Abstract
Background
Previous studies of children with homozygous sickle cell anemia (SCA) show impaired growth and maturation. The correlation of this suboptimal growth with metabolic and hematological factors during puberty is poorly understood.
Procedure
We studied a group of pre-adolescent children with SCA (19 males, 14 females) and healthy controls (16 males, 15 females) matched for race, sex, body size, and pubertal development. Height, weight, body mass index (BMI), and body composition changes were longitudinally assessed over a 2-year period and compared between the groups and with Z scores based on US growth charts. These changes were correlated with hemoglobin concentration and with energy expenditure measured using indirect whole-room calorimetry.
Results
Children with SCA progressed through puberty slower than control children. While, after 2 years, pubertal males with SCA were shorter, their annual increases in weight were not different from controls. The mean fat free mass (FFM) increments were significantly less in males and females with SCA than in control children. In males with SCA, growth in height declined over time and was significantly slower than in matched controls (p<0.05).
Conclusion
Growth delays were present during puberty in children with SCA. Decreased growth velocity in children with SCA was independently associated with decreased hemoglobin concentration and increased total energy expenditure.
Keywords: growth, energy expenditure, body composition
INTRODUCTION
Advances in the clinical care of children with sickle cell anemia (SCA), such as earlier diagnosis, penicillin prophylaxis, folate supplementation and hydroxyurea therapy have reduced morbidity and mortality related to this disease [1,2]. Suboptimal growth, however, remains a significant clinical problem. Compared to their healthy peers, studies suggest that children with SCA are smaller and have delayed pubertal development. Whether or not their ultimate adult weight and body mass index (BMI) remain lower is controversial; some studies have shown that young adults with sickle cell disease are smaller than their age-matched peers, [3-7] but the cohort study from Jamaica demonstrated normal adult height [8,9].
The importance of investigating the reasons for poor growth and delayed puberty and identifying possible modifiable factors that may promote more normal development in children with SCA has only recently been recognized. It has been reported that adequacy of dietary intake declines with age in children with SCA. However, changes in energy metabolism associated with SCA have not been fully studied. Further, there are controversial reports regarding association of hemoglobin levels with height and weight in females and males with SCA [7,10].
The objective of the present study was to systematically study the correlations between growth and hemoglobin and energy expenditure in children with SCA and developmentally matched healthy controls. We tested the hypothesis that growth rate in pubertal children with SCA is slower than in controls and is associated with hemoglobin concentration and total energy expenditure.
METHODS
Participants
A group of 33 African-American children with SCA were identified for participation in the study at the Pediatric Sickle Cell Clinic at Vanderbilt in Nashville, TN and at the MidSouth Sickle Cell Clinic in Memphis, TN from June 2001 to January 2006. The group screened and recruited for the study at the Clinical Center at Vanderbilt University Medical Center (CRC) included 19 males and 14 females, 10 to 13-years-old. Additionally, 31 African-American children from Nashville, who did not carry the sickle cell (HbS) gene or any other hemoglobinopathy were matched for sex, Tanner stage, and approximate height, weight, and fat mass to serve as a control for the study. The presence or absence of homozygous sickle cell disease (SCA) was confirmed through hemoglobin electrophoresis in all participants [11]. Patients with vaso-occlusive or pain crisis in the two months before screening, receiving chronic transfusion, on hydroxyurea therapy at baseline, or having apparent metabolic, skeletal, hepatic, or renal dysfunction, as well as pregnant females, were excluded from enrollment in the study. There were no subjects with disease characterized by stroke, multiple episodes of acute chest syndrome, or greater than three hospitalizations per year for pain due to the exclusion criteria of hydroxyurea or chronic transfusion. Self-reported complications occurring between the study visits were recorded. All children in the study were prescribed folic acid 1mg orally per day by their primary hematologist. Children and their parents or guardians received written information, verbal explanation about the nature and purpose of the study, and signed informed assent or consent according to the Declaration of Helsinki. The form was approved by both Vanderbilt University School of Medicine and Meharry Medical College for procedures to be performed at the CRC.
Procedures
Children were evaluated at baseline and annually for 2 years as part of a comprehensive study on energy balance that included annual measurement of total and resting energy expenditure, intake of energy and nutrients, and physical activity. Prior to participation in the study, subjects gave a medical history and underwent a complete physical examination. Children's Tanner pubertal staging was assessed on physical examination at baseline, at year 1, year 2 of the study using self-assessment questionnaire [12,13] used previously in our laboratory [14,15]. Hormone levels were measured at study entry and at each visit [12] and compared to normal ranges of hormone levels at puberty [16].
Anthropometrics and Body Composition
Body weight was measured to the nearest 0.05 kg with a monthly calibrated digital scale (Detecto-Medic, Detecto Scales, Inc, Northbrook, IL) with the participants wearing minimal clothing and no shoes. Height was measured using a wall-mounted stadiometer that was calibrated upon wall installation and recalibrated yearly (Perspective Enterprises, Portage, MI). Fat Mass, FFM and BMD were determined by dual energy x-ray absorptiometry (DXA, Lunar Prodigy, GE Medical Systems, Madison WI, children software, version 9.15) as previously described [17]. Our laboratory's intra-assay coefficient of variation for percent of FM using DXA is 0.79±0.49%.
Energy Expenditure
Resting and total energy expenditure were measured using whole-room indirect calorimetry described previously [18]. During the study, participants received a diet designed by a study dietitian and prepared at the CRC metabolic kitchen that contained 50±5% of the energy from carbohydrate, 30±3 % from fat, 15±3% from protein, and all required micronutrients. Participants spent 24 hours in the room calorimeter, in a strictly controlled environment (temperature and humidity), and followed a standardized protocol. The amount and intensity of physical activity performed was based on physical activity assessed in free living as described previously [15]. This approach minimized possible influence of physical activity and, at least in part, other environmental factors on outcome variables.
Resting energy expenditure (REE, kcal/min) was defined as the average energy expenditure (EE) during a 30-min period while the subject lay quietly in bed on the morning following an overnight sleep and 10 h of fasting as described previously [14,19]. Total energy expenditure (TEE, kcal/kg/day) was defined as the total energy per kilogram of body weight spent during an approximately 24 hour stay in the room calorimeter and extrapolated to 24 h (actual range was 22.5 - 23.5 hours).
Blood collection and analytical procedures
Hematological parameters that included whole blood hemoglobin concentration, packed cell volume, white blood cell count, reticulocyte count, ferritin, platelet count, red blood cells, and red blood cell folate were measured at Vanderbilt University Hospital Laboratory. Plasma albumin, thyroid-stimulating hormone, growth hormone, testosterone, estradiol, insulin, and leptin were measured at specialized Vanderbilt's Core Laboratories. All assays were performed using standard methodologies.
Statistical Analysis
Data are presented as mean ± standard deviation (SD). Continuous variables were compared using an unpaired Wilcoxon rank sum test between the SCA group and the control group. Since male and female children experience different growth patterns, they were compared separately. In addition, growth changes in height, weight, and BMI from baseline were characterized using Z scores calculated based on the U.S. growth charts webbed by the Center for Disease Control (http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm). Mixed effect models were used with disease status (SCA versus control), sex, hemoglobin concentration, total energy expenditure (per day per kilogram), and Tanner score as fixed effects and a random subject effect to analyze the endpoints. P values less than 0.05 were considered statistically significant and all tests were two-tailed. Analyses were performed using R-software version 2.6.2 (www.r-project.org) and SAS for Windows (Version 9.1.3, SAS, Cary, NC).
RESULTS
Participant characteristics at baseline
At the study entry, there were no significant differences in height, weight, BMI, FM, FFM, or Tanner staging between children with SCA and control children. Males with SCA were on average 0.9 years older and females with SCA were on average 1.7 years older than control children (p< 0.05); Table I). Males with SCA also had significantly lower bone mineral density (BMD) at study entry compared to control males (0.93±0.05 g/cm3 vs. 0.99±0.06 g/cm3, p< 0.05), females had BMD values that were not significantly different between females with SCA and controls. During the course of the study, 1 male and 3 females with SCA, and 1 control male and 1 control female dropped out of the study.
TABLE I.
Baseline characteristics of study participants
| Males | Females | |||
|---|---|---|---|---|
| Controls (n =16) | SCA (n =19) | Controls (n =15) | SCA (n =14) | |
| Age (years) | 10.43±0.93 | 11.29±1.21 * | 9.98±0.95 | 11.70±1.62 * |
| Weight (kg) | 36.36±5.65 | 37.11±7.60 | 41.27±8.29 | 39.33±9.37 |
| Height (cm) | 140.80±7.68 | 145.03±7.31 | 145.33±8.19 | 147.12±12.88 |
| Body mass index (kg/m2) | 18.28±2.05 | 17.55±2.72 | 19.50±3.63 | 17.93±2.31 |
| Fat-free mass (kg) | 28.59±4.00 | 28.66±4.35 | 29.23±3.22 | 28.82±6.27 |
| Body fat (%) | 16.94±5.92 | 19.63±7.63 | 23.97±10.61 | 21.21±7.74 |
| Bone mineral density (g/cm3) | 0.99±0.06 | 0.93±0.05 * | 0.99±0.066 | 0.96±0.09 |
| Hemoglobin (g/dL) | 13.56±1.21 | 8.93±1.62 | 13.82±1.32 | 9.14±1.33* |
Values are expressed as means ± standard deviation (SD)
significantly different from controls using Wilcoxon test (p<0.05).
Hematological indicators, hormone levels, and energy expenditure
As expected, hematological parameters at baseline were significantly different between controls and children with SCA in both males and females (Table I). Data for some less important parameters are not shown. There were no statistically significant differences between control children and children with SCA in levels of thyroid hormone, growth hormone, estradiol (females only), testosterone (Table II, p>0.05), leptin, or insulin (data not shown). Children with SCA had higher REE compared to control children, but there was no significant difference in TEE spent at baseline or at years 1 and 2 (P>0.05).
TABLE II.
Hormones level and energy expenditure in study participants
| Males | Females | |||
|---|---|---|---|---|
| Controls (n =16) | SCA (n =19) | Controls (n =14) | SCA (n =14) | |
| Thyroid hormone (mcU/ml) | 2.37±1.25 | 2.32±1.08 | 1.91±1.23 | 2.06±1.50 |
| Growth hormone (ng/ml) | 0.43±0.41 | 0.52±0.63 | 1.40±2.51 | 1.21±0.93 |
| Estradiol (pg/ml) | 27.5±15.2 | 35.6±44.1 | ||
| Year 1 | 46.25±39.10 | 59.0±52.2 | ||
| Year 2 | 82.08±36.95 | 48.0±53.3 | ||
| Testosterone (ng/ml) | 1.06±2.06 | 0.48±1.10 | 0.81±0.58 | 0.60±0.35 |
| Year 1 | 1.63±2.31 | 0.46±0.71 | 0.54±0.31 | 0.40±0.23 |
| Year 2 | 2.60±2.86 | 1.50±1.90 | 0.43±0.25 | 0.23±0.07 * |
| REE (kcal/kg FFM/day) | 45.92±2.45 | 57.03±5.94 * | 44.87±5.39 | 53.45±5.87 * |
| Year 1 | 43.95±3.94 | 55.62±4.25* | 43.36±3.50 | 52.35±7.12 |
| Year 2 | 41.89±3.86 | 51.84±5.59* | 40.49±5.02 | 49.04±4.17* |
| TEE (kcal/kg BW /day) | 47.41±5.77 | 50.49±4.66 | 45.86±4.15 | 45.94±6.39 |
| Year 1 | 47.83±6.28 | 47.05±5.87 | 43.64±5.92 | 42.85±8.12 |
| Year 2 | 45.85±7.60 | 44.22±4.58 | 39.07±4.80 | 38.04±6.45 |
REE – resting energy expenditure (kcal/kg fat free mass (FFM)/day); TEE- total energy expenditure (kcal/kg body weight (BW) per day); values are expressed as means ± SD
significantly different from controls using Wilcoxon test (p<0.05).
Longitudinal growth patterns and body composition changes
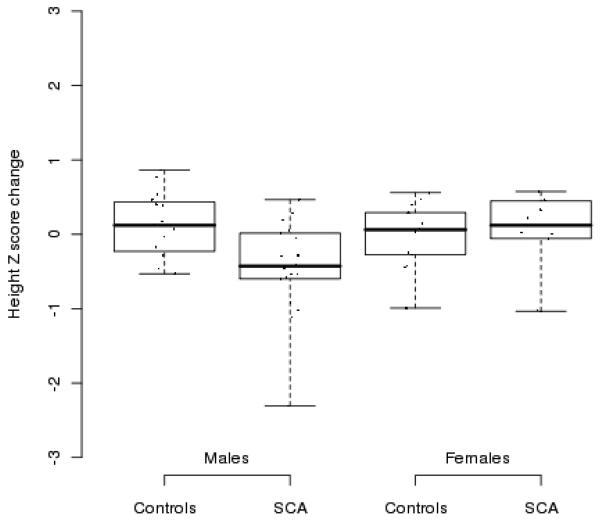
The changes in height, weight, and BMI are presented in Table III. Height: For females with SCA height change was lower than in healthy controls at year 1, but similar in year 2. For males with SCA, changes in height from baseline to year 2 were lower than changes in healthy controls due to significantly greater height change among controls in year 2. Differences between the groups in changes of height Z score are presented in Figure 1. Weight and BMI: There were no significant differences in weight or BMI at baseline or in years 1 or 2 for either males or females. Body composition: There were no significant differences in FM and FFM between males and females with SCA and control children at baseline; however, the mean FFM increments after 2 years were significantly smaller in males and females with SCA than in control children (Table III). Bone mineral density: Changes in bone mineral density (BMD) were not different in both groups, although males with SCA had significantly lower (BMD) at baseline. Pubertal development: Males and females were matched for Tanner staging at baseline, but both control males and control females progressed through puberty more rapidly than those with SCA (Table IV).
Table III.
Measures of growth status and body composition
| Males | Females | |||
|---|---|---|---|---|
| Controls (n =16) | SCA (n =19) | Controls (n =15) | SCA (n =14) | |
| Height (cm) | 145.80±7.68 | 145.03±7.31 | 145.33±8.19 | 147.12±12.88 |
| Year 1 – change from baseline (%) | 4.04±1.96 | 3.20±2.86 | 5.18±2.17 | 3.50±1.74 * |
| Year 2 – change from baseline (%) | 8.86±2.68 | 6.46±3.67* | 8.62±2.76 | 6.65±3.91 |
| Weight (kg) | 36.36±5.65 | 37.11±7.60 | 41.27±8.29 | 39.33±9.37 |
| Year 1 – change from baseline (%) | 14.56±7.13 | 13.33±7.19 | 16.81±6.90 | 14.37±5.32 |
| Year 2 – change from baseline (%) | 28.81±9.28 | 26.99±11.34 | 33.22±9.53 | 27.28±11.34 |
| Body Mass Index at baseline (kg/m2) | 18.28±2.05 | 17.55±2.72 | 19.50±3.63 | 17.93±2.31 |
| Year 1 – change from baseline (%) | 5.83±5.48 | 6.52±7.20 | 5.64±6.28 | 6.758±4.252 |
| Year 2 – change from baseline (%) | 8.75±7.69 | 12.10±9.08 | 12.97±7.97 | 12.10±11.05 |
| FFM at baseline (kg) | 28.65±3.85 | 28.66±4.35 | 29.23±3.22 | 28.82±6.27 |
| Year 1 – change from baseline (%) | 13.4±11.1 | 9.6±6.1 | 15.4±7.5 | 9.8±5.0* |
| Year 2 – change from baseline (%) | 30.5±14.7 | 20.3±9.1* | 27.9±8.97 | 19.3±10.4* |
| Bone mineral density at baseline (g/cm3) | 0.99±0.06 | 0.93±0.05 * | 0.99±0.07 | 0.96±0.09 |
| Year 1 – change from baseline (%) | 1.7±3.2 | 2.2±2.8 | 4.0±4.8 | 3.1±3.9 |
| Year 2 – change from baseline (%) | 5.0±5.1 | 3.6±3.8 | 6.8±6.2 | 6.3±3.4 |
Values are expressed as means ± SD (range)
significantly different from controls using Wilcoxon test (p<0.05); changes from baseline were summarized by means ± SD of the individual changes.
Figure 1.
Change from baseline in height Z score for boys and girls with SCA controls after 2 consecutive follow-up years. The figures illustrate individual Z score change (points), median (middle thick line), and a range between 1 and 3 quartiles (box). Lower and upper bars illustrate 1.5 interquartile range distance.
Table IV.
Number of children at each Tanner stage during the study
| Males | Females | ||||
|---|---|---|---|---|---|
| Controls (n =16) | SCA (n =19) | Controls (n =15) | SCA (n =14) | ||
| Baseline - | Tanner 2 | 16 (100%) | 19 (100%) | 13 (87%) | 13 (93%) |
| Tanner 3 | 0 | 0 | 2 (13%) | 1 (7%) | |
| Tanner 4 | 0 | 0 | 0 | 0 | |
| Year 1 - | Tanner 2 | 3 (20%) | 12 (67%) | 1 (7%) | 6 (55%) |
| Tanner 3 | 12 (80%) | 6 (33%) | 14 (93%) | 5 (45%) | |
| Tanner 4 | 0 | 0 | 0 | 0 | |
| Year 2 - | Tanner 2 | 0 | 0 | 0 | 0 |
| Tanner 3 | 8 (53%) | 17 (94%) | 2 (14%) | 7 (64%) | |
| Tanner 4 | 7 (47%) | 1 (6%) | 12 (86%) | 4 (36%) | |
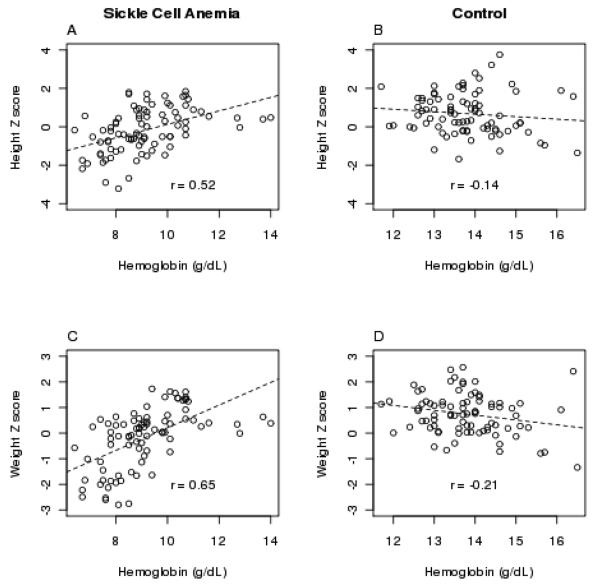
Mixed effect regression analysis (Table V) showed that after adjusting for sex, Tanner stage, and disease status, the height and weight were positively associated with Hgb concentration, and weight and BMI negatively associated with TEE. The correlations between Hob concentration, weight, and height are shown in Figure 2.
TABLE V.
Prediction of growth status during the study using multiple regression
| Coefficient | p-value | lower 95% C.I. | upper 95% C.I. | |
|---|---|---|---|---|
| Height (cm) | ||||
| Disease status (0-control, 1 – sickle cell anemia) | 4.45 | 0.10 | −0.92 | 9.81 |
| Sex (0- female, 1 male) | 0.34 | 0.85 | −3.40 | 4.09 |
| Hemoglobin (g/dL) | 0.23 | 0.59 | −0.61 | 1.07 |
| Total energy expenditure (kcal/kg/day) | −0.37 | <0.001 | −0.53 | −0.20 |
| Tanner stage | 5.81 | <0.001 | 4.78 | 6.83 |
| Weight (kg) | ||||
| Disease status (0-control, 1 – sickle cell anemia) | 2.04 | 0.42 | −2.94 | 7.02 |
| Sex (0- female, 1 male) | 0.92 | 0.60 | −2.54 | 4.37 |
| Hemoglobin (g/dL) | 0.31 | 0.43 | −0.47 | 1.10 |
| TEE (kcal/kg/day) | −0.46 | <0.001 | −0.62 | −0.31 |
| Tanner stage | 5.55 | <0.001 | 4.59 | 6.50 |
| BMI (kg/m2) | ||||
| Disease status (0-control, 1 – sickle cell anemia) | −0.58 | 0.54 | −2.46 | 1.30 |
| Sex (0- female, 1 male) | 0.26 | 0.71 | −1.13 | 1.65 |
| Hemoglobin (g/dL) | 0.03 | 0.83 | −0.25 | 0.31 |
| TEE (kcal/kg/day) | −0.11 | <0.001 | −0.16 | −0.05 |
| Tanner stage | 0.93 | <0.001 | 0.61 | 1.26 |
Models for the longitudinal mixed effects analysis predicting changes in growth over time; Tanner stage (1 to 4) were assessed at each visit and entered into the models as variables; hemoglobin (Hgb) concentration and total energy expenditure (TEE) were measured at each visit (baseline, year 1, and year 2).
Figure 2.
Spearman-rho (r) correlations between hemoglobin concentration (g/dL) and height Z score (A and B) and weight Z score (C and D) in children with sickle cell anemia (SCA) (A and C) and matched healthy controls (B and D) measured at baseline and after 1 and 2 years.
DISCUSSION
The major finding of this study is that during puberty growth patterns are different in children with SCA than in non-SCA pubertal development matched controls and this difference is associated with Hgb concentration and total energy expenditure. Males with SCA had lower growth in height and lower BMD and entry and after two years than controls. Both males and females with SCA had lower FFM gain than controls. Pubertal attainment was slower in children with SCA than in healthy controls.
In our study, Hgb concentration was longitudinally associated with height and weight Z scores in both males and females (Figure 2). While children with SCA as a group did not had lower changes in weight and height Z scores than controls, the height and weight of children with SCA were found to be associated with Hgb concentration (Figure 2). Zemel at al. [7] found in their cohort of children that Hgb, hematocrit, and hemoglobin F concentration were associated with weight, height, and BMI scores in females but not in males. In contrast, Singhal et al. [20] reported that Hgb concentration was associated with height and weight in prepubertal Jamaican males, but not females. We previously showed that gender differences exist in the association of Hgb concentration with REE [14] and physical activity in free-living conditions [15]. Since Hgb concentration and REE were highly correlated (0.7) we used less correlated (0.3) TEE as a factor in the final models for the longitudinal mixed effects analysis predicting changes in growth status over time and found increased TEE to be an independent risk factor for poor growth. We feel that TEE is an important variable for sickle cell studies because it includes REE, as well as physical activity, and it is easier to use in calculating energy balance (energy intake minus total energy expenditure). We found that although changes in weight and BMI were not different between the groups, the BMI and weight of individuals were associated with their TEE.
These findings answer some questions raised by the results from the Stroke Prevention Trial [21] which suggested that growth failure is not inherent to the genetic disease of SCA. That study showed that children with SCA who were chronically transfused to maintain a hematocrit greater than 30% had significant improvement in height, weight, and body mass index (BMI) Z scores [22], such that after a mean of 2 years of chronic transfusion therapy, they were of comparable size to their peers without SCA. These findings raised the question as to whether impaired growth is purely a function of anemia, or if it is related to other aspects of the chronic disease [23,24]. Our study confirms that energy expenditure, in addition to hematological status, are factors affecting growth in children with SCA.
We compared children with SCA to race, gender, body size, and stage of puberty matched healthy control children independent of age. Despite that all children with SCA and majority of controls (90%) were at Tanner 2 stage at baseline, the control children progressed through puberty stages faster than the children with SCA. After two years of the study, 47% of control males and 86% control females progressed to Tanner 4 stage. At that time the majority of males (94%) and females (64%) with SCA were at Tanner 3 stage and only 6% of males and 36% of females with SCA progressing to Tanner 4 stage of development..
Other indices of pubertal development such as linear growth is steady in childhood, but increases from 5.5 cm/year in pre-pubertal children to a peak velocity of 8.3 cm/year in females and 9.5cm/year for males during puberty [25]. Therefore, one would expect children with SCA to have continued pubertal height velocity and possibly achieve normal adult heights. The differences in height velocity between children with SCA and controls are well illustrated in Figure 1.
Poor increase in BMD may be due to hormonal differences between control males, who were progressing through puberty over the 2-year study period, and SCA males who remained in relatively early pubertal stages despite the passage of 2 years. While hormone levels were not statistically different due to a broad range in controls and males with SCA, the mean levels were higher in the control group. Another factor in BMD that has been investigated in other studies is calcium and vitamin D intake [26]. Calcium intake is often low in African Americans because of lactose intolerance and cultural dietary habits [27], as well as the poor calcium intake of this age-group in general [28]. Lal et al [29] found that low BMD in children with SCA was significantly associated with vitamin D status. In our study, vitamin D intake at baseline was lower in children with SCA than in controls (21.3 ± 16.2 vs. 34.3 ± 5.5 μmol/L; p<0.05). Because puberty is a critical time to increase BMD, it is encouraging that children with SCA have prolonged puberty and thus more time to intervene and improve their BMD and possibly decrease their risk for bone fracture as suggested by Buison et al.[30]. These authors also found lower whole body mineral content in SCA males than females with SCA also observed in our study. Further analysis of associations between BMD and other factors (i.e., Hgb, TEE) were limited by a relatively small cohort size that did not allow calculating population-specific BMD Z scores.
We followed our cohort for two consecutive years after baseline measurements. A longer follow-up period would very likely provide more comprehensive results. However, we documented important trends in growth patterns and their association with the hematological status and elements of energy balance. In this study, we did not evaluate the effects of hydroxyurea and long-term transfusion therapy on growth status in children with SCA. The benefits of these treatments have been reported in other studies [7,22,31,32].
Future intervention studies should assess potential benefits of both of these treatments and other medical strategies aimed to improve growth and development of children with SCA during puberty. Options include supplementation with protein for which requirements in SCA are unknown, and supplementation with micronutrients such as zinc, calcium and/or vitamin D [10,33]. The impact of increased requirements for energy, protein, and micronutrients in SCA combined with effects of possible endocrine abnormalities on pubertal growth in SCA needs to be further investigated.
Our study indicates that during puberty, children with SCA have a slower rate and different growth pattern than non-SCA controls, allowing a longer interval for interventions to improve height, weight, and BMD. Growth velocity in children with SCA is positively associated with hemoglobin concentration and negatively associated with total energy expenditure.
Acknowledgments
We acknowledge our participants and their families for their enthusiasm and commitment to this study. We thank staff of the Clinical Research Center at Vanderbilt University for help with this project. We also thank LeMonica Adkerson, BA, Andrea Buchholz, PhD, Kay Hongu, PhD, and Cindy Dorminy, MEd for their assistance. We also thank Dr. Winfred Wang from St. Jude Sickle Cell Center in Memphis, TN for his scientific input and help with study subjects' recruitment. The study was supported in part by Vanderbilt CTSA grant 1 UL1 RR024975 from NCRR/NIH. MSB was supported by HL677715 and HL82988.
Statement of financial support: Study supported in part by Vanderbilt CTSA grant 1 UL1 RR024975 from NCRR/NIH. MSB was supported by HL677715 and HL82988.
Abbreviations
- SCA
sickle cell anemia
- BMI
body mass index
- FFM
fat free mass
- Hgb
hemoglobin
- REE
resting energy expenditure
- TEE
total energy expenditure
References
- 1.Serjeant GR. The emerging understanding of sickle cell disease. British Journal of Haematology. 2001;112(1):3–18. doi: 10.1046/j.1365-2141.2001.02557.x. [DOI] [PubMed] [Google Scholar]
- 2.Scott JP, Hillery CA, Brown ER, et al. Hydroxyurea therapy in children severely affected with sickle cell disease. The Journal of Pediatrics. 1996;128(6):820–828. doi: 10.1016/s0022-3476(96)70335-9. [DOI] [PubMed] [Google Scholar]
- 3.Kramer M, Rooks Y, Washington L, et al. Pre- and postnatal growth and development in sickle cell anemia. Journal of Pediatrics. 1980;96(5):857–860. doi: 10.1016/s0022-3476(80)80557-9. [DOI] [PubMed] [Google Scholar]
- 4.Platt O, Rosenstock W, Espeland M. Influence of sickle hemoglobinopathies on growth and development. N Engl J Med. 1984;311(1):7–12. doi: 10.1056/NEJM198407053110102. [DOI] [PubMed] [Google Scholar]
- 5.Stevens M, Maude G, Cupidore L, et al. Prepubertal growth and skeletal maturation in children with sickle cell disease. Pediatrics. 1986;78(1):124–132. [PubMed] [Google Scholar]
- 6.Whitten C. Growth status of children with sickle-cell anemia. Am J Dis Child. 1964;102(9):355–364. doi: 10.1001/archpedi.1961.02080010357009. [DOI] [PubMed] [Google Scholar]
- 7.Zemel BS, Kawchak DA, Ohene-Frempong K, et al. Effects of Delayed Pubertal Development, Nutritional Status, and Disease Severity on Longitudinal Patterns of Growth Failure in Children With Sickle Cell Disease. Pediatric Research. 2007;61(5, Part 1):607–613. doi: 10.1203/pdr.0b013e318045bdca. [DOI] [PubMed] [Google Scholar]
- 8.Ashcroft M, Serjeant G, Desai P. Heights, weights, and skeletal age of Jamaican adolescents with sickle cell anaemia. Arch Dis Child. 1972;47(254):519–524. doi: 10.1136/adc.47.254.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas PW, Singhal A, Hemmings-Kelly M, et al. Height and weight reference curves for homozygous sickle cell disease. Arch Dis Child. 2000;82:204–208. doi: 10.1136/adc.82.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawchak DA, Schall JI, Zemel BS, et al. Adequacy of Dietary Intake Declines with Age in Children with Sickle Cell Disease. Journal of the American Dietetic Association. 2007;107(5):843–848. doi: 10.1016/j.jada.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Adams J., III . Clinical laboratory diagnosis. In: Embury SH, Hebbel RP, Mohandas N, Steinberg MH, editors. Sickle cell disease: basic principles and clinical practice. Lippincott-Raven; Pennsylvania: 1994. pp. 457–468. [Google Scholar]
- 12.Tanner J. Growth at adolescence, with a general consideration of the effects of hereditary and environmental factors upon growth and maturation from birth to maturity. Blackwell Scientific Publications; Oxford: 1962. [Google Scholar]
- 13.Peterson A, Crockett L, Richards M, et al. A self-report measure pf pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1982;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 14.Buchowski MS, Chen KY, Byrne D, et al. Equation to estimate resting energy expenditure in adolescents with sickle cell anemia. Am J Clin Nutr. 2002;76:1335–1344. doi: 10.1093/ajcn/76.6.1335. [DOI] [PubMed] [Google Scholar]
- 15.Buchowski MS, Townsend KM, Williams R, et al. Patterns and energy expenditure of free-living physical activity in adolescents with sickle cell anemia. J Pediatr. 2002;140:86–92. doi: 10.1067/mpd.2002.120689. [DOI] [PubMed] [Google Scholar]
- 16.Euling SY, Herman-Giddens ME, Lee PA, et al. Examination of US Puberty-Timing Data from 1940 to 1994 for Secular Trends: Panel Findings. Pediatrics. 2008;121(Supplement_3):S172–191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- 17.Buchholz AC, Majchrzak KM, Chen KY, et al. Use of Air Displacement Plethysmography in the Determination of Percentage of Fat Mass in African American Children. Pediatr Res. 2004;56(1):47–54. doi: 10.1203/01.PDR.0000130477.05324.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun M, Reed GW, Hill JO. Modification of a whole room indirect calorimeter for measurement of rapid changes in energy expenditure. J Appl Physiol. 1994;76(6):2686–2691. doi: 10.1152/jappl.1994.76.6.2686. [DOI] [PubMed] [Google Scholar]
- 19.Poehlman ET, Toth MJ. Mathematical ratios lead to spurious conclusions regarding age- and sex- related differences in resting metabolic rate. Am J Clin Nutr. 1995;61(3):482–485. doi: 10.1093/ajcn/61.3.482. [DOI] [PubMed] [Google Scholar]
- 20.Singhal A, Thomas P, Cook R, et al. Delayed adolescent growth in homozygous sickle cell disease. Arch Dis Child. 1994;71(5):404–408. doi: 10.1136/adc.71.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MT, Piomelli S, Granger S, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood. 2006;108(3):847–852. doi: 10.1182/blood-2005-10-009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang WC, Morales KH, Scher CD, et al. Effect of Long-term Transfusion on Growth in Children with Sickle Cell Anemia: Results of the Stop Trial. The Journal of Pediatrics. 2005;147(2):244–247. doi: 10.1016/j.jpeds.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 23.Akohoue S, Shankar S, Milne G, et al. Energy expenditure, inflammation, and oxidative stress in steady-state adolescents with sickle cell anemia. Pediatr Res. 2007;61:233–238. doi: 10.1203/pdr.0b013e31802d7754. [DOI] [PubMed] [Google Scholar]
- 24.Smiley D, Dagogo-Jack S, Umpierrez G. Therapy Insight: metabolic and endocrine disorders in sickle cell disease. Nat Clin Pract End Met. 2008;4(2):102–109. doi: 10.1038/ncpendmet0702. [DOI] [PubMed] [Google Scholar]
- 25.Veldhuis JD, Roemmich JN, Richmond EJ, et al. Endocrine Control of Body Composition in Infancy, Childhood, and Puberty. Endocr Rev. 2005;26(1):114–146. doi: 10.1210/er.2003-0038. [DOI] [PubMed] [Google Scholar]
- 26.Weaver CM. Vitamin D, Calcium Homeostasis, and Skeleton Accretion in Children. Journal of Bone and Mineral Research. 2007;22(s2):V45–V49. doi: 10.1359/jbmr.07s201. [DOI] [PubMed] [Google Scholar]
- 27.Johnson AO, Semenya JG, Buchowski MS, et al. Correlation of lactose maldigestion, lactose intolerance, and milk intolerance. Am J Clin Nutr. 1993;57(3):399–401. doi: 10.1093/ajcn/57.3.399. [DOI] [PubMed] [Google Scholar]
- 28.Pribila BA, Hertzler SR, Martin BR, et al. Improved Lactose Digestion and Intolerance Among African-American Adolescent Girls Fed a Dairy Rich-Diet. Journal of the American Dietetic Association. 2000;100(5):524–528. doi: 10.1016/S0002-8223(00)00162-0. [DOI] [PubMed] [Google Scholar]
- 29.Lal A, Fung E, Pakbaz Z, et al. Bone mineral density in children with sickle cell anemia. Pediatric Blood & Cancer. 2006;47(7):901–906. doi: 10.1002/pbc.20681. [DOI] [PubMed] [Google Scholar]
- 30.Buison AM, Kawchak DA, Schall JI, et al. Bone Area and Bone Mineral Content Deficits in Children With Sickle Cell Disease. Pediatrics. 2005;116(4):943–949. doi: 10.1542/peds.2004-2582. [DOI] [PubMed] [Google Scholar]
- 31.Fung EB, Barden EM, Kawchak DA, et al. Effect of hydroxyurea therapy on resting energy expenditure in children with sickle cell disease. J Pediatr Hematol Oncol. 2001;23(9):604–608. doi: 10.1097/00043426-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Wang WC, Helms RW, Lynn HS, et al. Effect of hydroxyurea on growth in children with sickle cell anemia: results of the HUG-KIDS Study. J Pediatr. 2002;140(2):225–229. doi: 10.1067/mpd.2002.121383. [DOI] [PubMed] [Google Scholar]
- 33.Zemel BS, Kawchak DA, Fung EB, et al. Effect of zinc supplementation on growth and body composition in children with sickle cell disease. Am J Clin Nutr. 2002;75:300–307. doi: 10.1093/ajcn/75.2.300. [DOI] [PubMed] [Google Scholar]