Abstract
Transient receptor potential melastatin 2 (TRPM2) proteins form multiple-subunit complexes, most likely homotetramers, which operate as Ca2+-permeable, nonselective cation channels activated by intracellular ADP-ribose (ADPR) and oxidative stress. Each TRPM2 channel subunit is predicted to contain two coiled-coil (CC) domains, one in the N-terminus and the other in the C-terminus. Our recent study has shown that the C-terminal CC domain plays an important, but not exclusive, role in the TRPM2 channel assembly. This study aimed to examine the potential role of the N-terminal CC domain. Domain deletion dramatically reduced protein expression and abolished ADPR-evoked currents but did not alter the subunit interaction. Deletion of both CC domains strongly attenuated the subunit interaction, confirming that the C-terminal CC domain is critical in the subunit interaction. Glutamine substitutions into individual hydrophobic residues at positions a and d in the heptad repeats to disrupt the CC formation had no effect on protein expression, subunit interaction, or ADPR-evoked currents. Mutation of Ile658 to glutamine, which did not perturb the CC formation, decreased ADPR-evoked currents without affecting protein expression, subunit interaction, or membrane trafficking. These results collectively suggest the requirement for the N-terminal CC domain for protein expression and function, but not subunit interaction, of the TRPM2 channel.
Keywords: TRPM2, Coiled coil, Subunit interaction, Expression, ADPR-evoked current
TRPM2 belongs to the melastatin subfamily of transient receptor potential (TRPM) proteins (Clapham 2003; Fleig and Penner 2004; Ramsey et al. 2006; Nilius et al. 2007; Venkatachalam and Montell 2007) and forms Ca2+-permeable, nonselective cation channels activated by ADP-ribose (ADPR) and oxidative stress such as H2O2 (Perraud et al. 2001; Sano et al. 2001; Naziroğlu and Lückhoff 2008a, b). Functional expression of TRPM2 or TRPM2-like channels has been documented in immune, cardiovascular, neuronal, and endocrine cells, where they mediate Ca2+ influx-dependent chemokine production (Yamamoto et al. 2008), increased endothelial permeability (Hecquet et al. 2008), insulin secretion (Togashi et al. 2006), and cytotoxicity in response to oxidative stress (Hara et al. 2002; Wehage et al. 2002; Zhang et al. 2003, 2006; Fonfria et al. 2005).
TRPM2 channels, like other TRP channels, are thought to assemble as homotetramers. Each TRPM2 channel subunit comprises large N- and C-termini residing intracellularly and six membrane-spanning segments (S1–S6), with a pore-forming domain between S5 and S6. The mechanisms governing assembly of the TRP channels including TRPM2 are not fully understood, nonetheless, distinct domains in the N-terminus, transmembrane region, and/or C-terminus have been identified as important molecular determinants in subunit interaction and assembly of TRP channels (Lepage and Boulay 2007; Jiang 2007; Schindl and Romanin 2007). Therefore, for the TRPM subfamily, the C-terminus and, particularly, the coiled-coil (CC) domain have been demonstrated to be critical for the subunit interaction and assembly of functional TRPM2, TRPM4, and TRPM8 channels (Launay et al. 2004; Erler et al. 2006; Mei et al. 2006a; Tsuruda et al. 2006). In addition to the C-terminal CC domain, these channel subunits contain another CC domain located in the N-terminus (Fig. 1a). For the TRPM2 channel, our recent study suggests that the C-terminal CC domain plays a primary, but not exclusive, role in subunit interaction and channel assembly (Mei et al. 2006a). The present study examined, using molecular, biochemical, and electrophysiological approaches, the potential contribution of the N-terminal CC domain in TRPM2 subunit interaction and formation of the functional TRPM2 channel.
Fig. 1.
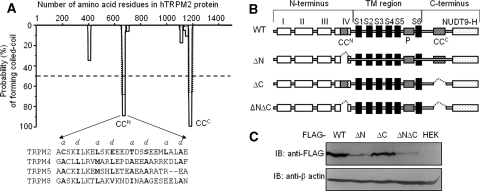
Coiled-coil (CC) domains in TRPM2 subunit and their role in TRPM2 protein expression. a Amino acid sequence analysis using the COILS program (http://www.ch.embnet.org/software/COILS_form.html) indicates two domains in the human TRPM2 subunit that exhibit a high probability of CC formation. Solid and dotted lines represent the results using 24- and 28-residue windows, respectively. CCN, N-terminal CC domain from Ala654 to Glu681; CCC, C-terminal CC domain from Gly1171 to Ala1200. Inset: Amino acid sequences of the N-terminal CC domains in human TRPM2, TRPM4, TRPM5, and TRPM8 subunits. b Linear representation of molecular/structural features of the human TRPM2 subunit. In the intracellular N-terminus, there are four domains (I–IV) that are highly conserved among the TRPM channels, and the CC domain is located within the IV domain. The transmembrane (TM) domain comprises six transmembrane segments (S1–S6) and a pore loop (P) between S5 and S6. The intracellular C-terminus contains another CC domain and TRPM2- specific NUDT9 homology domain, where ADPR binds. The TRPM2 deletion mutants examined in this study: ΔN, lack of residues Ala654–Ala686; ΔC, lack of residues Leu1167–Ser1201; ΔNΔC, lack of both domains. c Western blot analysis of whole-cell lysates prepared from cells expressing the wild type (WT), ΔN, ΔC, or ΔNΔC mutant, or nontransfected cells (HEK), detected using an anti-FLAG antibody (top) and an anti-β-actin antibody (bottom)
Materials and Methods
Reagents, Molecular Biology, and Cell Biology
All reagents or chemicals used in this study were from Sigma except those individually specified otherwise. Human embryonic kidney (HEK) 293 cells were cultured in Dulbecco’s modified Eagle medium (supplemented with 2 mM L-glutamine and 10% heat-inactivated fetal bovine serum) at 37°C under 5% CO2 humidified conditions. Deletion and point mutants were generated as described previously (Mei et al. 2006b) and confirmed by sequencing. TRPM2 subunits were C-terminally tagged with either an EE (EYMPME) or a FLAG epitope. HEK293 cells were transfected using Lipofectamine2000 reagent (Invitrogen) and used 24–48 h later.
Coimmunoprecipitation, Biotin Labeling, and Western Blotting
These experiments were described in detail in our previous studies (Mei et al. 2006a, b; Xia et al. 2008) and are briefly summarized below. For immunoprecipitation, transfected cells were lysed in 300 μl lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 2 mM EGTA, 1% Triton X-100, and 5% glycerol) containing protease inhibitor cocktail (Roche) at 4°C for 30 min. The lysates were then mixed with 3 μl anti-FLAG M2 antibody and incubated at 4°C for 2 h, and after the addition of 20-μl protein A/G-agarose bead suspension (Santa Cruz), the incubation continued overnight. The immunoprecipitated proteins were suspended in 40 μl electrophoresis buffer (50 mM Tris–HCl, pH 7.4, 100 mM DTT, 2% SDS, 10% glycerol, and 0.05% bromophenol blue) and resolved on 10% SDS-PAGE gels. The TRPM2 proteins were detected by Western blotting using primary mouse anti-FLAG M2 (at a dilution of 1:1000) or rabbit anti-EE (1:2000; Bethyl) antibodies, and secondary horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit IgG antibodies (1:2000, Santa Cruz), and the β-actin using primary mouse anti-β-actin antibody (1:1000; Santa Cruz) and secondary HRP conjugated with anti-mouse IgG antibody. Protein bands were quantified using a gel doc XR quantity system (Bio-Rad). The subunit interaction was assessed by the intensity of the EE-tagged wild-type (WT) subunit coimmunoprecipitated with the FLAG-tagged mutant subunit, normalized to the intensity of the EE-tagged WT subunit coimmunoprecipitated with the FLAG-tagged WT subunit. The difference in protein expression was corrected by normalizing the intensity of the mutant subunits to that of the parental WT subunit in parallel experiments. For biotin labeling, cells were incubated in phosphate-buffered saline (PBS) (136 mM NaCl, 1.4 mM KCl, 10 mM Na2HPO4, 1.7 mM KH2PO4, pH 8.0) containing 1 mg/ml sulfo-NHS-biotin (Pierce) for 30 min at 4°C, followed by extensive washing with PBS containing 50 mM glycine. The same immunoprecipitation and Western blotting protocols as described above were followed; cell lysates were prepared in 300 μl lysis buffer and immunoprecipitated with the anti-FLAG M2 antibody. Proteins were detected by Western blotting using HRP-conjugated streptavidin (1:1000; Pierce) and mouse anti-FLAG M2 antibody (1:1000) to detect the proteins at the cell surface and the total amount of proteins.
Electrophysiological Recording
Whole-cell recordings were made at room temperature using an Axopatch 200B amplifier in extracellular (147 mM NaCl, 2 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 13 mM glucose, pH7.3) and intracellular (147 mM NaCl, 0.05 mM EGTA, 10 mM HEPES, 1 mM Na2ATP, and 1 mM ADPR, pH 7.3) solutions as described previously (Mei et al. 2006a). Cells were held at −40 mV and voltage ramps of 1-s duration from −120 to 80 mV were applied every 5 s. Flufenamic acid (FFA; 0.5 mM) or N-(p-amylcinnamoyl)anthranilic acid (ACA; 20 μM), which gave rise to complete inhibition of TRPM2 channel currents (Hill et al. 2006; Kraft et al. 2006), was extracellularly applied to patched cells via a RSC160 rapid solution changer system (Bio-Logic Science Instruments, France) at the end of every recording in order to assess the leak currents, and recordings with leak currents < 100 pA or <5% of the total currents at −80 mV were used in analysis. The currents plotted in figures were taken at −80 mV.
Data Analysis
The data, where appropriate, are presented as mean ± SE values. Comparisons were made using Student’s t-test between two groups or analysis of variance (post hoc Turkey) between multiple groups.
Results
Effects of N-Terminal CC Domain Deletion on Subunit Interaction
We started with introduction deletion of the N-terminal CC domain into the FLAG-tagged WT, resulting in the ΔN mutant, and also into a FLAG-tagged mutant lacking the C-terminal CC domain (ΔC; Mei et al. 2006a), generating the ΔNΔC double-deletion mutant (Fig. 1b). We performed Western blotting, using an anti-FLAG antibody, to compare the protein expression of WT, ΔN, ΔC, and ΔNΔC mutants, following transient expression in HEK293 cells. Unlike deletion of the C-terminal CC domain, which had no significant effect on protein expression (Fig. 1c) (Mei et al. 2006a), deletion of the N-terminal CC domain led to a dramatic reduction in protein expression (Fig. 1c). These results suggest that the N-terminal CC domain is crucial for protein expression.
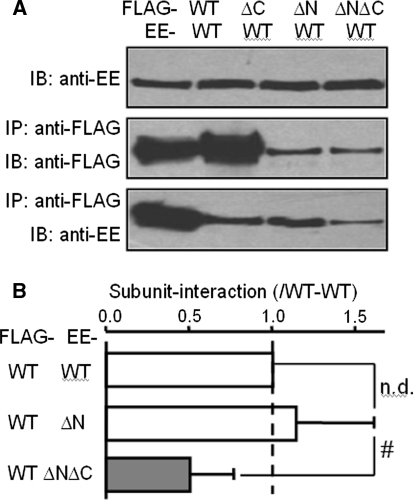
We then moved to coexpress the FLAG-tagged WT or deletion mutant subunits with the EE-tagged WT subunit and performed coimmunoprecipitation to examine whether the N-terminal CC domain was involved in subunit interaction. Figure 2 shows the results. The WT-ΔN subunit interaction appeared to be decreased (Fig. 2a), however, after correction of the reduced protein expression of the ΔN mutant (see Materials and Methods), it was in fact not significantly different from the WT-WT subunit interaction (n = 4; P > 0.1, paired t-test). This contrasted with the WT-ΔC subunit interaction, which was much weaker than the WT-WT subunit interaction (Fig. 2a) as we reported previously (Mei et al. 2006b). For the ΔNΔC double-deletion mutant, the expression of the ΔNΔC mutant protein was comparable to that of the ΔN mutant (middle lane in Fig. 2a). However, the WT-ΔNΔC subunit interaction, compared to the WT-ΔN subunit interaction, was reduced by 53% ± 7% (n = 4; P < 0.001, paired t-test) (Fig. 2b). These results, on one hand, provide further supporting evidence for our previous finding that the C-terminal CC domain is important in subunit interaction (Mei et al. 2006b) and, on the other hand, argue against a significant contribution of the N-terminal CC domain in subunit interaction.
Fig. 2.
Effects of deleting N- and C-terminal CC domains on protein expression and subunit interaction. a Western blot analysis of whole-cell lysate or protein samples immunoprecipitated with an anti-FLAG antibody from cells coexpressing the FLAG-tagged WT or mutant subunit with the EE-tagged WT subunit. Top: proteins in whole-cell lysate detected using an anti-EE antibody. Middle: immunoprecipitated proteins detected using an anti-FLAG antibody. Bottom: immunoprecipitated proteins detected using an anti-EE antibody. b Summary of the results of four independent experiments as shown in a. n.d. no statistically significant difference in subunit interaction between WT-WT and WT-ΔN. # P < 0.05, WT-ΔN versus WT-ΔNΔC
Effects of Mutating Hydrophobic Residues at Positions a and d of the Heptad Repeats on Protein Expression and Subunit Interaction
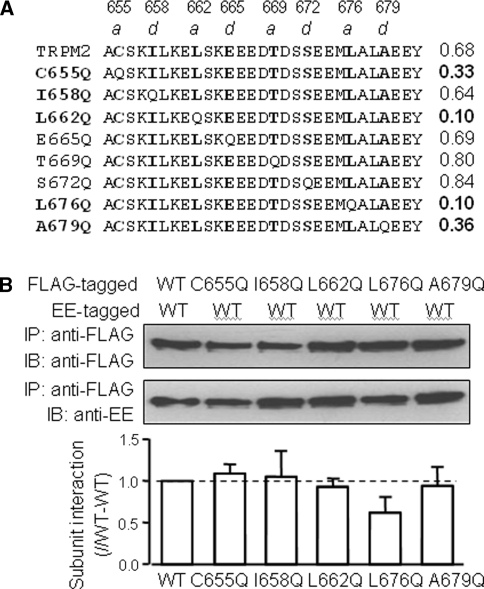
The canonical CC domain is composed of heptad repeats, denoted by abcdefg, in which positions a and d are preferentially occupied by hydrophobic residues that are critical in mediating protein–protein interactions (Lupas 1996). Amino acid sequence analysis indicates that single substitution with hydrophilic glutamine of Cys655, Leu662, Leu676, and Ala679 residues strongly disrupts the N-terminal CC formation (Fig. 3a). Thus, to further investigate the role of the N-terminal CC domain, we generated point mutants, in which each of these four residues was individually replaced with glutamine. We also introduced glutamine substitution of Ile658, which is expected to have no effect on CC formation (Fig. 3a). We examined the effect on protein expression and subunit interaction using the same experimental protocols described above for the deletion mutants. As shown in Fig. 3b, there was no reduction in protein expression by any of these mutations, whether expressed on their own (data not shown) or coexpressed with the EE-tagged WT subunit (top panel in Fig. 3b). Moreover, the mutations had no statistically significant effect on the protein interaction between EE-tagged WT and FLAG-tagged mutant subunits (bottom panel in Fig. 3b). Consistent with the deletion mutant, these results suggest that the hydrophobic residues in the N-terminal CC domain are not critical for subunit interaction.
Fig. 3.
Effects of mutational disruption of N-terminal coiled-coil (CC) formation on TRPM2 protein expression and subunit interaction. a Top: amino acid sequence of the TRPM2 N-terminal CC domain and predicted effects of glutamine substitution (denoted at the left) on CC formation probability (denoted by the numbers on the right). The mutations at positions a and d, in boldface, were investigated in this study. The numbers at the top indicate the location of residues in the human TRPM2 subunit. b Top: Western blot analysis of proteins immunoprecipitated with an anti-FLAG antibody from cells coexpressing the FLAG-tagged WT or point mutant subunit with the EE-tagged WT subunit. Bottom: summary of the results of three independent experiments. WT-mutant subunit interaction was normalized to the WT-WT subunit interaction in each parallel experiment. No significant difference was found in WT-mutant subunit interaction compared to WT-WT
Effects of Deleting and Point-Mutating N-Terminal CC Domain on Formation of Functional TRPM2 Channels
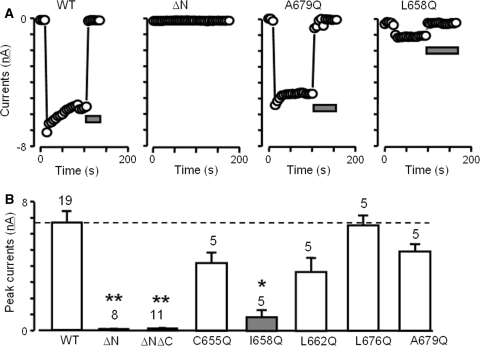
In parallel with the biochemical experiments described above, we used patch-clamp technique to measure ADPR-evoked whole-cell currents to investigate the effects of deleting the N-terminal CC domain and glutamine-substituting the hydrophobic residues in the heptad repeats on the formation or assembly of TRPM2 channels. Figure 4 shows the results. In cells expressing the WT, strong current responses were consistently elicited by ADPR at 1 mM, a supermaximal concentration (Mei et al. 2006a, b). The ADPR-evoked currents exhibited complete blockage by the TRPM2 channel inhibitors (Fig. 4a) and a linear relationship with voltage (data not shown), typical properties of TRPM2 channels. No or very little current was detectable in cells expressing the ΔN or ΔNΔC mutant. ADPR-evoked currents were observed in cells expressing C665Q, L662Q, L676Q, or A679Q mutants; the mean currents for C665Q, L662Q, and A679Q were modestly reduced compared to the WT but the reduction was not statistically significant (Fig. 4b). These functional results are consistent with coimmunoprecipitation and, taken together, suggest that these hydrophobic residues, which are critical for CC formation (Fig. 3a), are not essential for formation of functional TRPM2 channels. On the other hand, the mean currents were significantly reduced by I658Q mutation (Fig. 4a, b), which is predicted not to alter CC formation (Fig. 3a). We further examined the surface protein expression of this mutant in the biotin-labeling assay. The surface protein expression of the I658Q mutant was 136% ± 35% that of the WT (n = 3; P > 0.05, paired t-test), thus largely excluding the possibility that a mutational defect in membrane trafficking was responsible for the reduced channel function.
Fig. 4.
Effects of deleting and point-mutating N-terminal CC domain on formation of functional TRPM2 channels. a Example current recordings showing responses at −80 mV to intracellular ADPR (1 mM) in cells expressing WT or indicated mutants. Note the complete blockage of ADPR-evoked currents by flufenamic acid (FFA; 0.5 mM) or ACA (20 μM) indicated by the horizontal bars. b Summary of the peak currents from cells expressing WT or indicated mutants at −80 mV. The number of cells examined in each case is denoted at the top of the bars. * P < 0.01 and ** P < 0.001, versus the WT
Discussion
The present study examined the role of the N-terminal CC domain in TRPM2 protein expression and subunit interaction, and assembly of functional TRPM2 channels, and provided evidence indicating that this domain is important for protein expression and function, but not for subunit interaction of the TRPM2 channel.
The primary sequence of the TRPM2 channel subunit predicts the presence of two CC domains, one in the C-terminus and the other in the N-terminus (Fig. 1a). Our previous study showed a primary, but not exclusive, role for the C-terminal CC domain in the subunit interaction, which is confirmed in this study (Fig. 2a), and assembly of functional TRPM2 channels (Mei et al. 2006b). This study examined whether the N-terminal CC domain is also involved, as previously shown for this domain in the TRPC and some TRPV channels (Lepage and Boulay 2007; Schindl and Romanin 2007). However, the evidence obtained in this study argues against this idea. In our coimmunoprecipitation experiments with the domain deletion ΔN mutant, we found that the WT-ΔN subunit interaction was not different from the WT-WT subunit interaction (Fig. 2). In contrast, we observed a significant reduction in the WT-ΔNΔC subunit interaction compared to the WT-ΔN subunit interaction (Fig. 2a), providing further supporting evidence for our previous finding of a critical role of the C-terminal CC domain (Mei et al. 2006b). More evidently, disruption of the N-terminal CC domain formation by mutating the hydrophobic residues at positions a and d in the heptad repeats to hydrophilic residue glutamine (Fig. 3a) was without effect on the subunit interaction (Fig. 3b). Finally, these CC domain-disrupting mutations had no effect on the formation or assembly of functional TRPM2 channels, as indicated by the lack of a significant reduction in ADPR-evoked currents (Fig. 4). Taken together, these results provide clear evidence disregarding a significant role of the N-terminal CC domain in TRPM2 subunit interaction and assembly of functional TRPM2 channels. Therefore, the results of the present and previous studies by us and other groups strongly suggest that the molecular basis for assembly of TRPM, TRPC, and TRPV channels is fundamentally different (Lepage and Boulay 2007; Jiang 2007; Schindl and Romanin 2007).
This study instead revealed an important role of the N-terminal CC domain in TRPM2 expression. Deletion of this domain resulted in a dramatic reduction in protein expression (Figs. 1c and 2a), which in large part accounts for the complete loss of ADPR-evoked currents (Fig. 4). However, a minor contribution due to defective membrane trafficking cannot be excluded, considering the very low, yet detectable protein expression and subunit interaction. Further point mutation study showed that disruption of the CC formation caused no profound effect on protein expression (Fig. 3), suggesting that the importance of this domain in protein expression does not pertain to its being a CC domain.
Finally, we showed that substitution with glutamine of the Ile658 residue impaired channel function, as evidenced by a significant reduction in ADRP-evoked currents. This mutation was “negative” in the sense that it was predicted not to affect CC formation (Fig. 3a), which was consistent with a lack of any significant effect on the subunit interaction (Fig. 3b). Further biotin-labeling assay demonstrated that this mutation also had no effect on membrane trafficking (see Results). The ADPR binding site is located with the NUDT9-H domain in the C-terminus (Fig. 1b) (Perraud et al. 2001). Thus, the reduction in ADPR-evoked currents by I658Q mutation implies that Ile658 is important in normal TRPM2 channel function and, most likely, channel gating.
In summary, we showed that the N-terminal CC domain is important for protein expression but not for subunit interaction of the TRPM2 channel subunit and that the Ile658 residue in this domain is required for normal TRPM2 channel function. This N-terminal CC domain is also present in the TRPM4, TRPM5, and TRPM8 channels (Fig. 1a), and thus the results of this study on the TRPM2 channel should help evolve a better understanding of the molecular mechanisms controlling protein expression, subunit interaction, assembly, and function of these TRPM channels as well as TRPM2.
Acknowledgments
This work was supported by the Wellcome Trust. We are grateful to Dr. A. M. Scharenberg, University of Washington, Seattle, USA, for providing the hTRPM2 cDNA clone and Professor D. J. Beech for his generous support and suggestion during the project.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Clapham DE (2003) TRP channels as cellular sensors. Nature 426:517–524 [DOI] [PubMed]
- Erler I, Al-Ansary DM, Wissenbach U, Wagner TF, Flockerzi V, Niemeyer BA (2006) Trafficking and assembly of the cold-sensitive TRPM8 channel. J Biol Chem 281:38396–38404 [DOI] [PubMed]
- Fleig A, Penner R (2004) The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharmacol Sci 25:633–639 [DOI] [PubMed]
- Fonfria E, Marshall IC, Boyfield I, Skaper SD, Hughes JP, Owen DE, Zhang W, Miller BA, Benham CD, McNulty S (2005) Amyloid beta-peptide(1–42) and hydrogen peroxide-induced toxicity are mediated by TRPM2 in rat primary striatal cultures. J Neurochem 95:715–723 [DOI] [PubMed]
- Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9:163–173 [DOI] [PubMed]
- Hecquet CM, Ahmmed GU, Vogel SM, Malik AB (2008) Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res 102:347–355 [DOI] [PubMed]
- Hill K, Tigue NJ, Kelsell RE, Benham CD, McNulty S, Schaefer M, Randall AD (2006) Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology 50:89–97 [DOI] [PubMed]
- Jiang L-H (2007) Subunit interaction in channel assembly and functional regulation of transient receptor potential melastatin (TRPM) channels. Biochem Soc Trans 35:86–88 [DOI] [PubMed]
- Kraft R, Grimm C, Frenzel H, Harteneck C (2006) Inhibition of TRPM2 cation channels by N-(p-amylcinnamoyl)anthranilic acid. Br J Pharmacol 148:264–273 [DOI] [PMC free article] [PubMed]
- Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP (2004) TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109:397–407 [DOI] [PubMed]
- Lepage PK, Boulay G (2007) Molecular determinants of TRP channel assembly. Biochem Soc Trans 35:81–83 [DOI] [PubMed]
- Lupas A (1996) Coiled coils: new structures and new functions. Trends Biochem Sci 21:375–382 [PubMed]
- Mei ZZ, Mao HJ, Jiang L-H (2006a) Conserved cysteine residues in the pore region are obligatory for human TRPM2 channel function. Am J Physiol Cell Physiol 291:C1022–C1028 [DOI] [PMC free article] [PubMed]
- Mei ZZ, Xia R, Beech DJ, Jiang L-H (2006b) Intracellular coiled-coil domain engaged in subunit interaction and assembly of melastatin-related transient receptor potential channel 2. J Biol Chem 281:38748–38756 [DOI] [PMC free article] [PubMed]
- Naziroğlu M, Lückhoff A (2008a) Effects of antioxidants on calcium influx through TRPM2 channels in transfected cells activated by hydrogen peroxide. J Neurol Sci 270:152–158 [DOI] [PubMed]
- Naziroğlu M, Lückhoff A (2008b) A calcium influx pathway regulated separately by oxidative stress and ADP-ribose in TRPM2 channels:single channel events. Neurochem Res 33:1256–1262 [DOI] [PubMed]
- Nilius B, Owsianik G, Voets T, Peters JA (2007) Transient receptor potential cation channels in disease. Physiol Rev 87:165–217 [DOI] [PubMed]
- Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM (2001) ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411:595–599 [DOI] [PubMed]
- Ramsey IS, Delling M, Clapham DE (2006) An introduction to TRP channels. Annu Rev Physiol 68:619–647 [DOI] [PubMed]
- Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K (2001) Immunocyte Ca2+ influx system mediated by LTRPC2. Science 293:1327–1390 [DOI] [PubMed]
- Schindl R, Romanin C (2007) Assembly domains in TRP channels. Biochem Soc Trans 35:84–85 [DOI] [PubMed]
- Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M (2006) TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J 25:1804–1815 [DOI] [PMC free article] [PubMed]
- Tsuruda PR, Julius D, Minor DL (2006) Coiled coils direct assembly of a cold-activated TRP channel. Neuron 51:201–212 [DOI] [PMC free article] [PubMed]
- Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417 [DOI] [PMC free article] [PubMed]
- Wehage E, Eisfeld J, Heiner I, Jüngling E, Zitt C, Lückhoff A (2002) Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem 277:23150–23156 [DOI] [PubMed]
- Xia R, Mei ZZ, Mao HJ, Yang W, Dong L, Bradley H, Beech DJ, Jiang LH (2008) Identification of pore residues engaged in determining divalent cationic permeation in transient receptor potential melastatin subtype channel 2. J Biol Chem 283:27426–27432 [DOI] [PMC free article] [PubMed]
- Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, Kaneko S, Lange I, Fleig A, Penner R, Nishi M, Takeshima H, Mori Y (2008) TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 14:738–747 [DOI] [PMC free article] [PubMed]
- Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K, Miller BA (2003) A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem 278:16222–16229 [DOI] [PubMed]
- Zhang W, Hirschler-Laszkiewicz I, Tong Q, Conrad K, Sun SC, Penn L, Barber DL, Stahl R, Carey DJ, Cheung JY, Miller BA (2006) TRPM2 is an ion channel that modulates hematopoietic cell death through activation of caspases and PARP cleavage. Am J Physiol Cell Physiol 290:C1146–C1159 [DOI] [PubMed]