reflexive, highly stereotypical movements, antisaccades have variable durations and velocities (Hallett and Adams 1980), and may not comply with the notion of symmetric velocity profiles. This variability could introduce some error in our estimation of antisaccade peak velocities. As long as this error is not systematic in a given direction, or differs as a function of age, it would not lead to false positive findings. However, it could generate false negative findings, and may prevent us from detecting significant effects.
Analysis
Prosaccade and antisaccade trials were analyzed separately, since they engage different processes of motor control/execution and underlying neural substrates (Leigh and Kennard 2004). Trials were further stratified by correct and incorrect responses. Correct trials where defined as those trials in which the first saccade after target onset went to the correct side of the screen (to the target in case of prosaccades, to the mirror location of the target in case of antisaccades), and incorrect trials when the first saccade after target onset went to the incorrect side (away from the target in case of prosaccades, toward the target in case of antisaccades). A total of six elemental conditions were thus analyzed per accuracy: prosaccade-reward, prosaccade-punishment, prosaccade-neutral, antisaccade-reward, antisaccade-punishment, antisaccade-neutral. Each of these conditions comprised 24 trials.
Trials were analyzed with repeated measures ANOVAs with GROUP (adults vs. adolescents) as the between-subject factor and CONTINGENCY (reward, punishment, or neutral condition) as the within-subject factor. Post-hoc analyses of simple effects were conducted to clarify the nature of significant ANOVAs results: For significant main effects of GROUP, post-hoc independent t tests were performed. For significant main effects of CONTIGENCY, paired samples t tests were performed. For significant GROUP-by-CONTIGENCY interactions, independent samples t tests and paired samples t-tests per group were performed.
Results
Of the total of 24 trials per condition, an average of 23.19 ± 1.56 responses (96.62% ± 6.52) were recorded after target onset, with no difference between adults and adolescents (adults: 96.55% ± 7.01, adolescents: 96.71% ± 5.37; t(1,51) = 0.09, P = 0. 928, and no differences among conditions (antisaccade punishment: 96.15% ± 7.39, antisaccade reward: 95.60% ± 7.43; antisaccade neutral: 96.78% ± 6.20; prosaccade punishment: 97.01% ± 6.35; prosaccade reward: 97.56% ± 5.32 prosaccade neutral 96.62% ± 6.44). Thus, on average 0.81 ± 1.56 responses (3.38 % ± 6.52) were not detected by the eyetracker. This loss of data was due to blinking or to the eye camera losing the pupillary signal.
Of all recorded responses, 3.73% ± 5.46 responses were anticipatory (latency of 0-80 ms) and were not included in the analysis.
Prosaccades
Accuracy
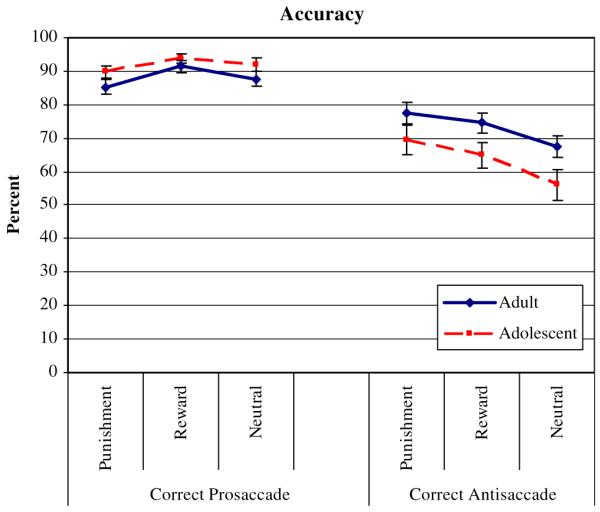
Mean percent of correct prosaccades across conditions did not differ between adults (88.1 ± 1.5) and adolescents (91.9 ± 8.1) (GROUP, F(1,51) = 2.44, P = 0.125, (Fig. 2; Table 1). Due to the low number of prosaccade errors, only correct prosaccades were analyzed.
Fig. 2.
Means (standard errors) of percent saccades in adults (n = 30) and adolescents (n = 23)
Table 1.
Mean (SD) accuracy percent by group and incentive
| Correct prossaccadesa |
Correct antisaccadesa,b |
|||
|---|---|---|---|---|
| Adult | Adolescent | Adult | Adolescent | |
| Reward | 91.39 (10.20) | 93.84 (06.14) | 74.58 (16.16) | 65.04 (18.28) |
| Punishment | 85.28 (12.41) | 89.86 (08.51) | 77.50 (17.69) | 69.38 (20.93) |
| Neutral | 87.64 (12.01) | 92.03 (09.56) | 67.39 (17.81) | 56.16 (22.43) |
Main effect of group, P < 0.05
Main effect of contingency, P < 0.001
There was a main effect of CONTIGENCY for percent of correct prosaccades: F(2,102) = 7.95, P = 0.001 (Fig. 2): Mean percent of correct responses was significantly greater in reward trials than in punishment trials, t(1,52) = 3.56, P < 0.001, and neutral trials, t(1,52) = 2.69, P = 0.005. Moreover, it was also higher in neutral trials than in the punishment trials, t(1,52) = 1.93, P = 0.029 (Table 1).
Latency
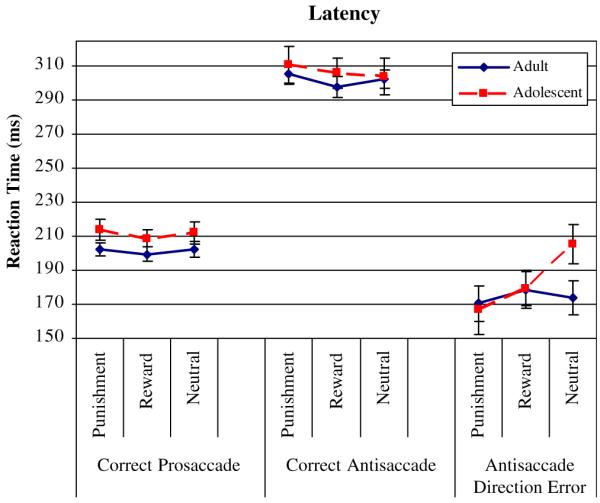
Main effects of GROUP and CONTINGENCY, and interaction of GROUP-by-CONTINGENCY on latency of correct prosaccades were not significant (Fig. 3; Table 2).
Fig. 3.
Means (standard errors) of saccade latency in adults (n = 30) and adolescents (n = 23)
Table 2.
Mean (SD) latency (ms) by group and incentive
| Correct prossaccades |
Correct antisaccades |
Direction errorsa |
||||
|---|---|---|---|---|---|---|
| Adult | Adolescent | Adult | Adolescent | Adult | Adolescent | |
| Reward | 199.55 (23.77) | 208.75 (23.36) | 298.16 (32.72) | 306.45 (42.51) | 178.85 (56.22) | 179.46 (46.07) |
| Punishment | 202.42 (22.37) | 214.00 (28.76) | 305.46 (31.14) | 311.25 (52.01) | 170.51 (51.65) | 166.58 (62.76) |
| Neutral | 202.39 (24.90) | 212.07 (321.67) | 302.86 (29.02) | 304.43 (51.52) | 173.99 (52.87) | 205.41 (52.87) |
Contingency by group interaction, P = 0.054
Peak velocity
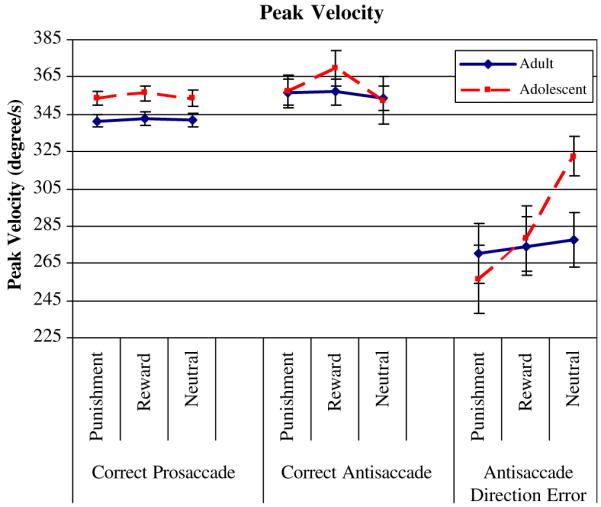
Peak velocity was significantly greater in adolescents than in adults (GROUP, F(1,51) = 6.55; P = 0.013, Fig. 4; Table 3). Post-hoc independent samples t tests indicated that this difference was significant in each contingency condition (punishment, t(1,51) = 2.57, P = 0.007; reward, t(1,51) = 2.56, P = 0.007; neutral, t(1,51) = 2.16; P = 0.018). However, there was no significant main effect of CONTINGENCY or CONTINGENCY-by-GROUP interaction (Table 3).
Fig. 4.
Means (standard errors) of saccade peak velocity in adults (n = 30) and adolescents(n = 23)
Table 3.
Mean (SD) peak velocity (deg/s) by group and incentive
| Correct prossaccadesa |
Correct antisaccadesb |
Direction errorsa,c |
||||
|---|---|---|---|---|---|---|
| Adult | Adolescent | Adult | Adolescent | Adult | Adolescent | |
| Reward | 342.56 (18.49) | 356.17 (19.97) | 356.73 (37.34) | 369.73 (44.98) | 274.29 (76.47) | 278.35 (82.02) |
| Punishment | 341.26 (17.79) | 353.86 (17.61) | 356.95 (37.51) | 357.14 (40.89) | 270.21 (79.36) | 256.71 (81.67) |
| Neutral | 341.92 (18.62) | 353.43 (20.01) | 353.64 (36.40) | 352.29 (62.05) | 277.68 (79.38) | 322.50 (49.02) |
Main effect of contingency, P < 0.05
Main effect of group, P < 0.05
Contingency by group interaction, P <0.05
Antisaccades
Correct antisaccades
Accuracy
Mean percent of correct antisaccades was significantly higher in adults (17.56 ± 4.13) than in adolescents (15.25 ± 4.93) across conditions (GROUP, F(1,51) = 4.40, P = 0.041). This GROUP difference was not influenced by the type of condition (no interaction effect of GROUP-by-CONTINGENCY). However, there was a main effect of CONTINGENCY, F(2,102) = 15.82, P < 0.001. Post-hoc paired t tests indicated that both groups made more correct antisaccades during the reward and the punishment condition than during the neutral condition (reward vs. neutral, t(1,52) = 3.43, P = 0.001; punishment vs. neutral: t(1,52) = 5.57, P < 0.001. Among the two contingent conditions, groups performed significantly better on punishment than on reward trials, t(1,52) = 1.88, P = 0.033.
Latency
Main effects of GROUP and CONTINGENCY, and interaction of GROUP-by-CONTINGENCY on latency of correct antisaccades were not significant (Table 2; Fig. 3).
Peak velocity
The two groups did not differ in peak velocity of correct antisaccades. However, there was a main effect of CONTINGENCY, F(2,102) = 4.53, P = 0.013: Peak velocities were faster during reward trials t(1,52) = 2.38, P = 0.010, and punishment trials t(1,52) = 2.23, P = 0.015 compared to neutral, but were not different between punishment and neutral trials, t(1,52) = 1.01, P = 0.159 (Table 3; Fig. 3).
Direction errors
Latency
Main effects of GROUP and CONTINGENCY were not significant. However, the CONTINGENCY-by-GROUP interaction showed a statistical trend F(2,80) = 3.03, P = 0.054, suggesting that whereas adolescents modulated latency of incorrect antisaccades in response to the reward manipulation, adults did not. In particular, adolescents had significantly longer saccade latencies during neutral trials than during punishment trials t(1,22) = 2.99, P = 0.004, and reward trials t(1,22) = 1.78, P = 0.045 (Table 2; Fig. 3). Independent samples t tests indicated that the two groups differed in saccade latency during the neutral condition. Adolescents had significantly longer saccade latencies during incorrect neutral antisaccades than adults t(1,49) = 2.10, P = 0.020 (Table 2).
Peak velocity
CONTINGENCY had a significant main effect, F(2,80) = 4.27, P = 0.017 and the CONTINGENCY-by-GROUP interaction was statistically significant, F(2,80) = 3.77, P = 0.027 (Fig. 4; Table 3). Relative to the neutral condition, incentives increased peak velocity in adolescents (punishment vs. neutral: t(1,19) = 3.82, P = 0.001; reward vs. neutral t(1,21) = 2.33, P = 0.015) (Fig. 4; Table 3). Of note, adolescents and adults differed significantly during the neutral condition (higher peak velocity in adolescents than in adults, t(1,49) = 2.33, P = 0.024, but not during the incentive conditions, suggesting that incentives permit adolescents to align their performance to the adult performance level (Fig. 4).
Discussion
The current study investigated developmental differences in task performance (accuracy, latency and peak velocity) on the reward saccade task (RST). As predicted, contingencies influenced performance on the RST and this influence differed in adolescents and adults. The key findings are three-fold: (1) Incentives improved accuracy performance on the task in both adults and adolescents. (2) Accuracy was superior in adults than in adolescents. (3) Adolescents and adults showed different reward-related modulation of dynamic saccadic characteristics, selectively in the context of incorrect responses (direction errors in antisaccade trials): incentives modulated response parameters in adolescents but not in adults. With incentives, adolescents aligned their performance to the adult level. This finding suggests that the preparation (saccade latency) and execution (saccade peak velocity) of an incorrect motor action can be influenced by the context of potential incentive in adolescents, whereas performance in adults may have reached a ceiling that cannot be modulated by context. This adds to the existing body of knowledge identifying developmental trajectories of cognitive processes that parallel maturational changes in brain function (see review, Casey et al. 2000) by providing evidence of adolescent capacity for competent cognitive control under conditions of enhanced motivation.
Saccadic eye movements across contingencies: adolescents versus adults
Saccadic eye movements require the integrity of (1) visual-spatial sensory processing, (2) sustained orienting, and attention shifting, (3) motor control and execution. Prosaccades, which are externally and visually guided reflexive movements, rely principally on attention and motor processes. Antisaccades, which are internally guided, intentional voluntary movements rely, in addition to the same processes engaged in prosaccades (attention and motor systems), on inhibitory control processes. The structure of the RST, which consists of the randomly mixed presentation of prosaccades and antisaccades, introduces an additional cognitive load in the form of working memory (remember the significance of the cue, i.e., gray for antisaccades and white for prosaccades). However, the use of only two target positions compared to multiple positions often used in antisaccade tasks in the literature (for example Luna et al. 2001) may mitigate the increased difficulty related to the additional working memory component.
Accuracy on prosaccade trials was close to perfect in both adolescents and adults and, as was saccade latency, similar between groups. The few prosaccade errors made by both adults and adolescents were likely related to task switching effects that resulted from the mixed pro and antisaccade structure of the task. In line with other developmental research showing adult level performance on prosaccades by age 10-12 (Fukushima et al. 2000; Klein and Foerster 2001; Munoz et al. 1998) our finding suggests that the overall efficiency of visually guided reflexive eye movements has reached maturity by adolescence. It also suggests that the additional cognitive load of working memory did not differentially influence adolescents and adults during preparation and initiation of prosaccades.
Interestingly however, groups differed in peak velocity of prosaccades, with adolescents showing significantly faster peak velocities than adults. This finding contrasts with other work showing no age-related differences in prosaccade peak velocity (Munoz et al. 1998; Fukushima et al. 2000). This discrepancy between studies could reflect differences in the definition of saccades. Whereas we defined saccades as the events occurring between two fixations, it is more typical to characterize saccades by changes in velocity of eye movements. Our less stringent definition does not allow us to rule out the inclusion of eye movements that do not qualify as saccades, such as square wave jerks. However, we minimized this possibility by excluding all events that did not fit saccade parameters and requiring saccades to meet specific latency, amplitude and duration criteria (see method section). Changes in saccade peak velocity have been observed under influence of different psychopharmacological agents such as clonidine, neuroleptics or benzodiazepines (e.g., Straube et al. 1999; Smith et al. 2003; Khan et al. 2000) and have been used as a biophysical index of alertness, and sedation during anaesthesia (Khan et al. 2000). Hence, the finding of higher prosaccade peak velocity in adolescents compared to adults may reflect enhanced arousal in adolescents relative to adults. Compared to saccade tasks previously used in children (Fukushima et al. 2000; Klein and Foerster 2001), the RST appears to be more complex and perhaps more motivating by virtue of the presence of incentives. These factors (complexity and motivation) might contribute to increase alertness and may have a greater impact on adolescents than adults.
Although adolescents were as accurate as adults on prosaccades, they were less accurate on antisaccades. Consistent with previous developmental work (Munoz et al. 1998), this dissociation in accuracy on prosaccade versus antisaccade tasks may reflect adolescent immaturity of inhibitory control systems within the prefrontal cortex (Casey et al. 2000; Luna and Sweeney 2004), since antisaccades, but not prosaccades, require intact inhibitory control.
Overall, antisaccade dynamics (latency and peak velocity) did not differ significantly between adolescents and adults. This finding partly contrasts with other developmental antisaccade research which reports slower saccade latency (but no developmental trend for peak velocity) of correct antisaccades for adolescents until age 15 years, with slight developmental improvements extending to the early 20s (Fischer et al. 1997b; Munoz et al. 1998, 2003). The lack of age-related difference in our study could reflect the relatively high mean age of the adolescent sample (15.68 ± 1.43), respective of the low age of our adult control (27.92 ± 5.71). Alternatively lack of differences could be related to the coupling of performance with incentives, suggesting improved performance in the context of contingencies in adolescents.
Influence of incentives on saccadic eye movements: main effect of contingencies and interaction of group by contingency
Overall, the presence of positive incentives (rewards) modulated accuracy of both pro- and antisaccades in both adults and adolescents. The prospect of a monetary gain improved accuracy in both types of saccades. This finding is partly consistent with the one study in adults (24 males, age 29.3 ± 6.2 years) that examined the effect of monetary incentives on saccade tasks (Duka and Lupp 1997). This study reported that monetary reward improved accuracy of antisaccades, but without affecting antisaccade dynamics or prosaccade performance. The monetary reward was a global “honorarium” at the end of testing for a “particularly good performance”, which may act differently than trial-by-trial incentives as used in the present study.
Incentives also influenced saccade dynamics, but during antisaccades, and only in adolescents. The most interesting pattern appeared in direction errors of the antisaccade trials (errant prosaccades). Specifically, adolescents showed significantly longer latencies and higher peak velocities than adults in the absence of incentives. However, in the presence of incentives (both the reward and punishment conditions), adolescent performance became undistinguishable from adult performance. This pattern signaled the adolescents’ capacity to modulate the control of preparation and execution of an erroneous action under incentives, which pushed their performance to the adult level.
Longer latencies during a direction error may indicate poorer capacity to inhibit the incorrect saccade, since, even with longer preparation time, an errant action is initiated. Similarly, lower peak velocities reflect smaller saccades, and in the context of an errant action, can reflect an attempt to inhibit the already initiated erroneous action. A better understanding of the neural mechanisms underlying this incentive-related effect can have critical implications for the development of neurobiologically based strategies to enhance adolescent inhibitory control. Examples for which such strategies could be useful include the control of potentially dangerous situations such as driving (particularly during the adolescent period), the prevention and treatment of conditions associated with impaired inhibitory control (such as attention-deficit/hyperactivity disorder) or impaired reward systems (such as depression).
As previously mentioned, interpretation of the present findings should be moderated by methodological limitations. First, the eye-tracking device had a relatively low sampling rate of 60 Hz. Thus, measurement error was ± 8 ms, and may have prevented us from detecting differences between groups or conditions. We plan to replicate this work using a higher sampling rate. Second, the measures of the saccade parameters were extrapolated from fixation periods identified by the eye-tracker (see Methods). A saccade was defined by the offset of the first fixation (after target appearance) and onset of the subsequent fixation. Although this method provided measures that were consistent with the literature, it may have introduced variability that was not directly related to saccadic movements per se. For example, saccade latencies and peak velocities could potentially be inflated because of the erroneous inclusion of eye movements that did not qualify as saccades (e.g., square wave jerks).
In conclusion, in addition to replicating maturational changes in performance on a task requiring intact inhibitory processes, this study demonstrated that incentives modulate a global measure of performance (accuracy) in adults and adolescents, and exert a more powerful influence on the control of incorrect motor responses in adolescents than in adults. These findings suggest that this task can be used in future neuroimaging studies to probe the influence of incentives on motor control, particularly inhibitory control, across development.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health. The authors thank Harvey Iwamoto for technical support.
References
- Abel LA, Troost BT, Dell’Osso LF. The effects of age on normal saccadic characteristics and their variability. Vision Res. 1983;23:33–37. doi: 10.1016/0042-6989(83)90038-x. [DOI] [PubMed] [Google Scholar]
- Amador N, Schlag-Rey M, Schlag J. Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. J Neurophysiol. 2000;84:2166–2170. doi: 10.1152/jn.2000.84.4.2166. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Reckless behavior in adolescence: a developmental perspective. Dev Rev. 1992;12:339–373. [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broerse A, Crawford TJ, den Boer JA. Parsing cognition in schizophrenia using saccadic eye movements: a selective overview. Neuropsychologia. 2001;39:742–756. doi: 10.1016/s0028-3932(00)00155-x. [DOI] [PubMed] [Google Scholar]
- Carpenter RHS. Movement of the eyes. 2 ed. Pion; London: 1988. [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Tylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Lupp A. The effects of incentive on antisaccades: is a dopaminergic mechanism involved? Behav Pharmacol. 1997;8:373–382. [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H. Characteristics of “anti” saccades in man. Exp Brain Res. 1992;89:415–424. doi: 10.1007/BF00228257. [DOI] [PubMed] [Google Scholar]
- Fischer B, Biscaldi M, Gezeck S. On the development of voluntary and reflexive components in human saccade generation. Brain Res. 1997a;754:285–297. doi: 10.1016/s0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- Fischer B, Gezeck S, Hartnegg K. The analysis of saccadic eye movements from gap and overlap paradigms. Brain Res Brain Res Protoc. 1997b;2:47–52. doi: 10.1016/s1385-299x(97)00027-5. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Hatta T, Fukushima K. Development of voluntary control of saccadic eye movements. I. Age-related changes in normal children. Brain Dev. 2000;22:173–180. doi: 10.1016/s0387-7604(00)00101-7. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud S, Vermersch AI, Pierrot-Deseilligny C. Cortical control of saccades. Exp Brain Res. 1998;123:159–163. doi: 10.1007/s002210050557. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Hallett PE, Adams BD. The predictability of saccadic latency in a novel voluntary oculomotor task. Vision Res. 1980;20(4):329–339. doi: 10.1016/0042-6989(80)90019-x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biol Psychiatry. 2005;58(8):632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Joos M, Rötting M, Velichkovsky BM. Die Bewegungen des menschlichen Auges: Fakten, Methoden, innovative Anwendungen. In: Rickheit G, Herrmann T, Deutsch W, editors. Psycholinguistik / Psycholinguistics Ein internationales Handbuch / An International Handbook. de Gruyter; Berlin New York: 2003. pp. 142–168. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and Schizophrenia for school-age children- present and lifetime version (K-SADS-PL) initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nat Neurosci. 1998;1:411–416. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- Khan OA, Taylor SR, Jones JG. Anaesthesia and saccadic eye movements. Anaesthesia. 2000;55:877–882. doi: 10.1046/j.1365-2044.2000.01529.x. [DOI] [PubMed] [Google Scholar]
- Klein C, Foerster F. Development of prosaccade and antisaccade task performance in participants aged 6 to 26 years. Psychophysiology. 2001;38:179–189. [PubMed] [Google Scholar]
- Klein CH, Raschke A, Brandenbusch A. Development of pro- and antisaccades in children with attention-deficit hyperactivity disorder (ADHD) and healthy controls. Psychophysiology. 2003;40:17–28. doi: 10.1111/1469-8986.00003. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Kennard C. Using saccades as a research tool in the clinical neurosciences. Brain. 2004;127:460–477. doi: 10.1093/brain/awh035. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz D, Eggan S, Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann N Y Acad Sci. 2004;1021:64–76. doi: 10.1196/annals.1308.008. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Matsuura M, Ohkubo T, Ohkubo H, Matsushima E, Inoue K, et al. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: cortical and subcortical networks. Psychiatry Res. 2004;131:147–155. doi: 10.1016/j.pscychresns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, et al. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the antisaccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Armstrong IT, Hampton KA, Moore KD. Altered control of visual Wxation and saccadic eye movements in attention-deffcit hyperactivity disorder. J Neurophysiol. 2003;90:503–514. doi: 10.1152/jn.00192.2003. [DOI] [PubMed] [Google Scholar]
- Rosano C, Krisky CM, Welling JS, Eddy WF, Luna B, Thulborn KR, et al. Pursuit and saccadic eye movement subregions in human frontal eye field: a high-resolution fMRI investigation. Cereb Cortex. 2002;12:107–115. doi: 10.1093/cercor/12.2.107. [DOI] [PubMed] [Google Scholar]
- Smith A, Brice C, Nash J, Rich N, Nutt DJ. Caffeine and central noradrenaline: effects on mood, cognitive performance, eye movements and cardiovascular function. J Psychopharmacol. 2003;17:283–292. doi: 10.1177/02698811030173010. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Jernigan TL. Further MRI evidence of late brain maturation: Limbic volume increases and changing asymmetries during childhood and adolescence. Dev Neuropsychol. 1998;14(4):599–617. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Straube A, Riedel M, Eggert T, Muller N. Internally and externally guided voluntary saccades in unmedicated and medicated schizophrenic patients. Part I. Saccadic velocity. Eur Arch Psychiatry Clin Neurosci. 1999;249:1–6. doi: 10.1007/s004060050058. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Levy D, Harris MS. Commentary: eye movement research with clinical populations. Prog Brain Res. 2002;140:507–522. doi: 10.1016/S0079-6123(02)40072-6. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Takarae Y, Macmillan C, Luna B, Minshew NJ. Eye movements in neurodevelopmental disorders. Curr Opin Neurol. 2004;17:37–42. doi: 10.1097/00019052-200402000-00007. [DOI] [PubMed] [Google Scholar]
- Takagi M, Abe H, Hasegawa S, Yoshizawa T, Usui T. Velocity profile of human horizontal saccades. Ophthalmologica. 1993;206:169–176. doi: 10.1159/000310386. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Itoh H, Nakahara H, Hikosaka O. Modulation of saccadic eye movements by predicted reward outcome. Exp Brain Res. 2002;142:284–291. doi: 10.1007/s00221-001-0928-1. [DOI] [PubMed] [Google Scholar]
- Trillenberg P, Lencer R, Heide W. Eye movements and psychiatric disease. Curr Opin Neurol. 2004;17:43–47. doi: 10.1097/00019052-200402000-00008. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. The Psychological Corporation; San Antonio: 1999. [Google Scholar]
- Yurgelun-Todd DA, Killgore, Cintron CB. Cognitive correlates of medial temporal lobe development across adolescence: a magnetic resonance imaging study. Percept Mot Skills. 2003;96:3–17. doi: 10.2466/pms.2003.96.1.3. [DOI] [PubMed] [Google Scholar]