Abstract
Gi/o protein-coupled receptors, signaling through G protein-dependent and -independent pathways, have prominent effects on secretion by modulating calcium signaling and regulating the size of the releasable secretory pool, the rates of exocytosis and endocytosis, and de novo synthesis. Pituitary cells fire action potentials spontaneously, and the associated calcium influx is sufficient to maintain prolactin (PRL) release, but not gonadotropin release, at high and steady levels for many hours. Such secretion, termed intrinsic, spontaneous or basal, reflects fusion of secretory vesicles triggered by the cell type-specific pattern of action potentials. In lactotrophs, activation of endothelin ETA and dopamine D2 receptors causes inhibition of spontaneous electrical activity and basal adenylyl cyclase activity, accompanied with inhibition of basal PRL release. Agonist-induced inhibition of cAMP production and firing of action potentials is abolished in cells with blocked pertussis toxin (PTX)-sensitive Gi/o signaling pathway. However, agonist-induced inhibition of PRL release is only partially relieved in such treated cells, indicating that both receptors also inhibit exocytosis downstream of cAMP/calcium signaling. The PTX-insensitive step in agonist-induced inhibition of PRL release is not affected by inhibition of PI3-kinase and GSK-3, but is partially rescued by down-regulation of the Gzα expression. Thus, ETA and D2 receptors inhibit basal PRL release not only by blocking electrical activity but also by desensitizing calcium-secretion coupling.
Keywords: action potential, calcium, cAMP, GPCR, lactotrophs, gonadotrophs, dopamine D2 receptors, endothelin ETA receptors, PI3-kinase, GSK-3
INTRODUCTION
The anterior pituitary functions are carried out by five cell types, defined by the hormone they produce and secrete: corticotrophs secreting adrenocorticotrophic hormone (ACTH), thyrotrophs secreting thyroid-stimulating hormone (TSH), somatotrophs secreting growth hormone (GH), lactotrophs secreting prolactin (PRL), and gonadotrophs secreting luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Although all these cells arise from progenitors in Rathke's pouch, the embryonic primordial of the pituitary gland, they differ with respect to their secretory patterns in vivo and in vitro 1. In vivo, removal of hypothalamo-pituitary connections leads to increased release of PRL but not gonadotropins. Animals bearing ectopic pituitary grafts also release high levels of PRL and low levels of LH for a prolonged period, leading to pseudo-pregnancy. In vitro, basal (in the absence of agonists) PRL and GH secretion from pituitary fragments, dispersed pituitary cells, and immortalized lacto-somatotrophs is high, occurs in an extracellular Ca2+-dependent manner and is also termed intrinsic or spontaneous secretory activity 2. Slow spontaneous secretion from single lactotrophs could be monitored with confocal microscopy using FM4-64 loaded cells 3, 4. By contrast, basal LH secretion in vitro is low and is not affected by removal of extracellular calcium 5. Consistent with the high basal levels of PRL and GH secretion, lactotrophs and somatotrophs are under negative hypothalamic control by the Gi/o-coupled dopamine and somatostatin receptors, respectively, in addition to positive regulation by Gq and Gs-coupled receptors 6, 7. Conversely, LH secretion from gonadotrophs is under positive hypothalamic control by Ca2+-mobilizing gonadotropin-releasing hormone (GnRH) receptors 8 and only in neonatal gonadotrophs is GnRH-stimulated gonadotropin secretion inhibited by the pineal hormone melatonin 9. Here we review roles of the Gi/o-coupled dopamine D2 and endothelin ETA receptors in control of signaling and secretion in pituitary cells.
In the late 1970s, it has been shown that the dopamine D2 receptor subtype mediates the tonic inhibitory control of hypothalamic dopamine on PRL release in lactotrophs 10. Later investigations showed that two subtypes of D2 receptors, termed D2S and D2L, are generated by alternative splicing in lactotrophs and other cell types 11, 12. Lactotrophs may express a different ratio of these two receptor subtypes, depending on the level of gonadal steroids 13. In addition to lactotrophs, dopamine D2 receptors have been identified in the intermediate lobe of the pituitary gland 10. Consistent with the expression of these receptors in two pituitary cells types, the knockout D2 mice showed chronic hyperprolactinemia, pituitary hyperplasia, and a moderate decrease in MSH content 14. The pituitary dopamine receptors are functionally associated with pertussis toxin (PTX)-sensitive G proteins 15. Two intracellular messengers affected by activation of PTX-sensitive pathways in pituitary cells, Ca2+ and cAMP, play major roles in controlling the fusion of secretory vesicles with the plasma membrane to release hormones in endocrine cells 16, 17. Dopamine D2S and D2L receptors also couple to the same extent to the PTX-sensitive Gi/o protein and to the PTX-insensitive Gz proteins in vitro 18 and in vivo 19, but the relevance of this coupling in pituitary hormone release has not been evaluated. The D2 type of dopamine receptors can also exert their actions independently of G proteins by promoting the formation of a signaling protein complex composed of β-arrestin, Akt, and protein phosphatase-2A 20.
The actions of endothelins (ETs) in pituitary cells are mediated by ETA receptors (ETARs), which are selective for ET-1 and ET-2 compared to ET-3 21, 22. Functional ETARs are expressed in all five secretory cell types 23, 24. In gonadotrophs, stimulation of these receptors leads to activation of the Gq/11 signaling pathway accompanied with the oscillatory Ca2+ release from intracellular pools and gonadotropin secretion 25. The stimulatory action of these receptors on Ca2+ signaling and secretion in gonadotrophs is transient due to their rapid desensitization and internalization 21. In somatotrophs and lactotrophs, ETs also activate the Ca2+-mobilization pathway and transiently stimulate hormone release 26, 27. In contrast to gonadotrophs, the stimulatory effect of ET is followed by inhibition of PRL and GH release below the basal levels 26, 28. This inhibitory phase lasts for several hours 24, 29, arguing against rapid desensitization of these receptors in lactotrophs and somatotrophs. ETRs also signal through Gs 30. Gi/o 31, Gz 32, and G12/13 33 pathways. Like dopamine D2 receptors 12, but in contrast to over 90% of all other GPCRs 34, the ETAR is encoded by an intron-containing gene 35. Our recent studies addressed the role of novel spliced forms of these receptors in G protein-specific coupling 36.
SPONTANEOUS ELECTRICAL ACTIVITY AND BASAL HORMONE SECRETION
The membrane potential (Vm) of cultured anterior pituitary cells is not stable but oscillates from a baseline potential of about -60 mV. When Vm oscillations reach the threshold level, pituitary cells fire action potentials (APs). This is a common feature of all secretory anterior pituitary cells. However, cells differ with respect to the pattern of electrical activity and AP-driven calcium signaling and secretion in vivo and in vitro 37-39. Cultured lactotrophs and somatotrophs frequently exhibit larger Vm oscillations, on top of which the depolarizing plateau and bursts of APs are generated, with spikes that do not usually reach the reverse potential. Such complex but organized superimposition of APs is termed plateau bursting activity. In contrast, Vm oscillations in cultured gonadotrophs are small and as yet uncharacterized pacemaker activity generates single APs that are sharp and short in duration, and reverse polarity 40, 41. Consequently, they are termed single axonal type APs (Fig. 1A). Immortalized GH pituitary cells can exhibit both single AP spikes and the plateau-bursting pattern of firing 42-44.
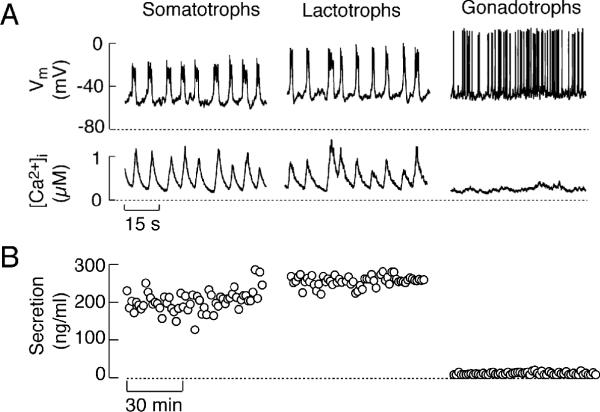
FIGURE 1.
Characterization of spontaneous firing of APs and basal hormone release in pituitary cells. (A) Simultaneous measurements of Vm and [Ca2+ m ]i in pituitary cells. (B) Basal hormone release in perifused pituitary cells. Basal secretion was normalized to account for the difference in the number of somatotrophs, lactotrophs, and gonadotrophs in mixed population of cells.
The differences in the patterns of spontaneous firing of APs among secretory pituitary cells are reflected in their respective pattern of calcium signaling, as demonstrated in experiments with simultaneous measurements of Vm, done by perforated patch clamp, and intracellular calcium concentration ([Ca2+]i), done by loading cells with the calcium indicator Indo-1. In somatotrophs and lactotrophs, slow resting Vm oscillations with superimposed bursts of APs, with an average duration of seconds, are accompanied with high amplitude calcium transients that ranges from 0.3 μM to 1.2 μM (Fig. 1A). These signals are termed global Ca2+ signals because they reflect increases in intracellular calcium concentration ([Ca2+]i) in all regions of cytosolic compartments and are accompanied with high basal GH and PRL release (Fig. 1B). Conversely, the high-amplitude, millisecond-lived single APs in spontaneously active gonadotrophs either cause no obvious changes in [Ca2+]i or generate low-amplitude signals ranging from 20 to 70 nM (Fig. 1A). Such APs generate localized Ca2+ signals below the plasma membrane, known also as calcium domains 45, 46. In parallel, basal LH release is low, at the level of detection by radioimmunoassay (Fig. 1B).
Pituitary gonadotrophs can also generate plateau-bursting type of electrical activity, but only during the activation of Ca2+-mobilizing GnRH receptors 47, 48. GnRH is associated with marked changes in the electrical activity in single gonadotrophs, including a transitory cessation of spontaneous AP firing, followed by a complex pattern of Vm oscillations in current-clamped cells. Oscillations occur over a frequency range (5-20/min) that is dependent on agonist concentration. Each cycle of Vm oscillations is initiated by rapid hyperpolarization, followed by slow depolarization that often leads to the firing of multiple APs. Such a pattern of electrical activity resembles the plateau-bursting type of Vm activity in spontaneously active somatotrophs and lactotrophs 39. At high nanomolar GnRH concentrations, a rapid and prolonged hyperpolarization of the plasma membrane is observed, and is followed by sustained oscillations in Vm 48.
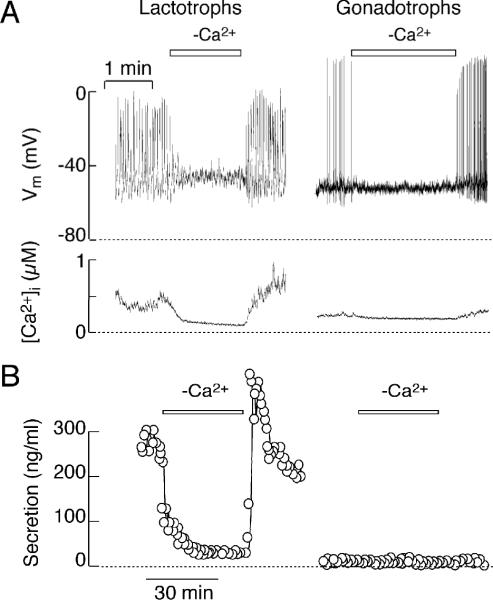
Firing of spontaneous APs in all secretory cell types depends on the presence of calcium in extracellular medium 40. Fig. 2A illustrates that a transient removal of calcium leads to abolition of spiking in both lactotrophs and gonadotrophs. In lactotrophs, but not in gonadotrophs, this was accompanied by abolition of calcium transients and basal hormone release (Fig. 2A and B). These results indicate that the prolonged duration of the AP wave form in lactotrophs accounts for the high amplitude [Ca2+]i signals and the high level of basal PRL release. Increase in the duration of the AP wave form in spontaneously active gonadotrophs induced by BayK 8644, an L-type calcium channel agonist, also generated global calcium signal in gonadotrophs and initiated LH secretion, comparable to that observed in spontaneously firing lactotrophs 40. Thus, basal pituitary hormone secretion is dependent on the duration of the AP wave form, which determines their capacity to drive calcium entry through voltage-gated calcium (Cav) channels.
FIGURE 2.
Extracellular calcium dependence of spontaneous electrical activity, calcium signaling and secretion in pituitary lactotrophs (left) and gonadotrophs (right). (A) Effects of removal of extracellular calcium on firing of APs (top) and [Ca2+]i (bottom). (B) Dependence of basal PRL release (left) but not basal LH release (right) on spontaneous electrical activity. In this and the next figure, horizontal bars indicate duration of removal of extracellular calcium.
DEPENDENCE OF DOPAMINE AND ET-1 SECRETORY ACTIONS ON PRE-STORED PRL
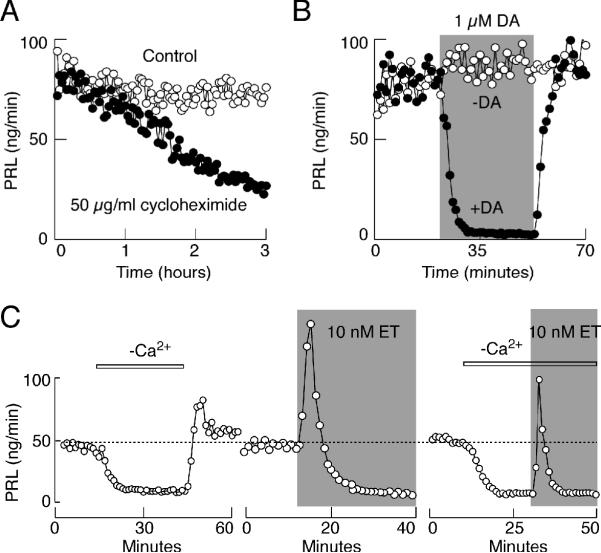
In our experiments, hormone secretion was monitored using a cell column perifusion system, with 1.2-1.5 × 107 cells per column. Prior to any treatment, cells were perifused at a flow rate of 0.8 ml/min at 37 °C for 2 h to establish stable basal secretion, followed by appropriate treatments of variable durations. Under such experimental conditions, perifusion of cells with cycloheximide, a protein synthesis inhibitor, was ineffective during the first 60 minutes of application, indicating that the pre-stored PRL predominantly accounts for AP-driven secretion during short-term experiments, whereas inhibition of de novo synthesis of hormone plays a major role in long-term experiments (Fig. 3A). To avoid contribution of de novo synthesis in secretory studies, in further experiments we focused on the short-term effects of two agonists, dopamine and ET-1, on PRL release.
FIGURE 3.
Dependence of dopamine and ET-1 actions on PRL release on pre-stored hormone. (A) Time-course of cycloheximide effects on basal PRL release. (B and C) Time course of dopamine (B) and ET-1 (C) induced inhibition of basal PRL release. Dotted line illustrates basal PRL release.
Dopamine-induced inhibition of basal PRL release in perifused pituitary cells occurred rapidly, within 6-7 minutes of application, and the washout of agonist was accompanied with the full recovery of basal secretion (Fig. 3B). Dopamine was fully effective in 1 and 0.5 μM concentrations, whereas at lower concentrations only partial inhibition was observed 49. Perifusion experiments also revealed the presence of two phases in the action of ET-1 on PRL release: a rapid and transient increase in PRL release and a sustained decrease in secretion below the basal levels (Fig. 3C, central). The level of inhibition of PRL release during the sustained agonist application was comparable to that observed in cells perifused with calcium-deficient medium (Fig. 3C, left and central). Consistent with this observation, ET-1 induced only monophasic response In cells perifused in calcium deficient medium (Fig. 3C, right). Several experiments revealed that the extracellular calcium-independent stimulatory action of ETA receptors on PRL release reflects coupling of these receptors to Gq/11 signaling pathway, leading to InsP3-mediated intracellular calcium mobilization and hormone secretion 32. Like dopamine and extracellular calcium removal, ET-1 induced inhibition of PRL release required about 5-8 minutes to reach the steady-state level (Fig. 3B and C).
INHIBITION OF PRL RELEASE IN CELLS WITH ELEVATED CYCLIC AMP
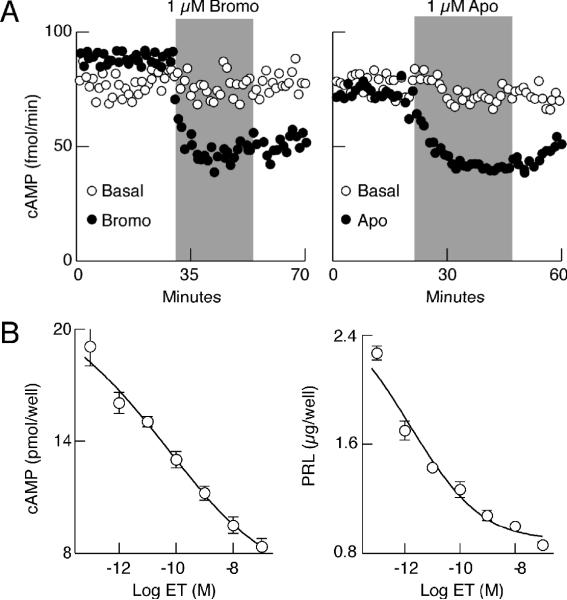
In order to study the dependence of agonist-induced inhibition of PRL release on cAMP production, we determined both PRL and cyclic nucleotide contents in samples obtained from perifusion experiments, using the appropriate radioimmunoassay techniques. Figure 4 illustrates such an experiment. In parallel to changes in the rate of PRL release, dopamine agonists bromocriptine and apomorphine inhibited cAMP release, which was sustained after their removal. This finding is consistent with the coupling of dopamine D2 receptors to Gi/o and inhibition of adenylyl cyclase (AC) activity by alpha subunits 50, 51. Endothelin also inhibited AC activity in a dose-dependent manner, with the EC50 value in a picomolar concentration range (Fig. 4B, left). Similar dose-dependence of ET-1 was observed in studies on PRL release (Fig. 4B, right). Our recent studies also revealed that inhibition of basal cAMP production in pituitary lactotrophs does not abolish spontaneous firing of APs, but partially inhibits basal PRL release 52, a finding consistent with previous work by Zorec's group showing stimulatory effects of cAMP downstream of voltage-gated calcium influx (VGCI) 16, 17. Thus, parallelism in the actions of dopamine and ET-1 on cAMP production and PRL release raised the possibility that agonist-induced inhibition of PRL release could reflect down regulation of cAMP production.
FIGURE 4.
Inhibition of basal adenylyl cyclase activity by activation of pituitary dopamine D2 and endothelin ETA receptors. (A) Time course of bromocriptine (Bromo) and apomorphine (Apo) induced inhibition of cAMP release in perifused pituitary cells. (B) Concentration-dependent effects of ET-1 on cAMP levels (released + cell content) in pituitary cells in static culture.
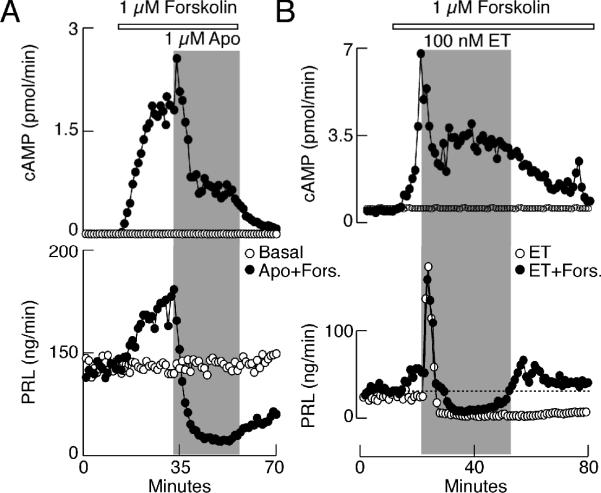
To test this hypothesis, AC activity was elevated by challenging anterior pituitary cells with forskolin in perfusion. Fig. 5A and B, top, shows that such a treatment elevates cAMP production within a few minutes of application. Measurements of PRL content in the same samples revealed that the increase in cAMP production was accompanied with stimulation of PRL release (Fig. 5A and B, bottom). Application of apomorphine and ET-1 attenuated AC activity in the presence of forskolin, but the residual cAMP production was several-fold higher than in untreated cells. At such elevated cAMP levels, both agonists inhibited PRL release in a manner highly comparable to that observed in controls. Forskolin treatment also elevated [Ca2+]i in a fraction of lactotrophs 52 and both apomorphine and ET-1 abolished stimulatory effect of forskolin on Ca2+ transients in a majority of lactotrophs, whereas in the absence of agonists, there was a sustained elevation in cAMP production, VGCI, and PRL release in forskolin-treated cells 32,52 These results do not argue against the relevance of AC signaling pathway in dopamine and ET-1-regulated PRL release, but demonstrate that both agonists can stop secretion through other signaling pathways as well.
FIGURE 5.
Agonist-mediated inhibition of basal PRL release in pituitary cells with forskolin-induced elevation of cAMP production. (A) Effects of forskolin and apomorphine on cAMP (top) and PRL (bottom) release. (B) Effects of forskolin and ET-1 on cAMP (top) and PRL (bottom) release. Horizontal bars indicate duration of forskolin treatment and gray areas represent duration of GPCR agonist application. Open circles, cells treated with agonists only.
INHIBITION OF SPONTANEOUS VGCI BY DOPAMINE AND ET-1
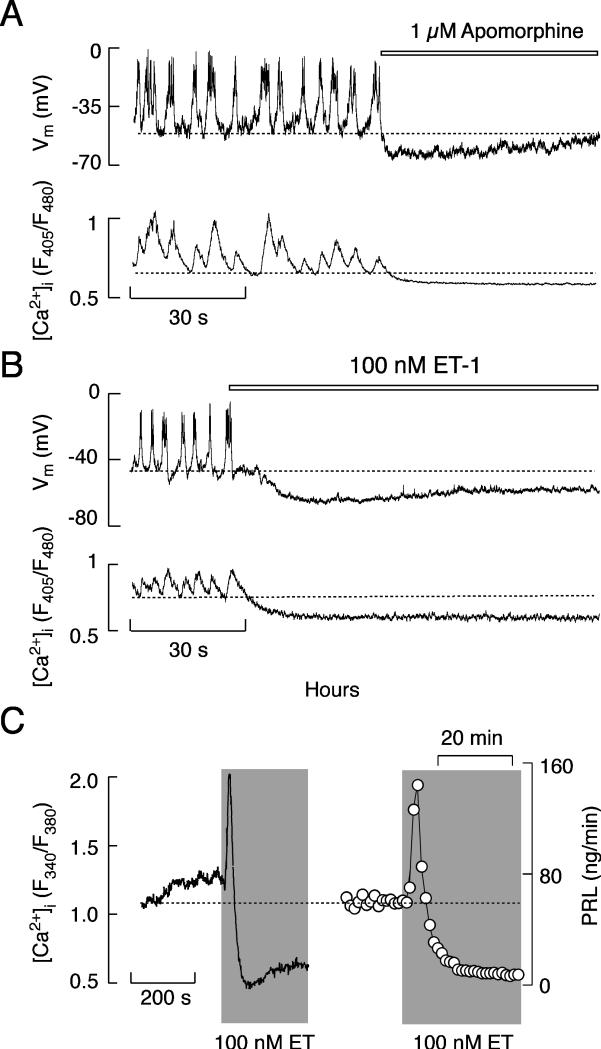
Simultaneous measurements of electrical activity and intracellular calcium in perforated cells revealed that apomorphine and ET-1 hyperpolarized the membrane, leading to the abolition of AP firing and a decrease in [Ca2+]i (Fig. 6A and B). Apomorphine and bromocriptine also inhibit spontaneous VGCI in intact cells loaded with Fura-2, indicating that inhibition of electrical activity in perforated cells does not reflect the side effects of the intrapipete chloride concentration 52. In contrast to apomorphine action on [Ca2+]i, ET-1 induced inhibition of VGCI was preceded by a transient elevation in [Ca2+]i (Fig. 6C), which was also preserved in cells bathed in calcium-deficient medium and was mediated by InsP3-induced calcium release 32. There was a strong parallelism in the actions of ET on spontaneous calcium transients and basal PRL release (Fig. 6C), consistent with the role of VGCI in basal PRL release 40.
FIGURE 6.
Agonist-induced inhibition of spontaneous electrical activity, calcium signaling, and secretion in pituitary lactotrophs. (A and B) Effects of apomorphine (A) and ET-1 (B) on spontaneous firing of APs (top) and calcium signaling (bottom) in identified lactotrophs. Horizontal bars indicate duration of GPCR agonist treatment. (C) Bidirectional effects of ET-1 on calcium signaling (left) and PRL release (right). Gray areas represent duration of ET-1 application.
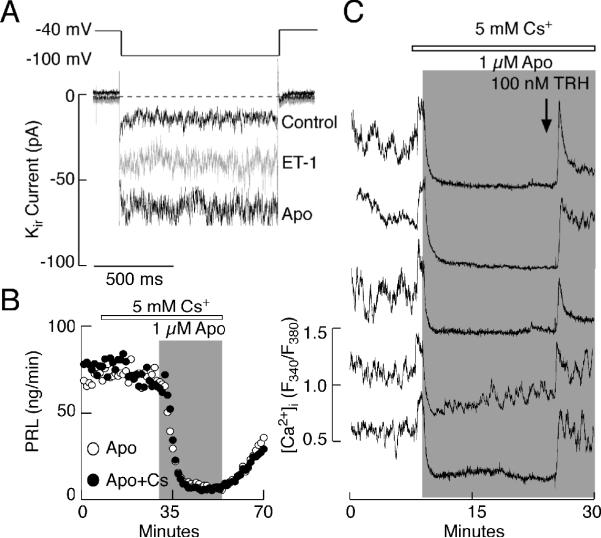
The role of dopamine in activation of inward rectifier potassium (Kir) channels in pituitary lactotrophs is well established 53, 54 and was also observed in our experimental conditions (Fig. 7A). ET-1 also increased the magnitude of the Kir current in lactotrophs, which probably contributes to the sustained hyperpolarization of the cells (Fig. 7A). Although extracellular cesium in 2-5 mM concentrations fully blocks Kir channels in these cells 31, the addition of 5 mM cesium did not affect apomorphine-induced inhibition of PRL release (Fig. 7B). In the presence of cesium, agonist-induced inhibition of AC activity is also preserved 49. In parallel with previously published data in immortalized lacto-somatotrophs 55, extracellularly added cesium elevated [Ca2+]i in single lactotrophs. Furthermore, in the presence of cesium apomorphine inhibited spontaneous Ca2+ transients in all lactotrophs (Fig. 7C). Same effects were also observed in the presence of 1 mM barium, another blocker of Kir channels 55. These observations are consistent with the finding that both dopamine 56, 57 and ET-1 27, 31 also inhibit Cav channels independently of the status of Kir channels. Taken together, these experiments do not argue against the relevance of Kir channels on dopamine and ET-1 action, but show that agonist-induced inhibition of Cav channel gating alone is sufficient to block spontaneous Ca2+ influx.
FIGURE 7.
Role of inwardly rectifying potassium channels (Kir) in spontaneous and agonist-controlled calcium signaling and secretion. (A) Activation of Kir channels by ET-1 and apomorphine. (B and C) Apomorphine-induced inhibition of basal PRL release (B) and calcium transients (C) in cells with inhibited Kir channels with cesium. Note increase in [Ca2+]i after application of cesium. Horizontal bars indicate duration of application of 5 mM cesium and gray areas represent duration of apomorphine treatment. Arrow indicates time of TRH addition.
AGONIST-INDUCED INHIBITION OF SECRETION DOWNSTREAM OF VGCI
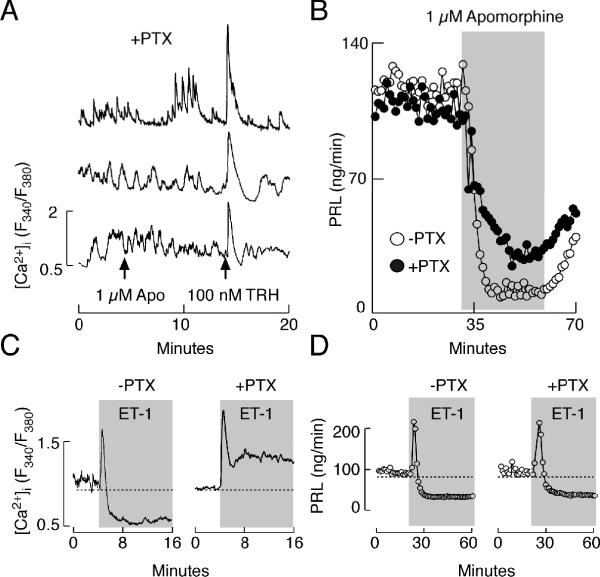
Earlier experiments have established that dopamine-induced inhibition of Cav channels 56, 57 and activation of Kir channels and consequent inhibition of VGCI 53, occurs through coupling of D2 receptors to Gi/o signaling pathway. Consistent with this, in cells treated with 250 ng/ml PTX overnight, the inhibitory effect of apomorphine on spontaneous Ca2+ transients was lost (Fig. 8A). However, the same PTX treatment could only exert a partial loss of inhibitory effects of apomorphine on PRL release (Fig. 8B). The dose-dependent studies with PTX revealed that 250 ng/ml overnight is a sufficient concentration to fully block Gi/o signaling pathway 49. We also reported recently that depolarization-induced Ca2+ influx was reduced by apomorphine in controls but not in PTX-treated TRH-responsive cells; although [Ca2+]i levels were elevated in PTX-treated cells during the sustained depolarization, apomorphine effectively decreased PRL release below the basal levels 49.
FIGURE 8.
Dependence of agonist-induced inhibition of spontaneous calcium transients and basal PRL release on pertussis toxin (PTX)-sensitive Gi/o signaling. (A) Lack of effects of apomorphine on calcium transients in anterior pituitary cells treated with 250 ng/ml PTX overnight. Arrows indicate time of apomorphine and TRH applications. (B) PTX-sensitive and -insensitive components of apomorphine-induced inhibition of basal PRL release. (C and D) Patterns of ET-1-induced calcium signaling (C) and PRL secretion (D) in controls (left) and PTX-treated cells (right). Gray areas indicate duration of GPCR agonist treatment.
Experiments with PTX further revealed that the coupling of ETA receptors to Gi/o proteins provides an effective mechanism for the inhibition of spontaneous VGCI 27, 31. As shown in Fig. 8C, the bidirectional response of [Ca2+]i typically observed in control cells during the application of ET-1 (left) was replaced with a biphasic response in cells treated overnight with PTX, composed of an early spike phase and a sustained plateau phase of elevated [Ca2+]i (right). In contrast to Ca2+ signaling, the bidirectional pattern of ET-induced PRL secretion was not obviously affected by PTX treatment (Fig. 8D, left vs. right). ET-1 also inhibited PRL release in PTX-treated static cultures of pituitary cells 32. In parallel with the experiments described above using dopamine as an agonist, ET-1 was unable to decrease [Ca2+]i in high potassium-depolarized PTX-treated cells, but inhibited PRL release to the levels observed in control cells 32. Thus, both agonists can inhibit basal PRL release downstream of VGCI.
G PROTEIN-INDEPENDENT SIGNALING BY GPCRs AND PRL SECRETION
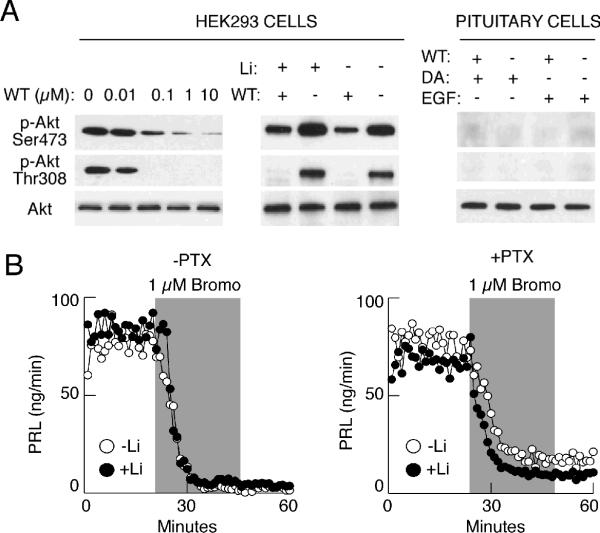
In general, dopamine and ET-1 could inhibit PRL release through G protein-dependent and G protein-independent, β-arrestin-dependent pathways, the later being discovered recently by Caron's group 20. The emerging components of this pathway include Akt (a member of serine/threonine kinase family), protein phosphatase-2A, and glycogen synthase kinase (GSK) 58. Via its pleckstrin homology domain, Akt is regulated by phosphoinositide 3 (PI3)-kinase 59, which is effectively blocked by wortmannin. In our hands, however, wortmannin did not influence dopamine-induced inhibition of PRL release 49. In themouse striatum, dopamine-stimulated dephosphorylation of Akt leads to a reduction in kinase activity and activation of GSK-3, and lithium antagonizes dopamine-dependent effects mediated by Akt/GSK-3 signaling cascade 60. In perifused pituitary cells with and without blocked Gi/o signaling pathway, we observed no effects of extracellular lithium on dopamine- and bromocriptine-induced inhibition of PRL release (Fig. 9B). To test the efficacy of wortmannin and lithium treatments to inhibit Akt phosphorylation, we used HEK293 cells that show constitutively high levels of Akt phosphorylation on Ser473 and Thr308. In these cells, wortmannin inhibited phosphorylation of Akt in a dose-dependent manner (Fig. 9A, left). On the other hand, lithium stimulated Akt phosphorylation (Fig. 9A, central), which is consistent with observations in other cell types 60. Akt is also present in pituitary cells, but we could not observe the presence of phosphorylated forms of this protein, suggesting that in our experimental conditions dopamine cannot signal through the β-arrestin signaling complex by dephosphorylating Akt on Thr308.
FIGURE 9.
Independence of dopamine D2 receptor action on PRL release of PI3-kinase and Akt/β arrestin signaling pathways. (A) Western blot analysis showing that wortmanin (WT) and lithium respectively inhibit and stimulate Akt phosphorylation in HEK293 cells but not in pituitary cells. (B) Lack of effects of lithium, an inhibitor of GSK-3, on bromocriptine-induced inhibition of basal PRL release in anterior pituitary cells without (left) and with (right) blocked Gi/o signaling pathway. Gray areas indicate duration of bromocriptine application.
Gz SIGNALING PATHWAY AND PRL SECRETION
Dopamine D2S and D2L receptors couple to the same extent to the PTX-sensitive Gi/o protein and to the PTX-insensitive sister protein Gz in vitro 18 and in vivo 19. Other subtypes of dopamine receptors also couple to Gz proteins 18, 61. Anterior pituitary cells also express Gzα protein 32, and the functional coupling of endothelin ETA receptor to this protein is suggested by the finding that ET-1-induced inhibition of AC activity is not abolished by PTX treatment 32. To prevent the coupling of ET receptors to Gz-dependent signaling pathways, we used two experimental approaches. In the first series of experiments, cells were stimulated with phorbol ester PMA, which should silence the Gz signaling pathway through the protein kinase C-dependent phosphorylation of its α subunit 62, 63. Consistent with the coupling of ETA receptors to Gz trimeric proteins, in PMA-treated cells ET-1-induced inhibition of AC was abolished, as well as the agonist-induced inhibition of sustained PRL release 32. Likewise, the inhibitory effect of dopamine on PRL secretion was dramatically reduced in PMA-treated cells with operative Gi/o signaling pathway, and agonist-induced inhibition of secretion was further reduced in PTX+PMA treated cells 49.
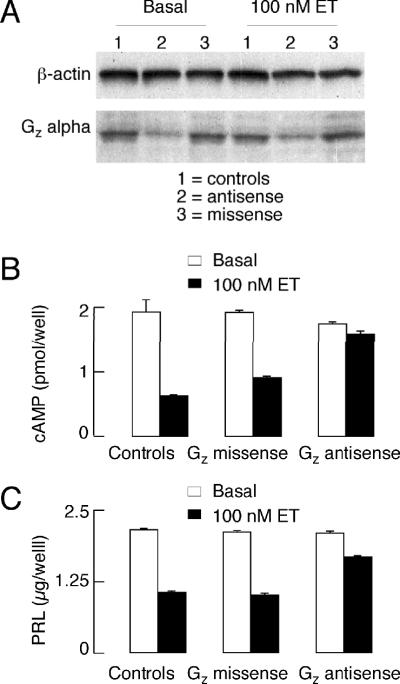
To down-regulate Gzα expression in lactotrophs, we continuously treated cells for 72 h with specific antisense phosphorothio-modified oligodeoxynucleotides, whereas control cells were treated with equivalent missense phosphorothio-modified oligodeoxynucleotides. Treatment with Gzα-specific antisense oligodeoxynucleotides, but not missense oligodeoxynucleotides, substantially decreased the integrated optical density value of the Gzα protein band (Fig. 10A). The negative coupling of ET receptors to the AC signaling pathway was practically abolished in Gzα-specific antisense oligodeoxynucleotide-treated cells (Fig. 10B). In the same samples, ET-induced inhibition of PRL release was substantially reduced (Fig. 10C), confirming the involvement of a Gz-signaling pathway in the inhibitory actions of ET receptors on AC activity and Ca2+-triggered exocytosis. Various experiments with AC activators in Gi/o silenced cells further revealed that both arms of Gz proteins, α and βγ, participate in the ET-induced inhibition of PRL release downstream of VGCI 32.
FIGURE 10.
Effects of down-regulation of Gz-alpha protein expression in pituitary cells (A) on ET-1-induced cAMP signaling (B) and PRL release (C). Primary cultures of anterior pituitary cells were treated overnight with Gzα-specific antisense and control missense phosphorothio-modified oligodeoxynucleotides prior to stimulation with 100nM ET-1. cAMP content, PRL release and Gzα expression were later assayed (see text for further details).
The contribution of Gzα-mediated attenuation of AC activity in pituitary cells appeared not to be sufficient in dopamine-induced rapid inhibition of PRL release, because such inhibition was preserved in forskolin-treated cells 32, 49. Thus, liberation of Gzβγ may represent the primary factor in dopamine-induced inhibition of calcium-secretion coupling. Such a conclusion is in accordance with the finding in other cell types about the role of Gβγ subunits in control of secretion independently of their actions on AC, phospholipase C-β2, and several tyrosine kinases 64. In fact, recent experiments by Martin's group in permeabilized PC12 cells also demonstrated that Gβγ directly regulates SNARE protein fusion machinery to modulate secretory granule exocytosis. This action occurs rapidly and affects ATP-primed secretory vesicles 65.
CONCLUSIONS
Both dopamine D2 and endothelin ETA receptors activate multiple signaling pathways in lactotrophs and such redundancy reinforces the blockade of basal PRL release (Fig. 11). Our results indicate that dopamine rapidly reduced basal cAMP production, a second messenger involved in both synthesis and release of PRL as well as in control of VGCI. Independently of the status of AC activity, however, these receptors inhibited spontaneous firing of APs and the associated VGCI in lactotrophs. Such inhibition was also observed in cells with blocked Kir channels, suggesting that inhibition of Cav channels per se by these agonists is enough to stop spontaneous calcium influx. Dopamine-induced inhibition of AC and electrical activity required the coupling of receptors to PTX-sensitive Gi/o proteins. In contrast, the coupling of dopamine receptors to both PTX-sensitive and -insensitive class of G proteins and/or to other signaling pathways was required for full inhibition of AC activity and PRL release. The presence of the PTX-insensitive step in dopamine action on PRL release does not argue against the relevance of AC, Kirchannels, and Cav channels in this process in vivo, but demonstrates that pharmacological exclusion of a single pathway does not provide a valid experimental model for a system simultaneously controlled by multiple pathways. In vitro, dopamine-mediated control of VGCI is more than sufficient to block PRL release, as it was shown in experiments with removal of extracellular calcium 40, 66. The triple control of VGCI by inhibiting AC and Cav and activating Kir channels, combined with the mechanism for desensitization of calcium-secretion coupling, not only secures inhibition of PRL release but also provides the possibility for maintaining such inhibition when cells are exposed to multiple agonists. For example, the Gz signaling pathway probably contributes to the control of PRL release by dopamine when the electrical activity in lactotrophs is stimulated by other factors. Based on the experimental observations outlined above with ET-1, an elegant theoretical model was developed to describe the complex signaling pathway in these cells, with the main idea that one receptor is coupled to many G proteins 67. More recent findings further suggested the existence of functional splice forms of ETA receptors, which could add to the remarkable complexity of G protein-specific coupling 36.
FIGURE 11.
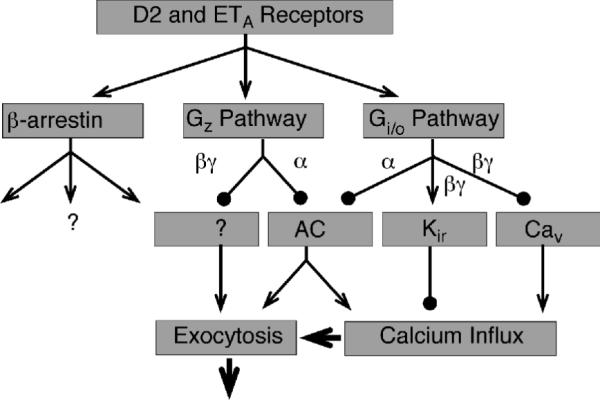
Activation of multiple signaling pathways by dopamine D2 and endothelin ETA receptors in anterior pituitary lactotrophs. Arrows indicate stimulatory actions and circles indicate inhibitory actions.
Acknowledgment
Supported by the Intramural Research Program of the NICHD, NIH
References
- 1.Zhu X, et al. Signaling and epigenetic regulation of pituitary development. Curr Opin Cell Biol. 2007;19:605–11. doi: 10.1016/j.ceb.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22:724–63. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- 3.Stenovec M, et al. Slow spontaneous secretion from single large dense-core vesicles monitored in neuroendocrine cells. Faseb J. 2004;18:1270–2. doi: 10.1096/fj.03-1397fje. [DOI] [PubMed] [Google Scholar]
- 4.Vardjan N, et al. Subnanometer fusion pores in spontaneous exocytosis of peptidergic vesicles. J Neurosci. 2007;27:4737–46. doi: 10.1523/JNEUROSCI.0351-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stojilkovic SS, Izumi S, Catt KJ. Participation of voltage-sensitive calcium channels in pituitary hormone release. J Biol Chem. 1988;263:13054–61. [PubMed] [Google Scholar]
- 6.Mayo KE, et al. Growth hormone-releasing hormone: synthesis and signaling. Recent Prog Horm Res. 1995;50:35–73. doi: 10.1016/b978-0-12-571150-0.50007-x. [DOI] [PubMed] [Google Scholar]
- 7.Freeman ME, et al. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 8.Stojilkovic SS, Reinhart J, Catt KJ. Gonadotropin-releasing hormone receptors: structure and signal transduction pathways. Endocr Rev. 1994;15:462–99. doi: 10.1210/edrv-15-4-462. [DOI] [PubMed] [Google Scholar]
- 9.Balik A, et al. Melatonin action in neonatal gonadotrophs. Physiol Res. 2004;53(Suppl 1):S153–66. [PubMed] [Google Scholar]
- 10.Missale C, et al. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 11.Giros B, et al. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989;342:923–6. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- 12.Monsma FJ, Jr., et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342:926–9. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- 13.Kukstas LA, et al. Different expression of the two dopaminergic D2 receptors, D2415 and D2444, in two types of lactotroph each characterised by their response to dopamine, and modification of expression by sex steroids. Endocrinology. 1991;129:1101–3. doi: 10.1210/endo-129-2-1101. [DOI] [PubMed] [Google Scholar]
- 14.Kelly MA, et al. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron. 1997;19:103–13. doi: 10.1016/s0896-6273(00)80351-7. [DOI] [PubMed] [Google Scholar]
- 15.Senogles SE, et al. The D2-dopamine receptor of anterior pituitary is functionally associated with a pertussis toxin-sensitive guanine nucleotide binding protein. J Biol Chem. 1987;262:4860–7. [PubMed] [Google Scholar]
- 16.Zorec R, Sikdar SK, Mason WT. Increased cytosolic calcium stimulates exocytosis in bovine lactotrophs. Direct evidence from changes in membrane capacitance. J Gen Physiol. 1991;97:473–97. doi: 10.1085/jgp.97.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sikdar SK, Kreft M, Zorec R. Modulation of the unitary exocytic event amplitude by cAMP in rat melanotrophs. J Physiol. 1998;511(Pt 3):851–9. doi: 10.1111/j.1469-7793.1998.851bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obadiah J, et al. Adenylyl cyclase interaction with the D2 dopamine receptor family; differential coupling to Gi, Gz, and Gs. Cell Mol Neurobiol. 1999;19:653–64. doi: 10.1023/A:1006988603199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leck KJ, et al. Gz proteins are functionally coupled to dopamine D2-like receptors in vivo. Neuropharmacology. 2006;51:597–605. doi: 10.1016/j.neuropharm.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–73. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Stojilkovic SS, et al. Endothelin ETA receptors mediate the signaling and secretory actions of endothelins in pituitary gonadotrophs. Endocrinology. 1992;130:465–74. doi: 10.1210/endo.130.1.1309344. [DOI] [PubMed] [Google Scholar]
- 22.Samson WK. The endothelin-A receptor subtype transduces the effects of the endothelins in the anterior pituitary gland. Biochem Biophys Res Commun. 1992;187:590–5. doi: 10.1016/0006-291x(92)91235-i. [DOI] [PubMed] [Google Scholar]
- 23.Stojilkovic SS, et al. Calcium signaling and secretory responses in endothelin-stimulated anterior pituitary cells. Mol Pharmacol. 1991;39:762–70. [PubMed] [Google Scholar]
- 24.Kanyicska B, Burris TP, Freeman ME. Endothelin-3 inhibits prolactin and stimulates LH, FSH and TSH secretion from pituitary cell culture. Biochem Biophys Res Commun. 1991;174:338–43. doi: 10.1016/0006-291x(91)90525-c. [DOI] [PubMed] [Google Scholar]
- 25.Stojilkovic SS, et al. Endothelin stimulation of cytosolic calcium and gonadotropin secretion in anterior pituitary cells. Science. 1990;248:1663–6. doi: 10.1126/science.2163546. [DOI] [PubMed] [Google Scholar]
- 26.Lachowicz A, et al. Uncoupling of calcium mobilization and entry pathways in endothelin-stimulated pituitary lactotrophs. J Biol Chem. 1997;272:28308–14. doi: 10.1074/jbc.272.45.28308. [DOI] [PubMed] [Google Scholar]
- 27.Tomic M, et al. Expression of Ca(2+)-mobilizing endothelin(A) receptors and their role in the control of Ca(2+) influx and growth hormone secretion in pituitary somatotrophs. J Neurosci. 1999;19:7721–31. doi: 10.1523/JNEUROSCI.19-18-07721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanyicska B, Livingstone JD, Freeman ME. Long term exposure to dopamine reverses the inhibitory effect of endothelin-1 on prolactin secretion. Endocrinology. 1995;136:990–4. doi: 10.1210/endo.136.3.7867609. [DOI] [PubMed] [Google Scholar]
- 29.Samson WK, et al. Pituitary site of action of endothelin: selective inhibition of prolactin release in vitro. Biochem Biophys Res Commun. 1990;169:737–43. doi: 10.1016/0006-291x(90)90393-2. [DOI] [PubMed] [Google Scholar]
- 30.Rebsamen MC, et al. Role of cAMP and calcium influx in endothelin-1-induced ANP release in rat cardiomyocytes. Am J Physiol. 1997;273:E922–31. doi: 10.1152/ajpendo.1997.273.5.E922. [DOI] [PubMed] [Google Scholar]
- 31.Tomic M, et al. Ca(2+)-mobilizing endothelin-A receptors inhibit voltage-gated Ca(2+) influx through G(i/o) signaling pathway in pituitary lactotrophs. Mol Pharmacol. 2002;61:1329–39. doi: 10.1124/mol.61.6.1329. [DOI] [PubMed] [Google Scholar]
- 32.Andric SA, et al. Endothelin-induced, long lasting, and Ca2+ influx-independent blockade of intrinsic secretion in pituitary cells by Gz subunits. J Biol Chem. 2005;280:26896–903. doi: 10.1074/jbc.M502226200. [DOI] [PubMed] [Google Scholar]
- 33.Gohla A, Schultz G, Offermanns S. Role for G(12)/G(13) in agonist-induced vascular smooth muscle cell contraction. Circ Res. 2000;87:221–7. doi: 10.1161/01.res.87.3.221. [DOI] [PubMed] [Google Scholar]
- 34.Gentles AJ, Karlin S. Why are human G-protein-coupled receptors predominantly intronless? Trends Genet. 1999;15:47–9. doi: 10.1016/s0168-9525(98)01648-5. [DOI] [PubMed] [Google Scholar]
- 35.Miyamoto Y, et al. Alternative RNA splicing of the human endothelin-A receptor generates multiple transcripts. Biochem J. 1996;313(Pt 3):795–801. doi: 10.1042/bj3130795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatae N, et al. Cloning and functional identification of novel endothelin receptor type A isoforms in pituitary. Mol Endocrinol. 2007;21:1192–204. doi: 10.1210/me.2006-0343. [DOI] [PubMed] [Google Scholar]
- 37.Kwiecien R, Hammond C. Differential management of Ca2+ oscillations by anterior pituitary cells: a comparative overview. Neuroendocrinology. 1998;68:135–51. doi: 10.1159/000054360. [DOI] [PubMed] [Google Scholar]
- 38.Stojilkovic SS, Zemkova H, Van Goor F. Biophysical basis of pituitary cell type-specific Ca2+ signaling-secretion coupling. Trends Endocrinol Metab. 2005;16:152–9. doi: 10.1016/j.tem.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Stojilkovic SS. Pituitary cell type-specific electrical activity, calcium signaling and secretion. Biol Res. 2006;39:403–23. doi: 10.4067/s0716-97602006000300004. [DOI] [PubMed] [Google Scholar]
- 40.Van Goor F, et al. Dependence of pituitary hormone secretion on the pattern of spontaneous voltage-gated calcium influx. Cell type-specific action potential secretion coupling. J Biol Chem. 2001;276:33840–6. doi: 10.1074/jbc.M105386200. [DOI] [PubMed] [Google Scholar]
- 41.Van Goor F, Li YX, Stojilkovic SS. Paradoxical role of large-conductance calcium-activated K+ (BK) channels in controlling action potential-driven Ca2+ entry in anterior pituitary cells. J Neurosci. 2001;21:5902–15. doi: 10.1523/JNEUROSCI.21-16-05902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlegel W, et al. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987;329:719–21. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- 43.Kwiecien R, et al. Endogenous pacemaker activity of rat tumour somatotrophs. J Physiol. 1998;508(Pt 3):883–905. doi: 10.1111/j.1469-7793.1998.883bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giraldez T, et al. Correlation between electrical activity and intracellular Ca2+ oscillations in GH3 rat anterior pituitary cells. Cell Calcium. 2002;31:65–78. doi: 10.1054/ceca.2001.0260. [DOI] [PubMed] [Google Scholar]
- 45.Li YX, et al. Spontaneous electrical and calcium oscillations in unstimulated pituitary gonadotrophs. Biophys J. 1995;69:785–95. doi: 10.1016/S0006-3495(95)79952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li YX, et al. Sensing and refilling calcium stores in an excitable cell. Biophys J. 1997;72:1080–91. doi: 10.1016/S0006-3495(97)78758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tse A, Hille B. GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science. 1992;255:462–4. doi: 10.1126/science.1734523. [DOI] [PubMed] [Google Scholar]
- 48.Kukuljan M, et al. Apamin-sensitive potassium channels mediate agonist-induced oscillations of membrane potential in pituitary gonadotrophs. FEBS Lett. 1992;301:19–22. doi: 10.1016/0014-5793(92)80201-q. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Iglesias AE, et al. Dopamine inhibits basal prolactin release in pituitary lactotrophs through pertussis toxin-sensitive and -insensitive signaling pathways. Endocrinology. 2008;149:1470–9. doi: 10.1210/en.2007-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enjalbert A, Bockaert J. Pharmacological characterization of the D2 dopamine receptor negatively coupled with adenylate cyclase in rat anterior pituitary. Mol Pharmacol. 1983;23:576–84. [PubMed] [Google Scholar]
- 51.McDonald WM, et al. Dopaminergic inhibition of adenylate cyclase correlates with high affinity agonist binding to anterior pituitary D2 dopamine receptors. Mol Cell Endocrinol. 1984;36:201–9. doi: 10.1016/0303-7207(84)90037-6. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez-Iglesias AE, et al. Dependence of electrical activity and calcium influx-controlled prolactin release on adenylyl cyclase signaling pathway in pituitary lactotrophs. Mol Endocrinol. 2006;20:2231–46. doi: 10.1210/me.2005-0363. [DOI] [PubMed] [Google Scholar]
- 53.Einhorn LC, Gregerson KA, Oxford GS. D2 dopamine receptor activation of potassium channels in identified rat lactotrophs: whole-cell and single-channel recording. J Neurosci. 1991;11:3727–37. doi: 10.1523/JNEUROSCI.11-12-03727.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gregerson KA, et al. Identification of G protein-coupled, inward rectifier potassium channel gene products from the rat anterior pituitary gland. Endocrinology. 2001;142:2820–32. doi: 10.1210/endo.142.7.8236. [DOI] [PubMed] [Google Scholar]
- 55.Charles AC, et al. L-type Ca2+ channels and K+ channels specifically modulate the frequency and amplitude of spontaneous Ca2+ oscillations and have distinct roles in prolactin release in GH3 cells. J Biol Chem. 1999;274:7508–15. doi: 10.1074/jbc.274.11.7508. [DOI] [PubMed] [Google Scholar]
- 56.Lledo PM, et al. Dopamine inhibits two characterized voltage-dependent calcium currents in identified rat lactotroph cells. Endocrinology. 1990;127:990–1001. doi: 10.1210/endo-127-3-990. [DOI] [PubMed] [Google Scholar]
- 57.Malgaroli A, et al. Dopamine inhibits cytosolic Ca2+ increases in rat lactotroph cells. Evidence of a dual mechanism of action. J Biol Chem. 1987;262:13920–7. [PubMed] [Google Scholar]
- 58.Bibb JA. Decoding dopamine signaling. Cell. 2005;122:153–5. doi: 10.1016/j.cell.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 59.Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 60.Beaulieu JM, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sidhu A, et al. Multiple coupling of human D5 dopamine receptors to guanine nucleotide binding proteins Gs and Gz. J Neurochem. 1998;70:2459–67. doi: 10.1046/j.1471-4159.1998.70062459.x. [DOI] [PubMed] [Google Scholar]
- 62.Fields TA, Casey PJ. Phosphorylation of Gz alpha by protein kinase C blocks interaction with the beta gamma complex. J Biol Chem. 1995;270:23119–25. doi: 10.1074/jbc.270.39.23119. [DOI] [PubMed] [Google Scholar]
- 63.Wang J, et al. Reciprocal signaling between heterotrimeric G proteins and the p21-stimulated protein kinase. J Biol Chem. 1999;274:31641–7. doi: 10.1074/jbc.274.44.31641. [DOI] [PubMed] [Google Scholar]
- 64.Blackmer T, et al. G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292:293–7. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- 65.Blackmer T, et al. G protein betagamma directly regulates SNARE protein fusion machinery for secretory granule exocytosis. Nat Neurosci. 2005;8:421–5. doi: 10.1038/nn1423. [DOI] [PubMed] [Google Scholar]
- 66.Lamberts SW, Macleod RM. Regulation of prolactin secretion at the level of the lactotroph. Physiol Rev. 1990;70:279–318. doi: 10.1152/physrev.1990.70.2.279. [DOI] [PubMed] [Google Scholar]
- 67.Bertram R, et al. Endothelin action on pituitary lactotrophs: one receptor, many GTP-binding proteins. Sci STKE. 2006:er2. doi: 10.1126/stke.3212006er2. 2006. [DOI] [PubMed] [Google Scholar]